Volatile organic compounds (VOCs) are a class of organic compounds that, due to their low boiling points, are easily detectable as vapors. They are released in the atmosphere by many anthropogenic sources including transportation, industrial processing, biomass burning, organic solvent, and natural sources such as microbial production and emissions from vegetation [
43]. Concentrations of VOCs are much higher indoors compared to outdoors because a wide range of consumer and personal care products, during their use, can release significant amounts of VOCs into the air. Therefore, in addition to CO
2 level, VOCs concentration is used as a parameter to establish the air quality in an indoor environment [
44,
45].
The monitoring of VOCs by nanostructured metal-oxide-based sensors has been widely investigated and many of them have proven to be effective for this purpose. Among them, ZnO has been one of the best performing sensitive materials for the detection of different species including ethanol, acetone, formaldehyde, acetylene, toluene, benzene, and n-butanol [
46]. For this reason, most of the works related to 2D ZnO have been aimed at the investigation of the sensing properties and the development of conductometric sensors for the monitoring of VOCs.
3.1.1. Ethanol
Liu et al. [
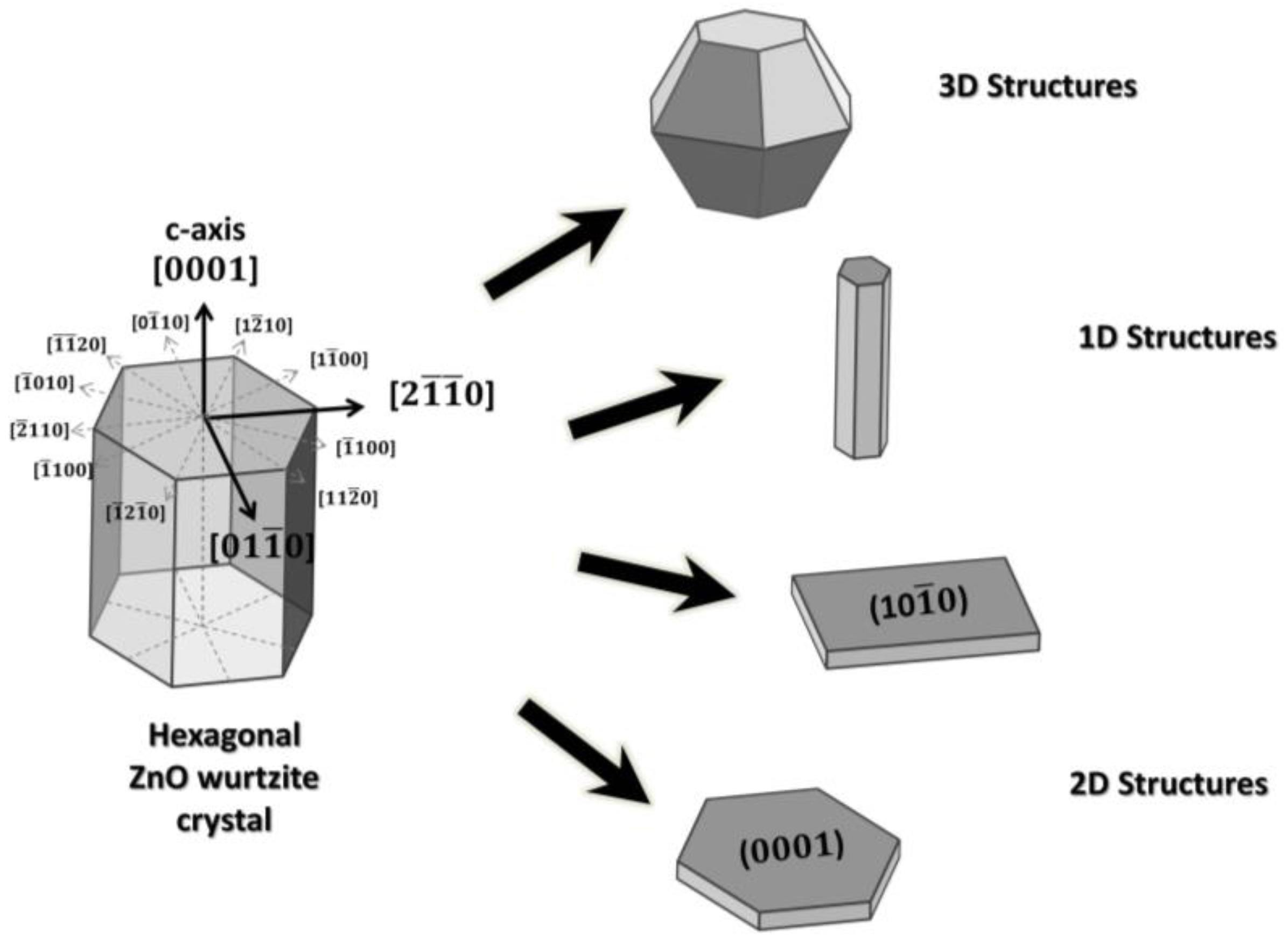
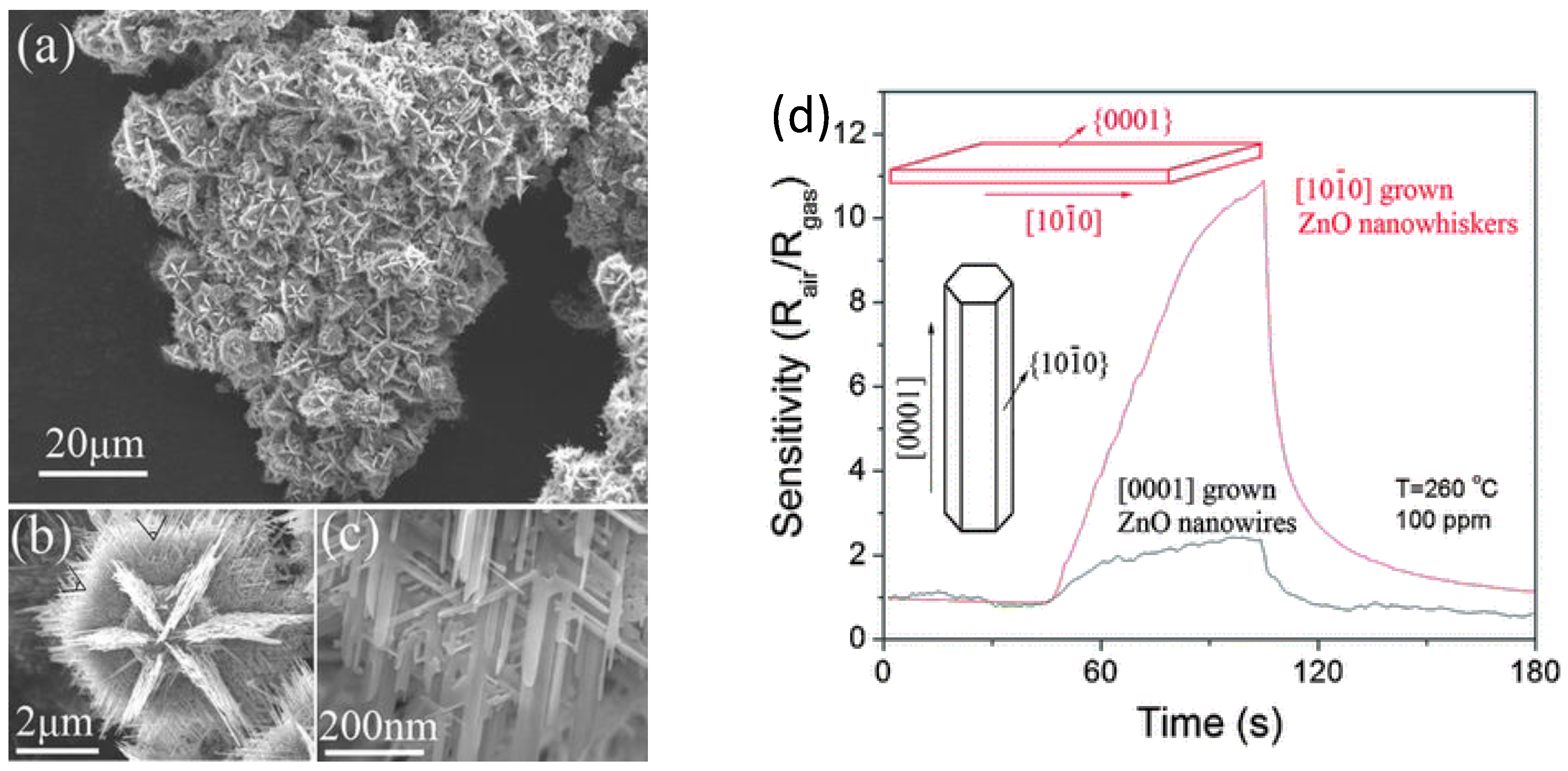
29] reported a simple gas-phase process to synthesize 3D hierarchical nanostructure based on ZnO nanowhiskers. By the adopted method it was possible, through controlling the ZnO growth along [
] direction, to grow nanowhiskers with exposed reactive {0001} facets. These nanowhiskers, self-assembling to form ZnO hollow spheres, stabilized the materials against agglomeration (
Figure 2a–c). Comparing the ethanol sensing responses of such structures with typical [0001] grown ZnO nanowires, a great enhancement of gas sensitivities for ZnO nanowhiskers based material was observed (
Figure 2d). It was also shown by calculation of the chemisorption energy of oxygen, that reactive {0001} facets energetically favored the chemisorption of oxygen species, which enhanced ethanol sensing properties compared to ZnO nanowires, which exhibited {
} side surfaces.
Similarly to the previous work, Kaneti et al. [
11] demonstrated the strong dependence of the ZnO crystal facets exposed to the gas on the sensing performance. Both ZnO nanoplates and nanorods with exposed (0001) and (
) crystal surfaces, respectively, were synthesized through facile solvothermal methods. ZnO products were mixed with polyvinylidene fluoride (PVDF) and 1-methyl-2-pyrollidone to prepare a paste, then spread onto an alumina ceramic tube with a pair of previously printed Au electrodes. The coated ceramic tube was subsequently aged at 450 °C to improve the stability of the film. The gas-sensing tests showed that ZnO hexagonal nanoplates exhibited sensitivity that was twice as high towards ethanol than ZnO nanorods at a similar optimum operating temperature of 300 °C. This difference was attributed to the larger surface area of the nanoplates compared to the nanorods (14.4 m
2/g vs. 7.43 m
2/g) and to the exposed (0001) planes of the nanoplates. By DFT simulation, the authors demonstrated a stronger adsorption of ethanol molecules on the (0001) ZnO planes as an effect of both the stronger adsorption energy of ethanol on O
− species and stronger hybridization between the adsorbed O2p orbital and the HOMO, HOMO − 1 molecular orbitals.
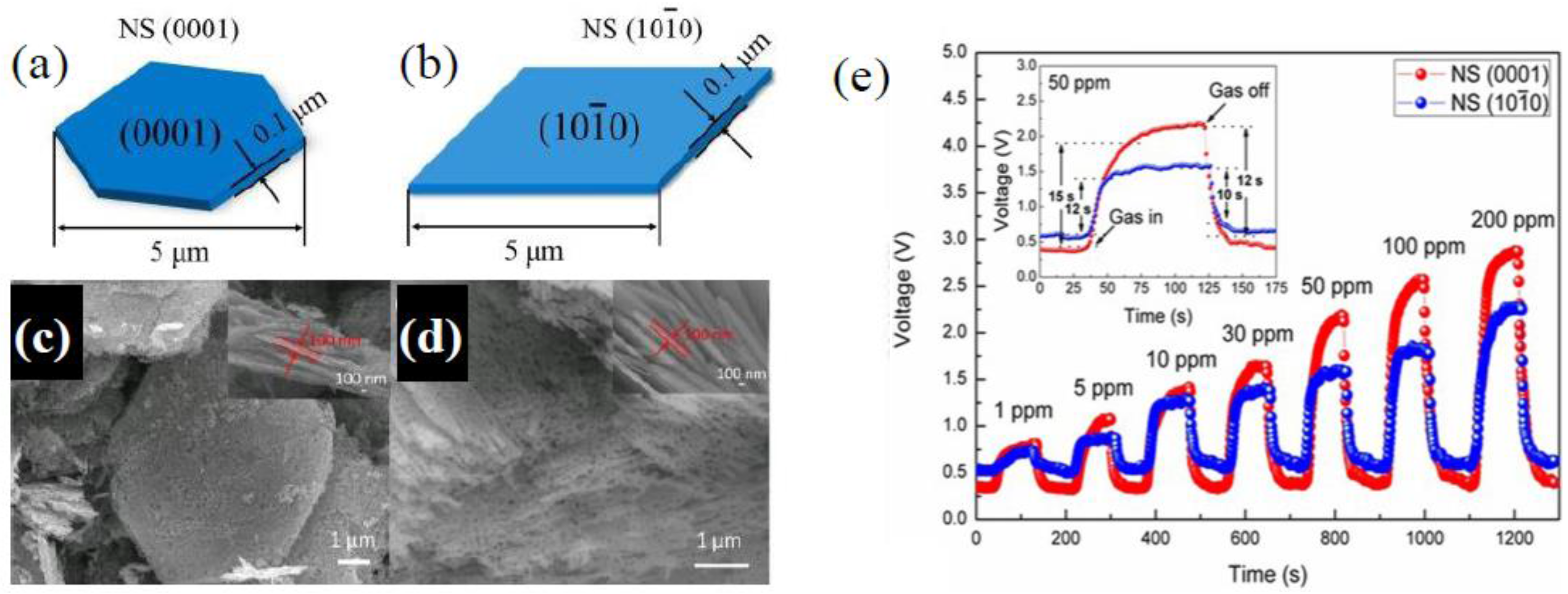
More recently, Xu et al. [
34] by facile hydrothermal routes, synthesized two porous ZnO nanosheets with different exposed facets. The synthesized nanosheets had similar specific surface areas of about 7.5 m
2/g, thickness about 100 nm, lateral size about 5 µm, and pore size of 10 nm; however, their dominating exposed crystal facets were (0001) and (
), respectively (
Figure 3a–d). For the sensing measurements, a paste made of ZnO powders mixed with terpineol was coated on a ceramic tube with Au electrodes, subsequently sintered at 500 °C. According to what was already observed in previous works, the best sensing performance was obtained for nanosheets with exposed (0001) facets (
Figure 3e), as also demonstrated by DFT simulation. In addition, by photoluminescence (PL) analysis the authors proved the presence of a large amount of oxygen vacancy defects and unsaturated dangling bonds existing in the ZnO nanosheets with exposed (0001) crystal facets. These, favoring the adsorption of gas molecules onto the material surface, resulted in the improvement of the gas response to ethanol.
Xie et al. [
47] fabricated a 3D porous hierarchical architecture through annealing of a zinc hydroxide carbonate precursor, which was obtained by a one-pot hydrothermal process with the assistance of carbon nanofibers and cetyltrimethyl ammonium bromide (CTAB). The ZnO hierarchical architectures were assembled from porous single crystal ZnO nanosheets with {0001} exposed crystal facets. The carbon nanofibers favored the formation of regular 3D hierarchical nanostructures and their stability after calcination. The gas sensor was fabricated coating a slurry of ZnO material onto an alumina tube with Au electrodes. Comparing the sensing properties of 3D hierarchical nanostructures with ZnO nanoclusters obtained in the absence of carbon nanofibers, a large improvement of sensing performances towards both ethanol and acetone were obtained. The enhanced gas-sensing properties of as-prepared porous ZnO hierarchical architectures were attributed to the unique structure, single crystal nature, and exposed polar crystal facets.
Zhang et al. [
48] employed a hydrothermal method followed by annealing of the zinc carbonate hydroxide hydrate precursors at 300 °C in air, to synthesized porous 2D ZnO single crystal nanosheets with hexagonal wurtzite and mesoporous structures. A gas sensor based on these ZnO nanosheets, fabricated using an alumina tube with Au electrode, exhibited high response and fast response/recovery times in the range 0.01–1000 ppm of ethanol, a detection limit as low as 10 ppb (R
a/R
g = 3.05), and excellent selectivity and stability. The high ethanol response of the as-synthesized ZnO nanosheets was attributed to the large specific surface area due to single crystal porous structure, plane contact between nanosheets, 3D network architecture, and characteristically small thickness.
Liu et al. [
49] reported the synthesis of a 2D ZnO structure made of single crystal flakes, which were grown on a silicon substrate using a combustion chemical vapor deposition (CVD) process with the assistance of methane flame. ZnO flakes, grown along <
> directions, showed a cactus-like shape with thickness ranging from 150 to 250 nm. A gas sensor, fabricated by printing the ZnO flakes on patterned platinum electrodes, showed excellent sensitivity and fast response towards ethanol working at 400 °C. The authors attributed the excellent behavior only to the high surface to volume ratio of the structure.
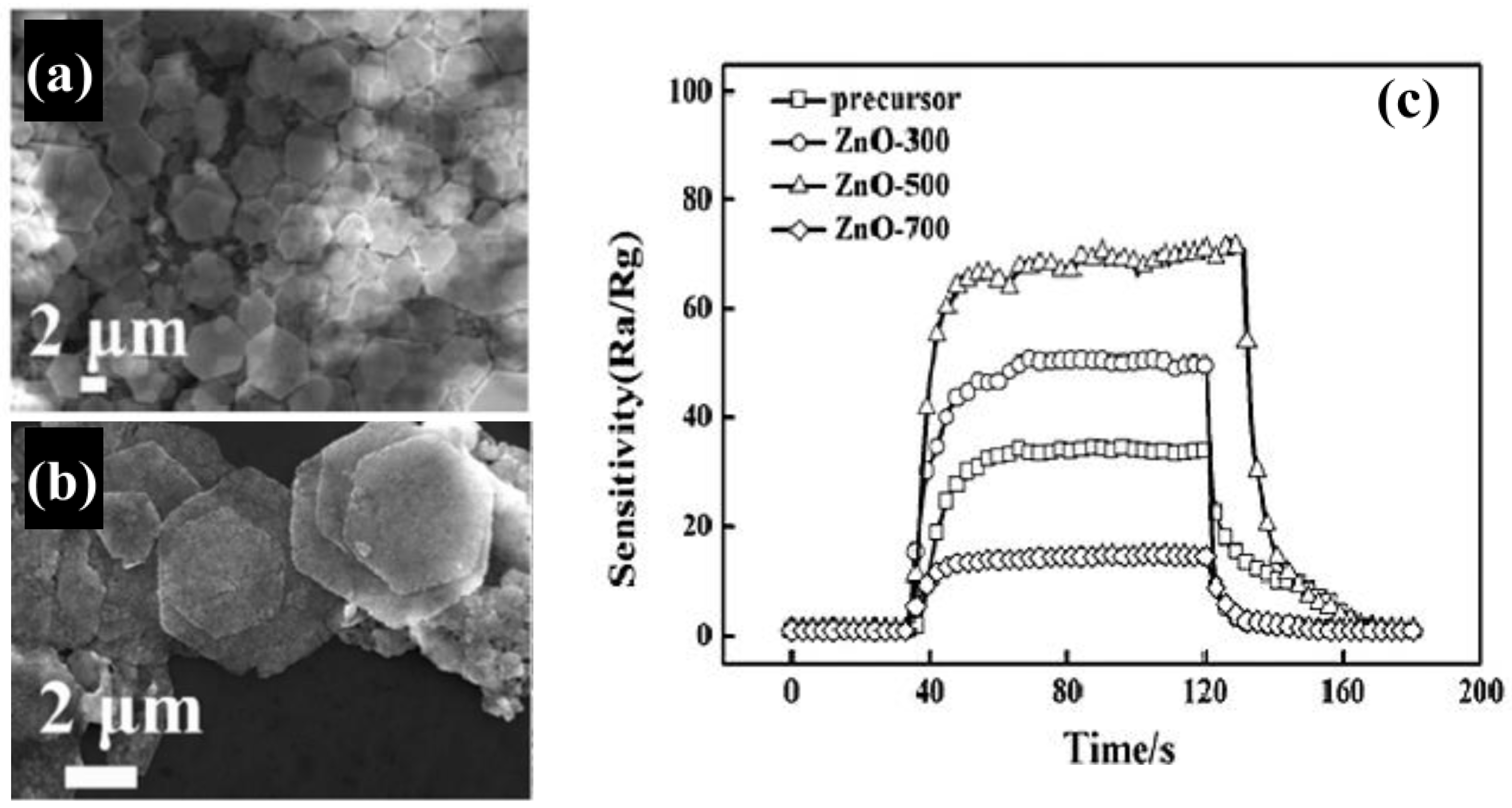
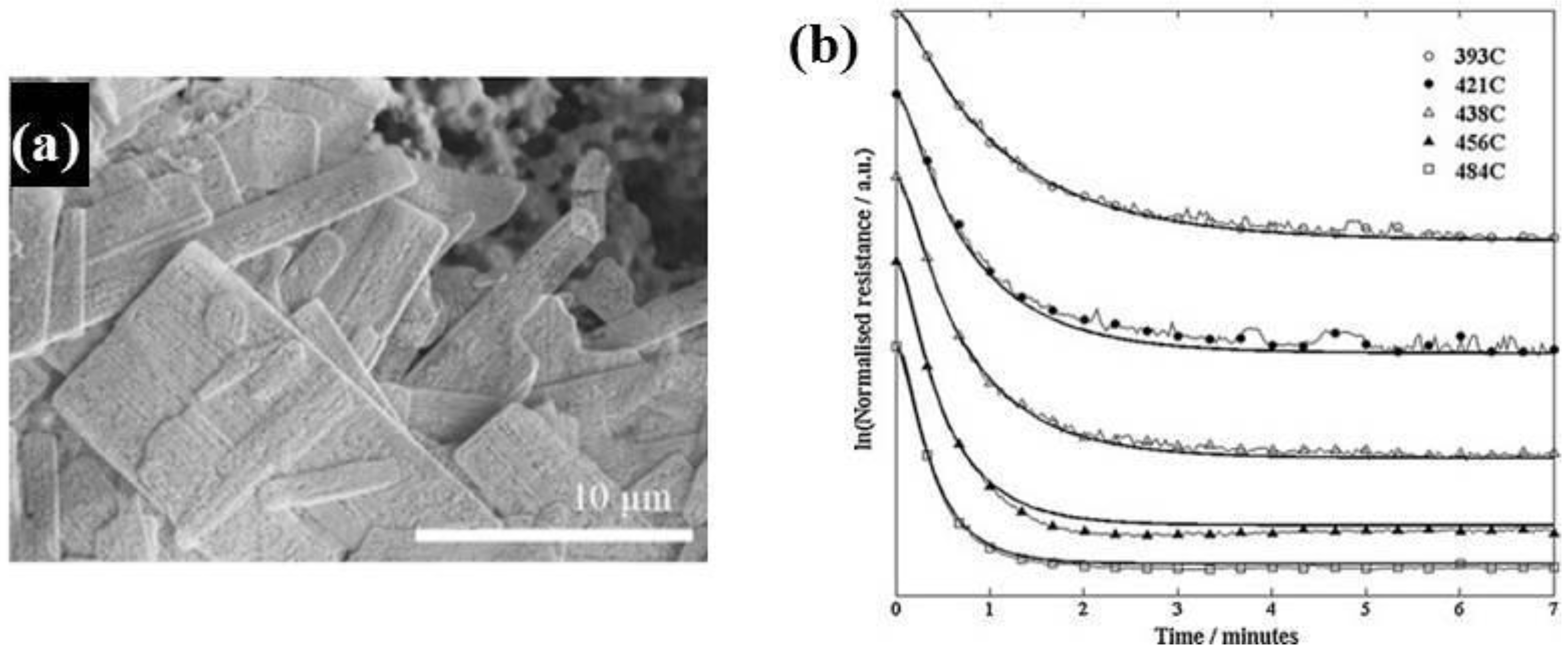
Zhang et al. [
50] prepared, on a large scale, ZnO hexagonal nanosheets by thermal annealing of simonkolleite (Zn
5(OH)
8Cl
2·H
2O) nanosheets synthesized via a one-pot hydrothermal method (
Figure 4a,b). ZnO nanosheets were mixed with terpineol to form a slurry, which was coated on a ceramic tube and then annealed at 300 °C. They found that the annealing temperature dramatically affects the gas-sensing performance; in particular, the ZnO nanosheets annealed at 500 °C exhibited the best sensitivity to ethanol, with shorter response and recovery times (
Figure 4c) than other samples. The enhanced gas-sensing performance was attributed to the optimal combination of porous structure, grain size, and the Cl element remaining in the structure of the material.
Chen et al. [
51] synthesized porous co-doped Sn–Rh ZnO nanosheets through a simple hydrothermal reaction process without any surfactant or template at 180 °C. A gas sensor was fabricated by coating the Au electrodes on a ceramic tube, with a paste of the ZnO product. The co-doped ZnO showed the best sensing performance for ethanol compared to pure and Sn doped ZnO nanosheets. The authors attributed the sensing performance of this material to the introduction of Sn ions in the ZnO structure, which leads to oxygen vacancy formation. In addition, due to the possible precipitation of rhodium oxide nanoparticles on the ZnO sheets, the formation of new Schottky barriers at the Rh
2O
3/ZnO interfaces could contribute to an enhancement in the sensing performance.
Fan et al. [
52] prepared a 3D porous rod-like ZnO nanostructure by a simple precipitation method followed by calcination at 300 °C. These rod-like hierarchical structures were made by plenty of nanosheets with a thickness of 5–16 nm, interleaved with each other to form a net-like and wide open structure (
Figure 5a). The ZnO powder mixed with ethanol was coated onto an alumina tube with a pair of Au electrodes and then aged at 200 °C. An excellent sensitivity to ethanol (
Figure 5b) was reported, much higher than smooth 1D nanorods. The authors attributed the superior gas-sensing performance of the 3D hierarchical porous ZnO to its highly open, porous structure and the high specific surface area conferred by the interconnected 2D nanosheets.
Yu et al. [
53] reported a facile preparation of 3D mesoporous ZnO film on planar Ag interdigitated electrodes substrate, which consisted of a uniform network of nanowalls ranging from 2 to 5 nm of thickness. Finally, the ethanol-sensing performance of the fabricated sensor was investigated. The authors considered that the contact resistance derived from the potential barriers at the nanowall junctions dominated the electrical properties of the material. Because the response of the gas sensor can be attributed to the apparent change of the potential barriers, more nanowalljunctions significantly increases the number of potential barriers, which results in an increased gas response.
Table 1 summarizes the gas-sensing properties of the above discussed 2D ZnO-based ethanol sensors.
3.1.2. Acetone
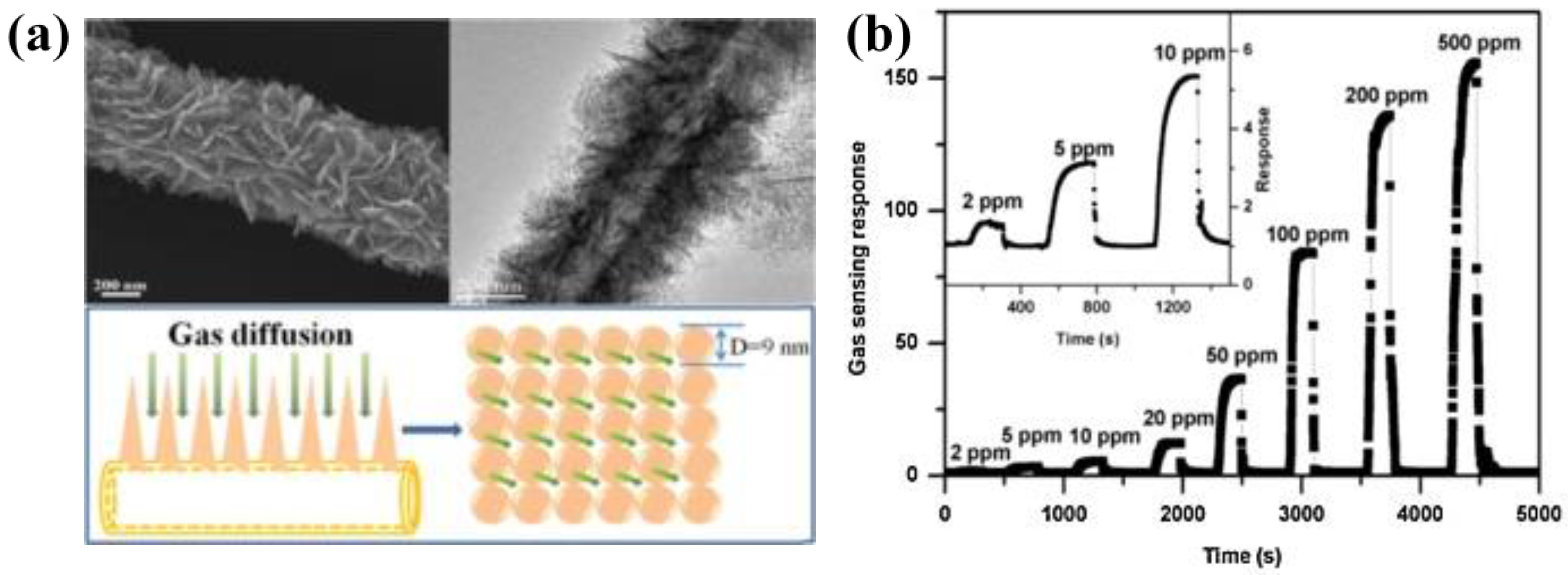
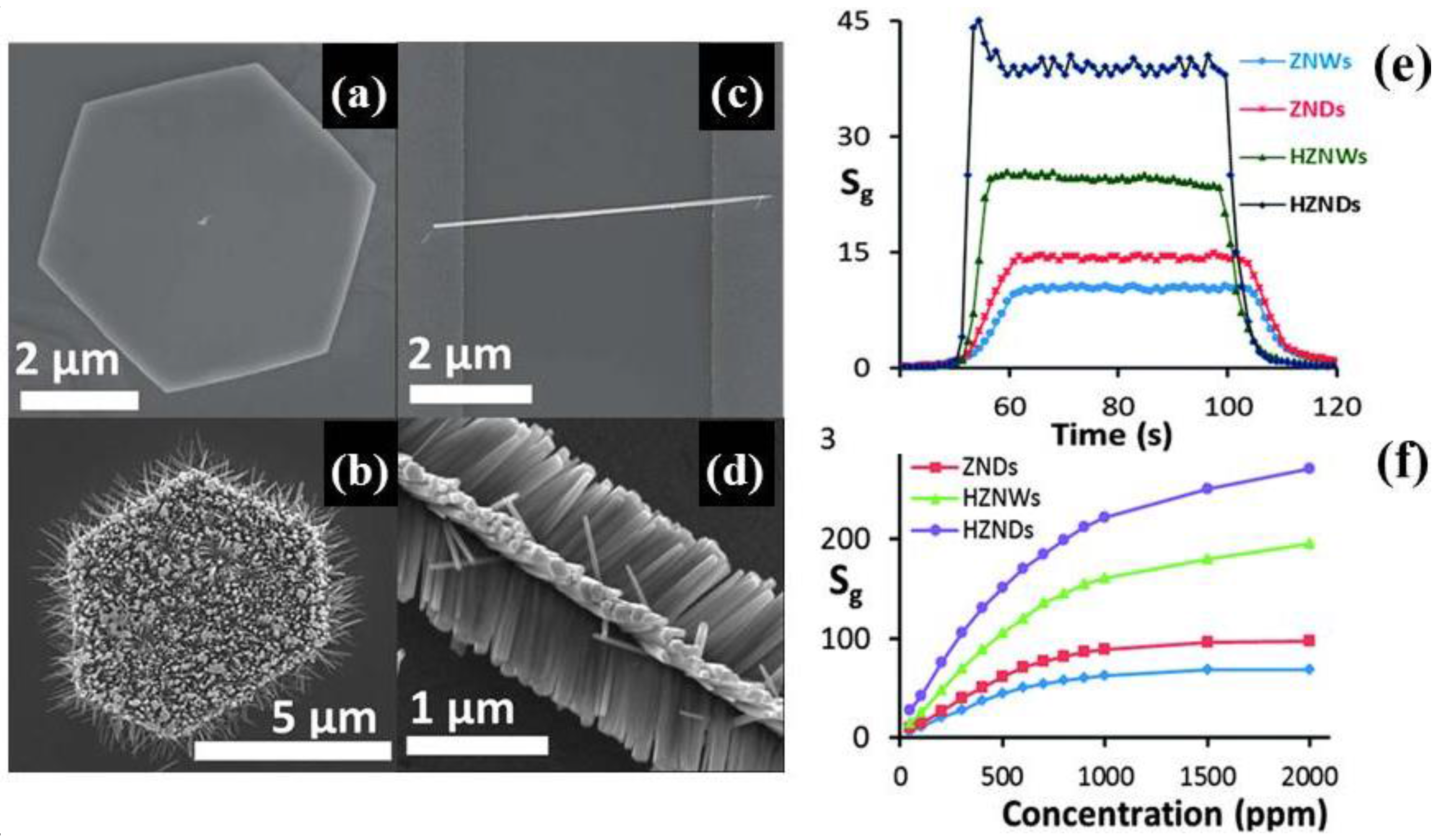
Alenezi et al. [
54], employing a facile hydrothermal route, developed three-dimensional hierarchical ZnO structures with a high surface-to-volume ratios and an increased fraction of (0001) polar surfaces. Starting from the synthesis of 1D single crystal nanowires and 2D nanodisks, followed by sequential nucleation and growth, elegant and extreme high surface to volume ratio hierarchical structures controlling the concentration of solution and the time of growth were prepared. In the first case, single nanowires with secondary rods (HZNWs) grown on them were obtained (
Figure 6a,b). Similarly, starting from 2D nanodisks, secondary nanowires were grown along the [0001] direction on the top, bottom, and lateral sides of the nanodisks (HZNDs) (
Figure 6c,d). ZnO nanostructures were deposited by spin coating on SiO
2/Si substrates with pre-patterned Au electrodes in order to fabricate the gas sensors. Both hierarchical ZnO structures displayed an enhancement of gas-sensing performance to acetone in comparison to the relative mono-morphological nanowires’ and nanodisks’ ZnO structures. In particular, HZNDs nanostructure showed the highest sensitivity, high saturation level, and shortest response and recovery times (
Figure 6e,f). In addition to the high surface-to-volume ratio due to its small size, the authors ascribed the enhanced sensing properties to the increased proportion of exposed active (0001) planes that favors the chemisorption of oxygen species, and the formation of many nanojunctions at the interface between the initial ZnO nanostructure and secondary nanowires.
Xiao et al. [
30] investigated the acetone sensing performance of pure and Pd nanoparticles decorated single-crystalline ZnO nanosheets with exposed (100) facets, synthesized by annealing of hydrozincite Zn
5(CO
3)
2(OH)
6 nanoplates produced with a water/ethylene glycol solvothermal method. Gas sensors were fabricated by printing a ZnO slurry on ceramic tubes with two Pt electrodes. The sensor made of Pd–ZnO nanosheets exhibited excellent sensitivity and selectivity to acetone. They suggested that the high percentage of exposed (100) facets may favor the selective adsorption of acetone molecules, while the Pd nanoparticles contributed to increase the sensitivity.
Al-Hadeethi et al. [
55] synthesized 2D Sn-doped ZnO ultrathin nanosheets with sharp pointed edges and thickness about 20 nm (
Figure 7a), by a simple hydrothermal method. A sensor, fabricated by printing a thick film of ZnO on alumina substrate decorated with Au electrodes, was tested towards acetone gas, which exhibited a maximum sensitivity of 5.556 (R
a/R
g) for 200 ppm of acetone at an optimal temperature of 320 °C (
Figure 7b). The authors proposed that Sn doping resulted in distortions of lattice and O
− vacancy defects, which were the most susceptible adsorption sites for the generation of oxygenated anionic species from atmospheric molecular O
2.
Fan and Jia [
56], via a simple mixed hydrothermal synthesis in the presence of CTAB and 1,2-propanediol, synthesized 2D ZnO nanosheets with dimensions of several microns in length and 10–20 nm in thickness that are organized to form nanoflowers. A ZnO paste was coated on an alumina tube with a pair of Au electrodes used as the sensor. The gas-sensing tests showed the double ability to selectively detect acetone at a temperature of 360 °C and gasoline at 180 °C.
Wen et al. [
57] developed a simple top-down route to prepare large porous ZnO flakes via template-free solution combustion synthesis followed by subsequent calcination in air. By this simple method, highly porous ZnO flakes, with tens to hundreds of micrometers in length and about 10 nm in thickness were obtained. The porous ZnO flakes were mixed with deionized water and then coated onto the outside of an alumina tube with two Au electrodes. The gas-sensing tests showed excellent sensitivity to both acetone and ethanol vapors as a result of the highly accessible 2D architecture, however, working at temperature as high as 485 °C. The highly porous structure shortens the diffusion distance and provides abundant transport channels and active surfaces for the target gas.
Behera and Chandra [
58] presented a simple and cost-effective MEMS sensor incorporating ZnO–CuO nanoflakes. The material was synthesized by thermal oxidation in pure oxygen of a brass film deposited by radio frequency (RF) diode sputtering on silicon substrate. By this technique the authors obtained a homogenous film with flower-like structures consisting of sheets with a thickness of about 20–25 nm. The fabricated sensor was able to detect acetone, ammonia, and ethanol at a relatively low temperature (50 °C) although it showed an optimal response at 300 °C with improved selectivity to acetone.
Table 2 summarizes the gas-sensing properties of the above discussed 2D ZnO-based acetone sensors.
3.1.3. Formaldehyde
Guo [
59] fabricated ZnO nanosheet–spheres with various Fe-doping concentrations by a one-step hydrothermal method. The synthesized materials were made of 3D nanospheres assembled by many single-crystal Fe–ZnO nanosheets. A paste of the sensitive materials was dropped onto alumina ceramic plate substrates to form a thick film between a pair of Ag–Pd interdigitated electrodes. The sensing properties towards 1–10 ppm formaldehyde were investigated for pure and Fe-doped ZnO nanosheets. The authors found the best performance at 300 °C for 2.5 wt% Fe doping with significantly enhanced sensitivity and selectivity, and short response and recovery times. The excellent performance was attributed to the large specific surface area of the 2.5 wt% Fe-doped sample that was damaged, increasing the dopant concentration. In addition, the key factor of Fe doping to enhance ZnO gas-sensing performance was attributed to structural and electronic sensitization such as narrowed bandgap, more electron donors, and oxygen vacancies (Vo), which were confirmed by UV-vis, PL, and XPS analysis.
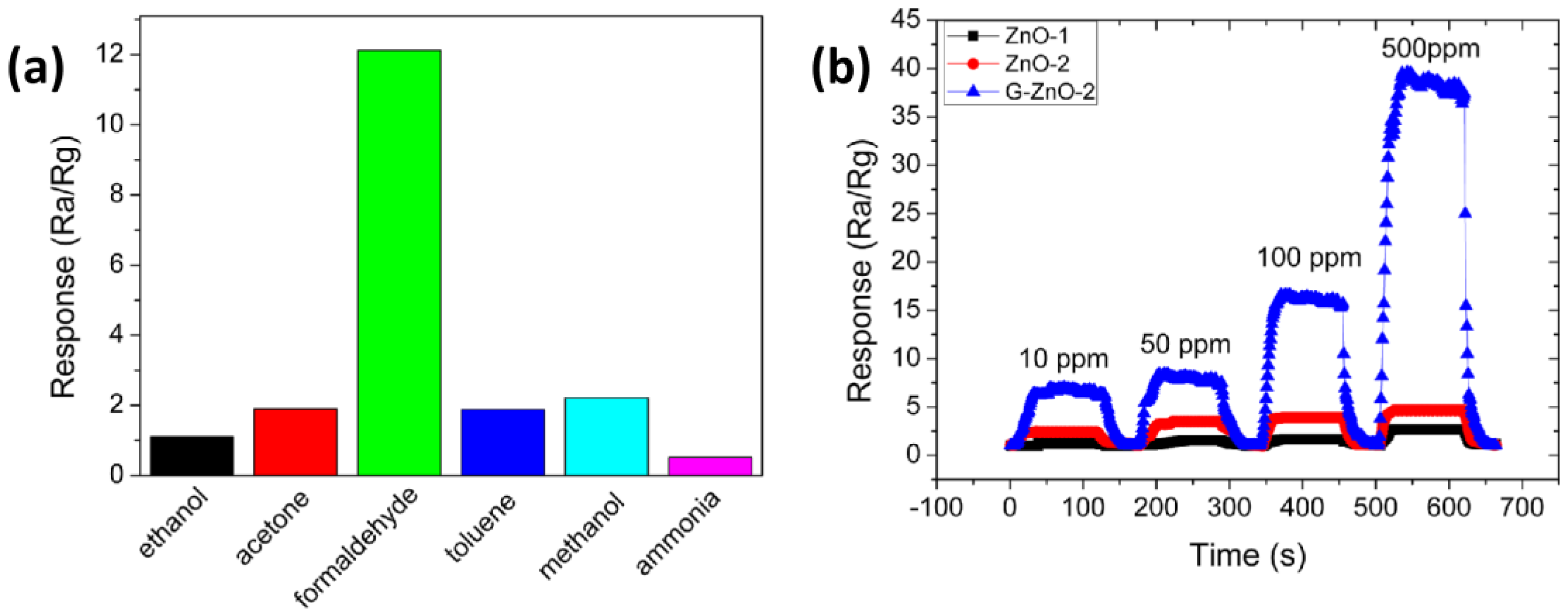
Chen et al. [
60] synthesized pure ZnO and graphene-modified ZnO using simple hydrothermal processes at 150 °C. Gas sensors were prepared by coating a paste of the materials onto an alumina tube with Au electrodes. Improving the regularity of pure ZnO nanosheets (ZnO-2), the sensitivity towards a low concentration of formaldehyde (10–500 ppm) was enhanced. In addition, by combining the best ZnO nanosheets with graphene, a large improvement of the sensing performance was obtained. In particular, loading ZnO with 2 wt% graphene (G-ZnO-2), the sensor exhibited excellent sensing properties such as higher response, faster response/recovery times, as well as good selectivity at a lower temperature than pure ZnO nanosheets (200 °C) (
Figure 8a,b). The improved gas-sensing properties of graphene-modified ZnO were mainly attributed to the larger work function of ZnO compared to graphene, which facilitates the migration of electrons from ZnO to the graphene sheets. Moreover, other benefits were attributed to the ability of ZnO nanosheets to adsorb oxygen species onto its surface and the narrower band gap, which gives rise to a higher carrier concentration than pure ZnO nanosheets at the same working temperature, thus facilitating the capture of oxygen.
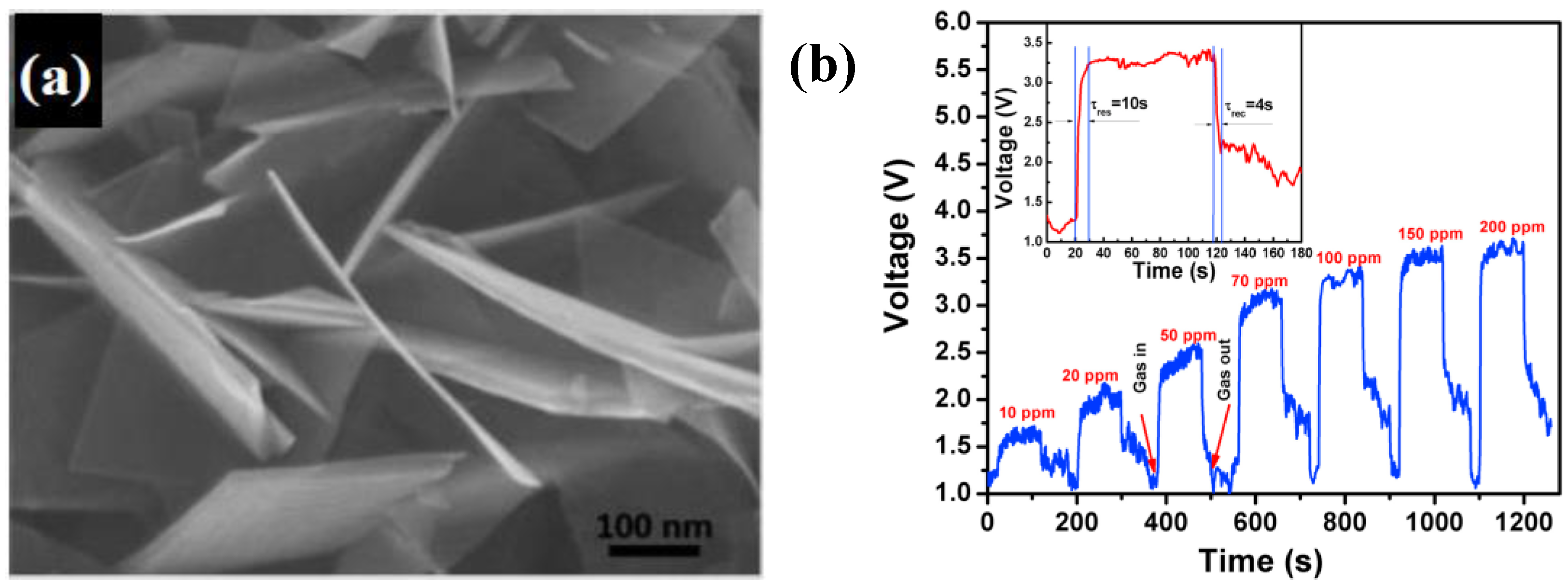
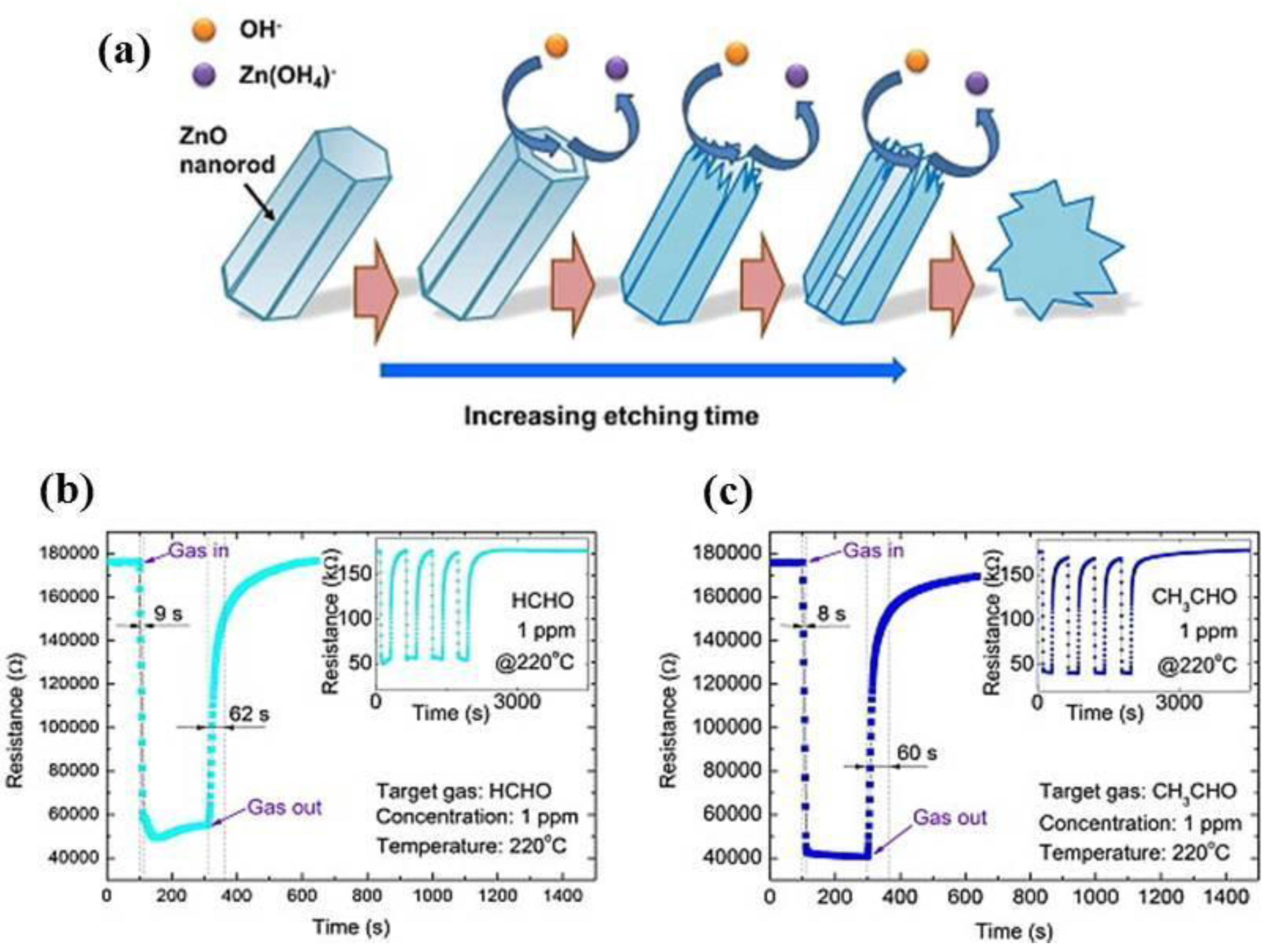
Zhang et al. [
61] proposed a simple method for the large-scale production of dispersed single-crystalline ZnO nanosheets through a two-step process.
In the first step, ZnO nanorods were synthesized by a sonochemical method at room temperature using zinc nitrate hydrate without any surfactant, catalyst, or template. Then, ZnO nanosheets were fabricated by chemical etching of the ZnO nanorods in alkaline 1.5 M NaOH solution for 24 h (
Figure 9a). The nanosheets had a size of 200–400 nm and thickness of about 10–60 nm. A microsensor substrate (1.8 mm × 1.8 mm with a 400 µm × 400 µm sensing area) was developed using MEMS technology where platinum electrodes and a micro-heater were deposited by sputtering with a patterned mask. Finally, a paste of ZnO nanosheet powder mixed with ethyl cellulose and terpineol was coated onto the MEMS substrate by a dropping method. Investigating the sensing performance, the authors showed a good ability of the ZnO nanosheets to detect the ppb-level of formaldehyde and acetaldehyde at the optimal operation temperature of 220 °C with a linear range from 50 ppb to 1 ppm and both response and recovery times lower than 1 min (
Figure 9b,c).
Guo et al. [
62] synthesized, by a simple hydrothermal method, ultrathin hexagonal ZnO nanosheets with a thickness of 17 nm and length of about 90 nm. They found that CTAB additive plays a critical role in producing such ultrathin ZnO nanosheets with exposed (0001) surfaces. A paste was next coated onto an alumina ceramic tube to realize a gas sensor. The sensing measurements revealed that the sensor made of these hexagonal ZnO nanosheets exhibited an excellent response to 50 ppm of formaldehyde with both response and recovery times close to 10 s at an optimal temperature of 350 °C.
Table 3 summarizes the gas-sensing properties of the above-discussed 2D ZnO-based formaldehyde sensors.
3.1.4. n-Butanol
Kaneti et al. [
28] presented a simple one-step hydrothermal approach under mild conditions (150–180 °C) and without high-temperature calcination, for the synthesis of ZnO nanoflakes with exposed (
) surfaces. The ZnO nanoflakes mixed with PVDF were coated on a ceramic tube with printed Au electrodes. A treatment at 450 °C was adopted to remove the binder. The gas-sensing properties of the as-synthesized ZnO nanoflakes were excellent and they also demonstrated good stability towards different VOCs—with the highest sensitivity and selectivity to n-butanol—at the optimal working temperature of 330 °C. The authors showed that the sensing mechanism was related to surface reactions with O
2−, also confirming that at temperatures above 300 °C, these are the predominant oxygen species absorbed onto the surface of the ZnO. In addition, employing a molecular dynamics (MD) simulation, the diffusion and adsorption of different VOCs onto (
) (
) and (0001) surfaces of ZnO at 330 °C were investigated. They found that the lowest diffusivity was shown by n-butanol on the (
) facet due to the heavier molecular weight, lower than other ZnO surfaces. This result may explain the higher sensitivity to n-butanol of the prepared ZnO nanoflakes in comparison to other alcohol molecules with smaller molecular weight.
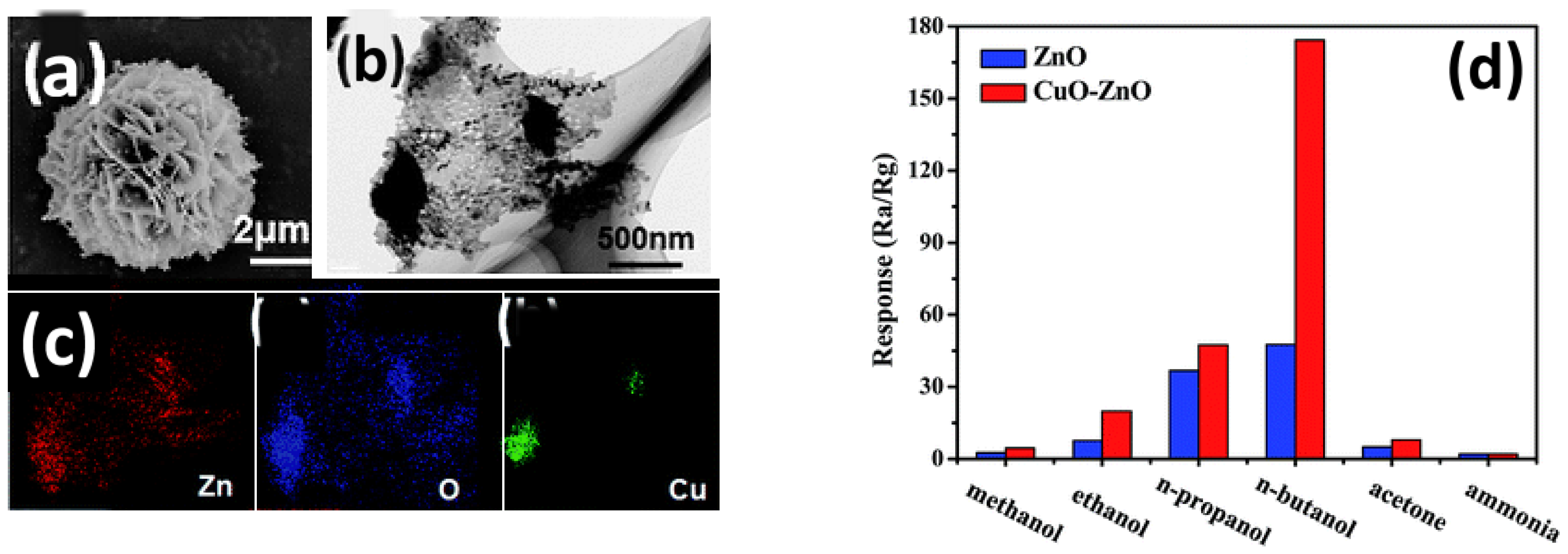
Chen et al. [
63] prepared by a two-step hydrothermal method a p–n junction ZnO–CuO nanostructure. In the first step, 2D porous ZnO nanosheets with exposed (0001) facets were assembled to form spheres. During the second hydrothermal process, leaf-like 2D CuO structures were grown outward on the surface of ZnO nanosheets (
Figure 10a–c). A proper amount of ZnO or CuO–ZnO composite powders were mixed with distilled water to form a paste, which was then coated onto an alumina tube with a pair of Au electrodes. Comparing the sensing performance towards different VOCs, an increased sensitivity of ZnO–CuO nanostructures was found in comparison to pure ZnO. In particular, the sensitivity to n-butanol of the nanocomposite was 2.7-fold higher than pure ZnO at 220 °C, with excellent selectivity (
Figure 10d). The authors attributed the enhanced sensitivity to the electronic sensitization due to the p–n junction. In addition, the porous and open 2D/2D heterostructure, favoring the fast diffusion and easy adsorption of gas molecules, contributed to the superior sensing performance of the composite.
Wang et al. [
64], by a hydrothermal method, prepared ZnO microsheets with exposed (0002) crystal planes functionalized with Au nanoparticles. A paste was coated onto an alumina tube with a pair of Au electrodes to realize the gas sensors. The sensing tests showed that the Au NPs-functionalized porous ZnO microsheets sensor exhibited enhanced performance towards several VOCs including, ethanol, methanol, acetone, benzene, and, markedly to n-butanol. The enhanced sensing properties were related to the unique structure of porous ZnO microsheets and the strong spillover effect of small Au nanoparticles that catalyze the sensing reactions, as well as the increase of Schottky barriers caused by the electronic interaction between Au and ZnO.
Huang et al. [
65], by a very simple solution method at near room temperature, prepared a flower-like ZnO nanostructure self-assembled by thin and uniform 2D nanosheets with a thickness of approximately 18 nm. The as-prepared flowerlike ZnO nanostructures were directly coated onto the outer surface of an alumina tube with a pair of Au electrodes. The gas-sensing measurements showed that the flowerlike ZnO nanostructures exhibited high sensitivity, fast response/recovery, and good reversibility towards some reducing gases, with the best sensitivity to both n-butanol and ethanol at a working temperature of 320 °C. The authors simply attributed the good sensing performance to the small thickness of ZnO nanosheets and 3D structures of the flowerlike ZnO nanostructures.
Table 4 summarizes the gas-sensing properties of the above-discussed 2D ZnO-based n-butanol sensors.
3.1.5. Other VOCs
Gu et al. [
66], via a one-pot hydrothermal method followed by a liquid phase reduction process, synthesized Pt-decorated porous single-crystalline ZnO nanosheets. Adjusting the amount of reduction agent (NaBH
4), a homogenous dispersion of Pt nanoparticles was obtained that improved the sensing performance of the synthesized Pt-decorated ZnO nanosheets. The sensing materials were dispersed in ethanol and then coated onto the ceramic tube to fabricate a sensor. The authors showed that Pt decoration leads to a better sensing performance to chlorobenzene than either Au- or Ag-decorated ZnO nanosheets. In particular, the Pt–ZnO nanosheets showed fast response/recovery times, and high sensitivity and selectivity to ppb levels of chlorobenzene vapor in comparison to other VOCs at 300 °C. The excellent sensing performance was attributed to the electronic and chemical sensitization effects of Pt nanoparticles.
Jing and Zhan [
67], by simple microwave synthesis followed by annealing at 400 °C, synthesized ZnO nanoplates with an edge thickness lower than 19 nm. The synthesized ZnO nanoplates were mixed with water and then coated onto an alumina tube. Investigating the sensing performance of the synthesized ZnO nanoplates, the authors observed excellent sensitivity to chlorobenzene in the range 100–250 ppm at an optimal temperature of 200 °C. In addition, the same material showed excellent sensitivity in the same range of concentration to ethanol but at higher temperature.
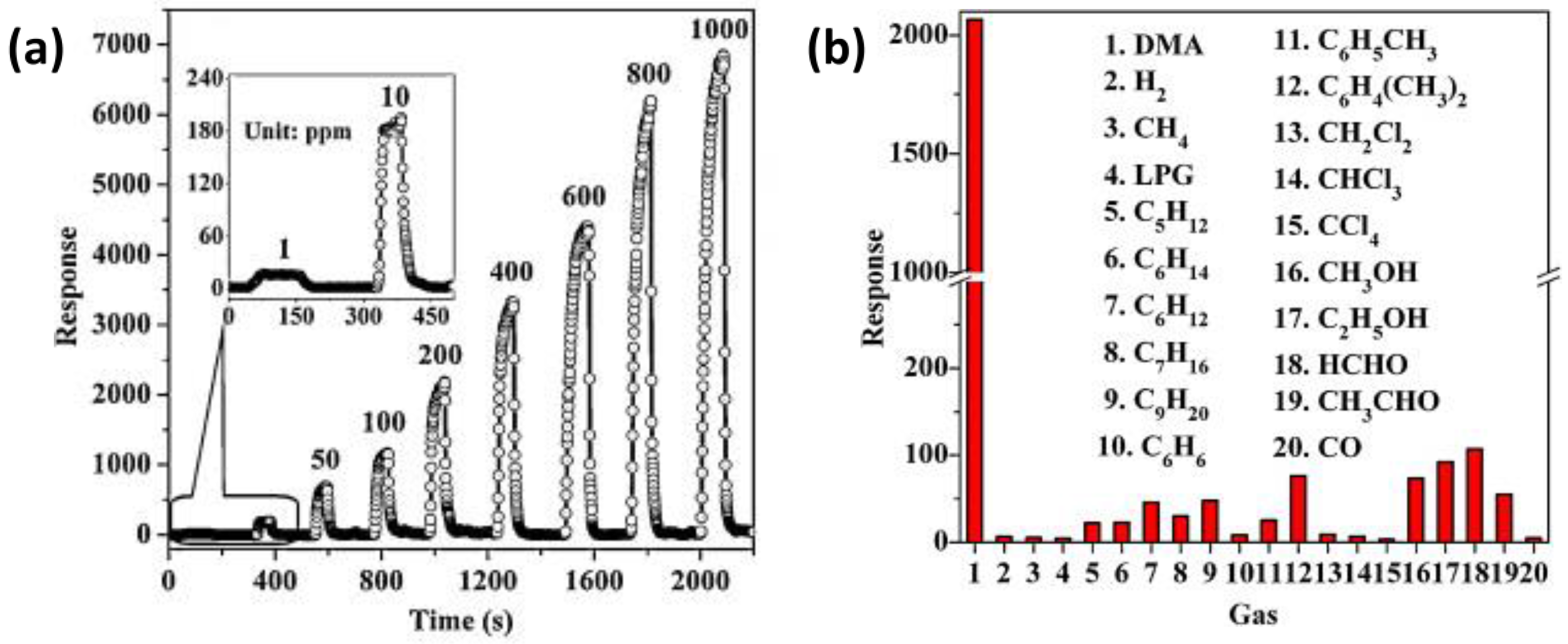
Zhang et al. [
68] synthesized ZnO architectures composed of nanorods and nanosheets-assembled microspheres through a one-pot hydrothermal process at low temperature. The ZnO products were ground with Triton X-100 and then the paste was coated on an alumina tube with Au electrodes. The gas-sensing property of the ZnO architectures displayed high response, fast response/recovery times, good selectivity, and long-term stability to 1–1000 ppm dimethylamine at 370 °C (
Figure 11a).
In particular, the synthesized ZnO architecture showed better selectivity to this analyte than many other VOCs and homologous compounds such as trimethylamine, methylamine, and ammonia (
Figure 11b). By photoluminescence and XPS analysis, the authors observed a large amount of chemisorbed oxygen species induced by a high content of electron-donor defects, to which was attributed the high response to dimethylamine.
Tonezzer et al. [
69] grew directly, on a silicon substrate, 1D ZnO nanowires and 2D ZnO nanosheets, then investigated their sensing performance towards liquid petroleum gas (LPG). Under the same operating conditions, the ZnO nanowires showed a better response when compared to ZnO nanosheets of similar thickness, although the latter nanostructure demonstrated an improved limit of detection (LoD). Although the authors attributed the improved sensitivity of the nanowires to the higher surface to volume ratio, they explained the lower LoD by the more stable base current shown by this nanostructure. However, there was no discussion of the exposed crystal faces.
Kaneti et al. [
70], by a hydrothermal method, synthesized ZnO nanoflakes with exposed (
) facets and high porous surface with diameters ranging from 2 to 20 nm. The ZnO nanoflakes were also decorated with Au nanoparticles using three different loads of 1.37, 2.87, and 5.41 wt%, respectively. ZnO samples were mixed with PVDF and 1-methyl-2-pyrrolidone to form a white slurry, which was then coated on ceramic tubes with Au electrodes. A treatment at 350 °C was used to remove the polymeric binder. Testing the gas-sensing behavior towards n-butylamine, the authors found that, compared to pure ZnO nanoflakes, 2.87 wt% Au-decorated samples exhibited greatly enhanced selectivity, a decrease in optimal operating temperature from 300 to 240 °C, about 6-fold higher response and up to 2.5-fold faster response and recovery times. By DFT simulation the interaction between the nitrogen atom of n-butylamine and (
) ZnO surfaces was demonstrated to favor the adsorption of this species over other VOCs. In addition, Au decoration increased the quantity of adsorbed oxygen species onto the ZnO surface, leading to the formation of a depletion layer at the ZnO/Au interface. This resulted in a significantly improved response of the ZnO/Au sensors towards n-butylamine.
Table 5 summarizes the gas-sensing properties of the above-discussed 2D ZnO-based VOCs sensors.