Oxygen Sensing by the Hybrid Langmuir-Blodgett Films of Iridium(III) Complexes and Synthetic Saponite on the Basis of Energy Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation and Characterization
2.3. Electronic Absorption and Stationary Emission Measurements
2.4. Measurements of Emission Lifetime
2.5. Sensing of Oxygen
3. Results and Discussion
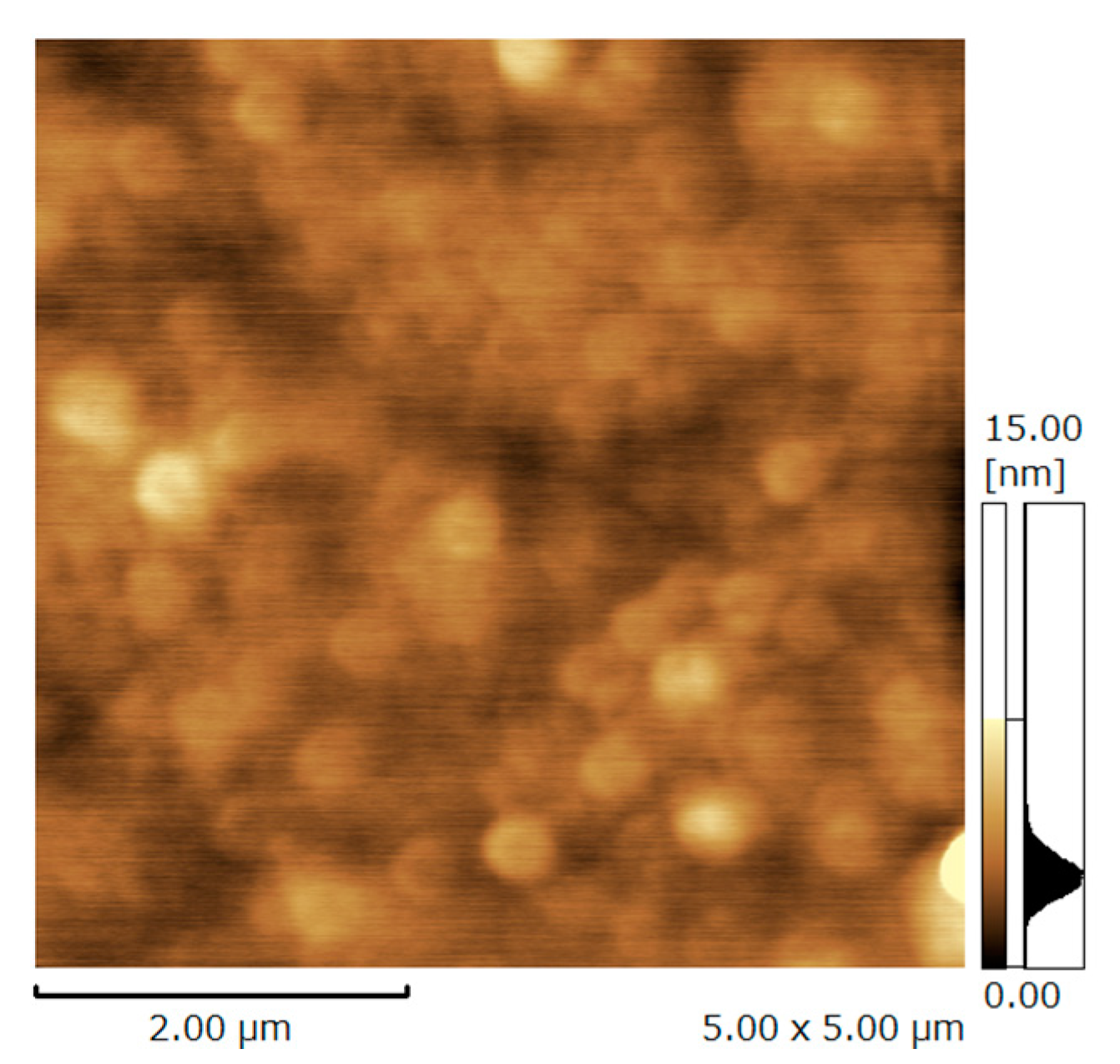
3.1. Characterization of Deposited Hybrid Films
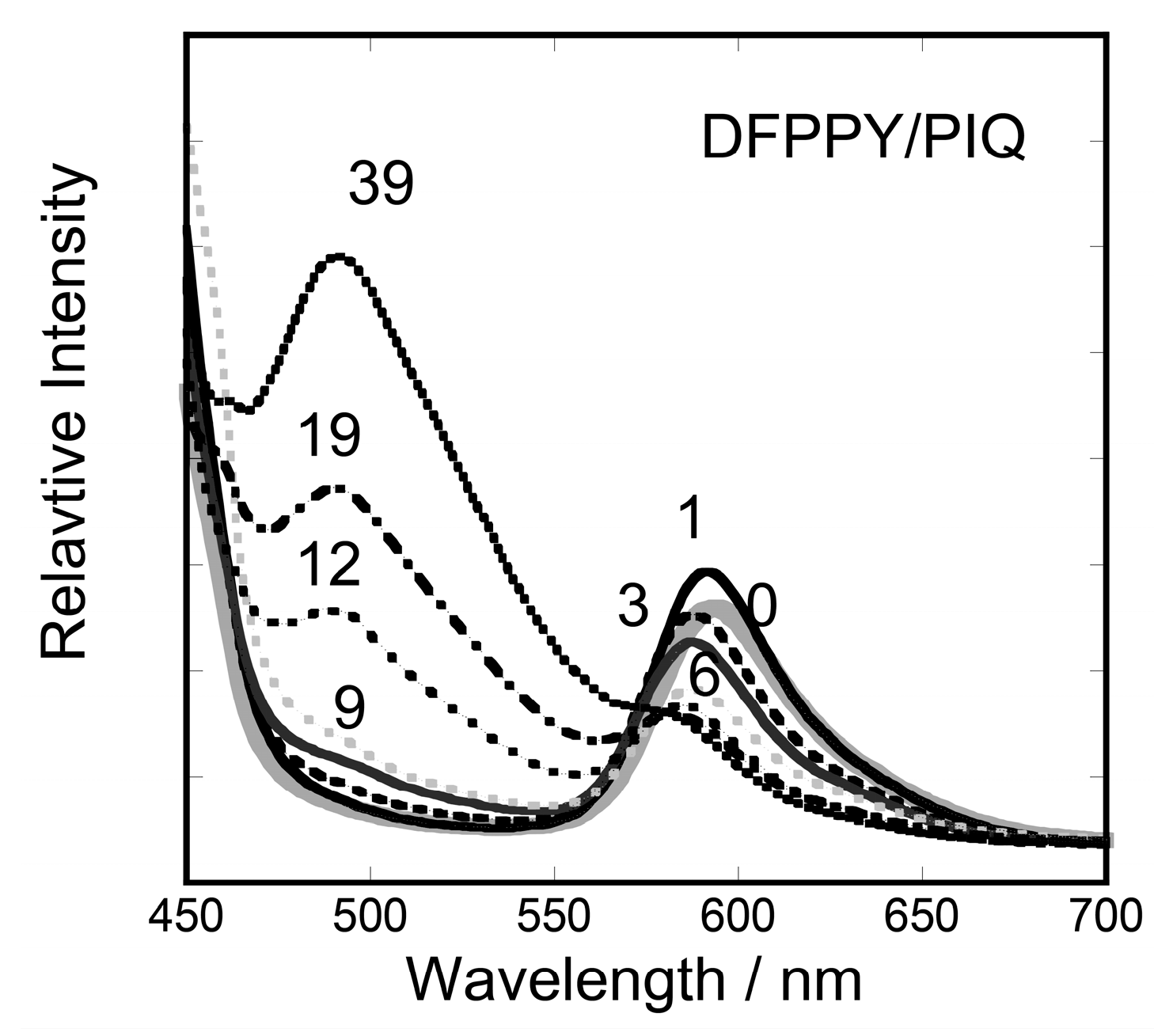
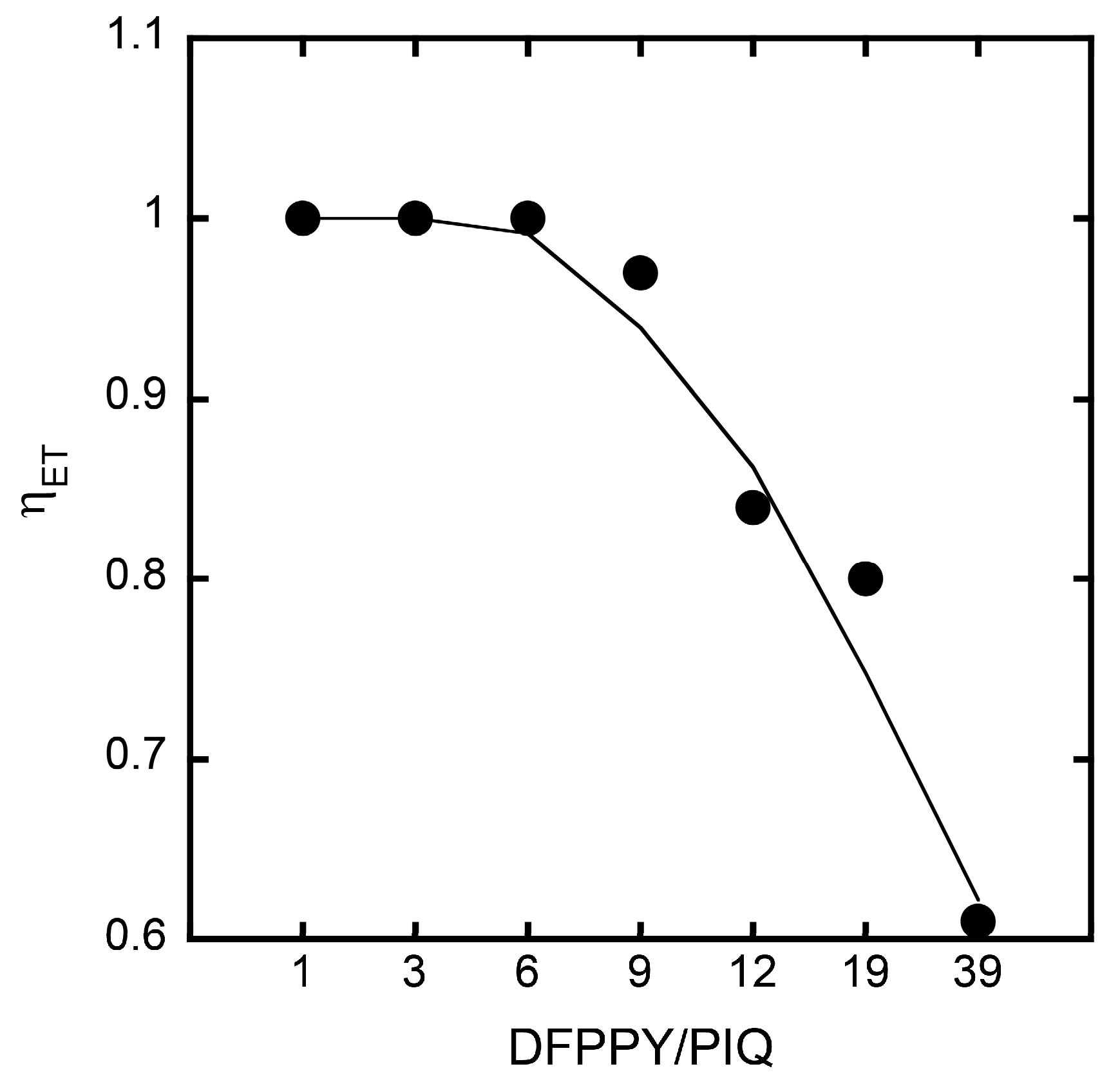
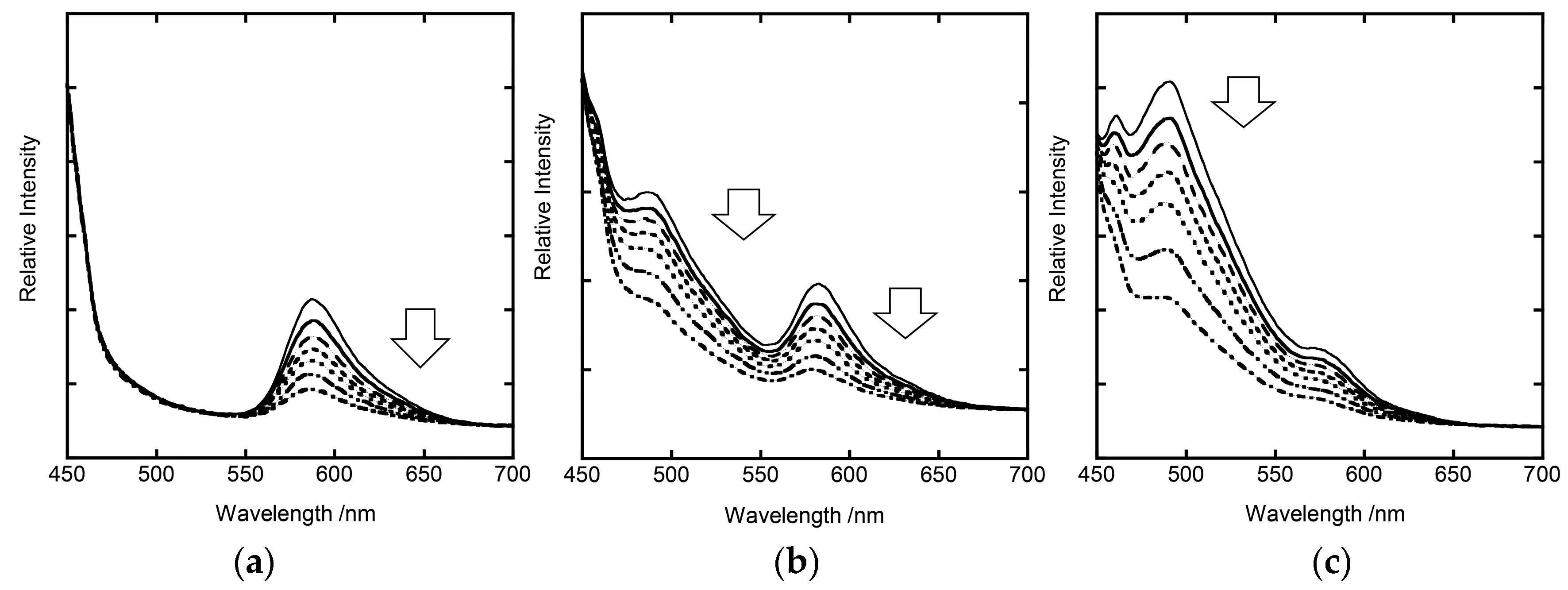
3.2. Stationary Measurements on Energy Transfer Processes
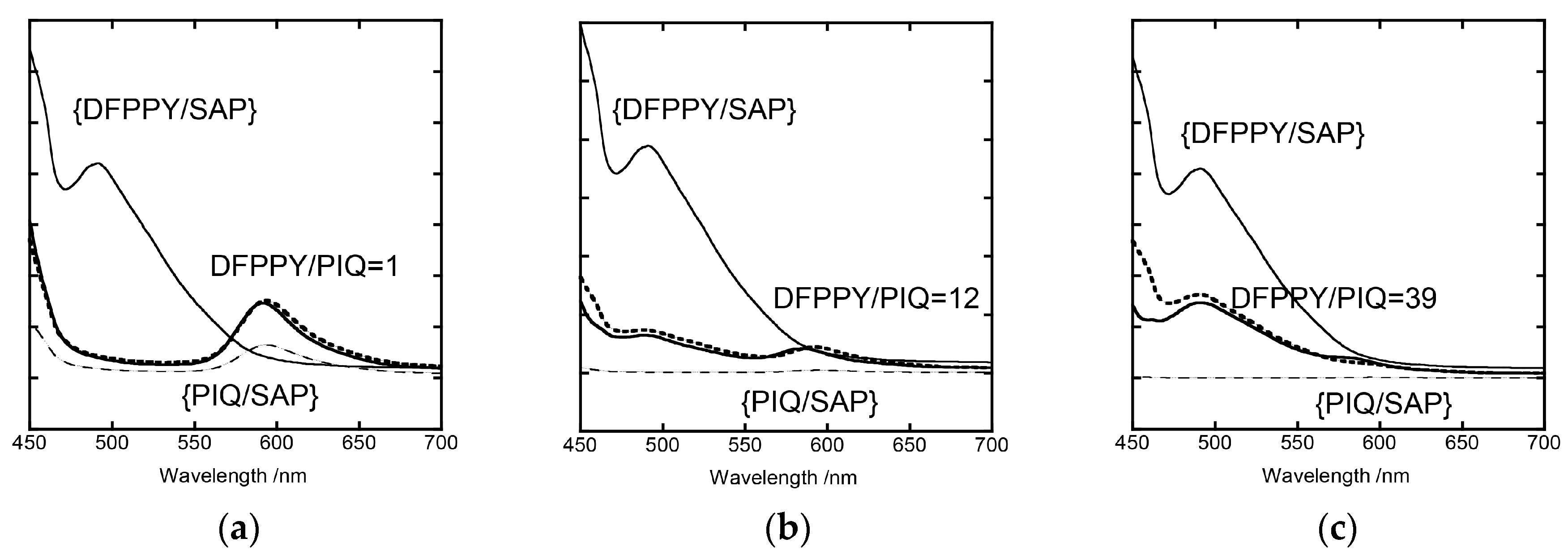
3.3. Analyses of Stationary Emission Spectra on the Basis of the Förster-Type Mechanism
3.4. Transient Measurements of the Energy Transfer Processes
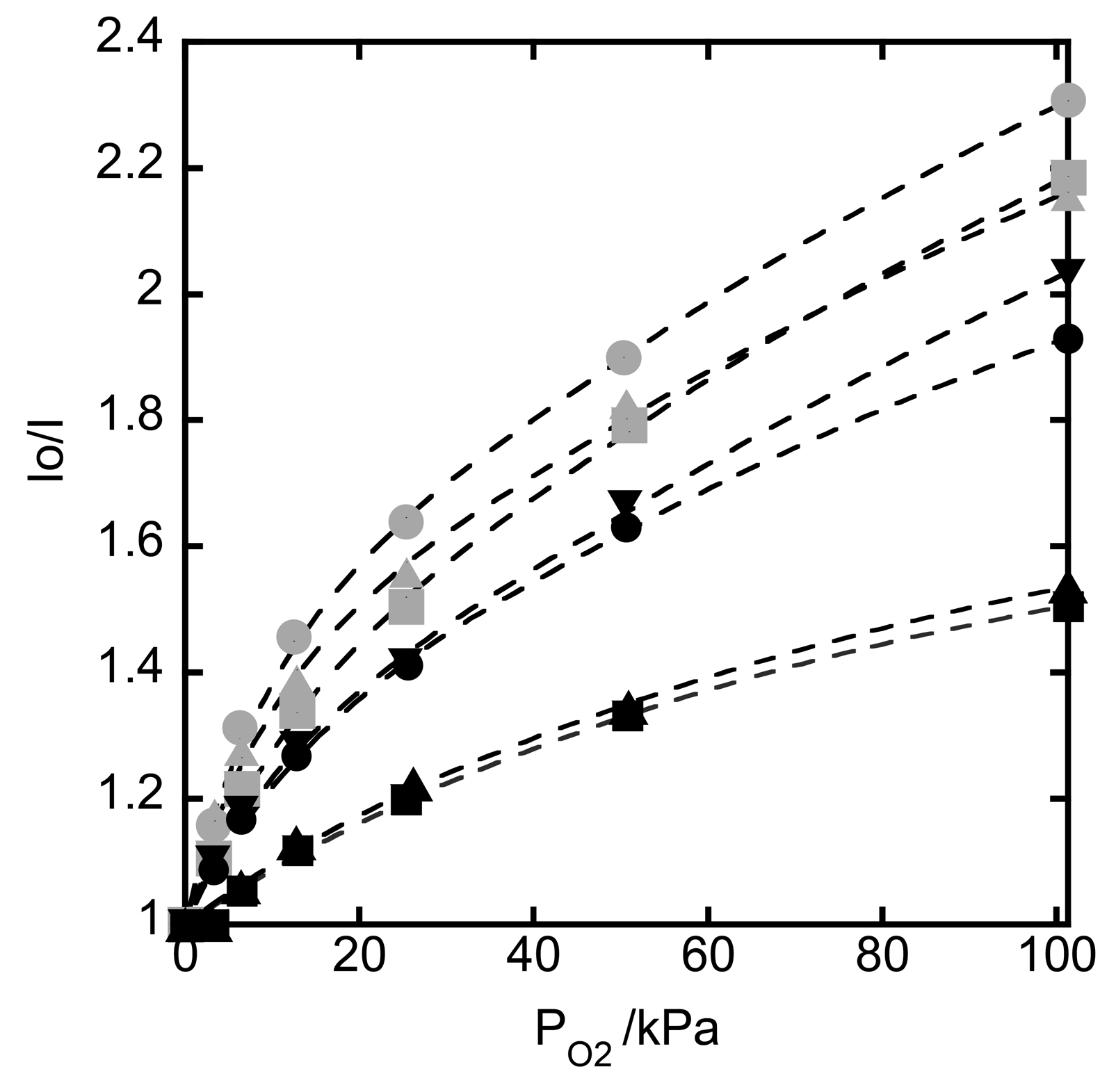
3.5. Oxygen Sensing by Hybrid Films Deposited onto a Quartz Plate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guan, W.; Zhou, W.; Lu, J.; Lu, C. Luminescent films for chemo- and biosensing. Chem. Soc. Rev. 2015, 44, 6981–7009. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors 2017, 5, 21. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, K.; Yamagishi, A. Luminescent Oxygen Gas Sensors Based on Nanometer-Thick Hybrid Films of Iridium Complexes and Clay Minerals. Chemosensors 2014, 2, 41–55. [Google Scholar] [CrossRef]
- Sato, H.; Yamagishi, A. Application of the ΔΛ isomerism of Octahedral Metal Complexes as a Chiral Source in Photochemistry. J. Photochem. Photobiol. C 2007, 8, 67–84. [Google Scholar] [CrossRef]
- Ras, R.H.; Umemura, Y.; Johnston, C.T.; Yamagishi, A.; Schoonheydt, R.A. Ultrathin hybrid films of clay minerals. Phys. Chem. Chem. Phys. 2007, 9, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Inukai, K.; Hotta, H.; Taniguchi, M.; Tomura, S.; Yamagishi, A. Formation of a clay monolayer at an air–water interface. J. Chem. Soc. Chem. Commun. 1994, 959–960. [Google Scholar] [CrossRef]
- Kotov, N.A.; Meldrum, F.C.; Fendler, J.H.; Tombácz, E.; Dèkány, I. Spreading of Clay Organocomplexes on Aqueous Solutions: Construction of Langmuir-Blodgett Clay Organocomplex Multilayer Films. Langmuir 1994, 10, 3797–3804. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamagishi, A.; Schoonheydt, R.; Persoons, A.; de Schryver, F. Langmuir−Blodgett Films of a Clay Mineral and Ruthenium(II) Complexes with a Noncentrosymmetric Structure. J. Am. Chem. Soc. 2002, 124, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Shinohara, E. Formation of Langmuir−Blodgett Films of a Clay and a Water-Soluble Alkylammonium Cation. Langmuir 2005, 21, 4520–4525. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, E.; Umemura, Y. Molecular orientation of Ru(II) complexes introduced in hybrid Langmuir-Scaefer filmsa of clay nanosheets and alkylammonim cations. Thin Solid Films 2013, 542, 373–379. [Google Scholar] [CrossRef]
- Hirahara, M.; Umemura, Y. Fabrication of Three-Layer-Component Organoclay Hybrid Films with Reverse Deposition Orders by a Modified Langmuir–Schaefer Technique and Their Pyroelectric Currents Measured by a Noncontact Method. Langmuir 2016, 31, 8346–8353. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C.-L.; Coronado, E.; Ángel, L.-M.; Repetto, D.; Ito, T.; Konya, T.; Yamase, T.; Constable, E.C.; Housecroft, C.E.; Doyle, K.; et al. Dual-Emissive Photoluminescent Langmuir-Blodgett Films of Decatungstoeuropate and an Amphiphilic Iridium Complex. Langmuir 2010, 26, 1316–1324. [Google Scholar]
- Huang, M.; He, S.; Liu, W.; Yao, Y.; Miao, S. Spectral Inspections on Molecular Configurations of Nile Blue Adsorbed on the Elementary Clay Sheets. J. Phys. Chem. B 2015, 119, 13302–13308. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Chakraborty, S.; Deb, S.; Nath, J.; Bhattacharjee, D.; Hussain, S.A. Reversible Transition between Excimer and J-Aggregate of Indocarbocyanine Dye in Langmuir–Blodgett (LB) Films. J. Phys. Chem. C 2015, 119, 9429–9441. [Google Scholar] [CrossRef]
- De Barros, A.; Ferreira, M.; José, C.; Constantino, L.; Bortoleto, J.R.R.; Ferreira, M. Synergy between Polyaniline and OMt Clay Mineral in Langmuir−Blodgett Films for the Simultaneous Detection of Traces of Metal Ions. ACS Appl. Mater. Interfaces 2015, 7, 6828–6834. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tamura, K.; Ohara, K.; Nagaoka, S.; Yamagishi, A. Hybridization of clay minerals with the floating film of a cationic Ir(III) complex at an air–water interface. New J. Chem. 2011, 35, 394–399. [Google Scholar] [CrossRef]
- Ohtani, Y.; Nishinaka, H.; Hoshino, S.; Shimada, T.; Takagi, S. Anisotropic photochemical energy transfer in clay/porphyrin system prepared by size-matching effect and Langmuir–Blodgett technique. J. Photochem. Photobiol. A 2015, 313, 15–18. [Google Scholar] [CrossRef]
- Hussain, S.A.; Chakraborty, S.; Bhattacharjee, D.; Schoonheydt, R.A. Fluorescence Resonance Energy Transfer between organic dyes adsorbed onto nano-clay and Langmuir–Blodgett (LB) films. Spectrochim. Acta Part A 2010, 75, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Schoonheydt, R.A.; Umemura, Y. Clay Minerals as Natureal Nanosheets. In Inorganic Nanosheets and Nanoshhet-Based Materials; Nakato, T., Kawamata, J., Takagi, S., Eds.; Springer: Tokyo, Japan, 2017; pp. 33–53. ISBN 978-4-431-56494-2. [Google Scholar]
- Lowry, M.S.; Bernhard, S. Synthetically Tailored Excited States: Phosphorescent, Cyclometalated Iridium(III) Complexes and Their Applications. Chem. Eur. J. 2006, 12, 7970–7977. [Google Scholar] [CrossRef] [PubMed]
- Martí, A.A. Metal complexes and time-resolved photoluminescence spectroscopy for sensing applications. J. Photochem. Photobiol. A 2015, 307–308, 35–47. [Google Scholar] [CrossRef]
- Morimoto, K.; Nakae, T.; Ohara, K.; Tamura, K.; Nagaoka, S.; Sato, H. Dual emitting Langmuir–Blodgett films of cationic iridium complexes and montmorillonite clay for oxygen sensing. New J. Chem. 2012, 36, 2467–2471. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, K.; Ohara, K.; Nagaoka, S. Multi-emitting Properties of Hybrid Langmuir–Blodgett Films of Amphiphilic Iridium Complexes and the Exfoliated Nanosheets of Saponite Clay. New J. Chem. 2014, 8, 132–138. [Google Scholar] [CrossRef]
- Sato, H.; Ochi, M.; Kato, M.; Tamura, K.; Yamagishi, A. Energy Transfer in Hybrid Langmuir-Blodgett Films of Iridium Complexes and Synthetic Saponite: Dependence of Transfer Efficiency on Interlayer Distance. New J. Chem. 2014, 38, 5715–5720. [Google Scholar] [CrossRef]
- Tokieda, D.; Tsukamoto, T.; Ishida, Y.; Ichihara, H.; Shimda, T.; Takagi, S. Unique fluorescence behavior of dyes on the clay minerals surface: Surface Fixation Induced Emission (S-FIE). J. Photochem. Photobiol. A 2017, 339, 67–79. [Google Scholar] [CrossRef]
- Eguchi, M.; Shimada, T.; Inoue, H.; Takagi, S. Kinetic analysis by laser flash photolysis of porphyrin molecules’ orientation change at the surface of silicate nanosheet. J. Phys. Chem. C 2016, 120, 7428–7434. [Google Scholar] [CrossRef]
- Ishida, Y.; Shimada, M.; Masui, D.; Tachibana, H.; Inoue, H.; Takagi, S. Efficient Excited Energy Transfer Reaction in Clay/Porphyrin Complex toward an Artificial Light-Harvesting System. J. Am. Chem. Soc. 2011, 133, 14280–14286. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, D.; Yoshida, H.; Sato, K.; Saito, K.; Yagi, M.; Takagi, S.; Yui, T. Light energy accumulation from pyrene derivative to tris(bipyridine)ruthenim on clay surface. Langmuir 2017, 33, 3680–3684. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hagiwara, S.; Morimoto, D.; Saito, K.; Yagi, M.; Takagi, S.; Yui, T. Emission amplicaiton of Ru(bpy)32+ via energy transfer from pyrene derivatives on synthesized clay. J. Photochem. Photobiol. A 2015, 313, 9–14. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, K.; Taniguchi, M.; Yamagishi, A. Efficient Energy Transfer of Cationic Iridium(III) Complexes on the Surface of a Colloidal Clay. Appl. Clay Sci. 2014, 497–498, 84–90. [Google Scholar] [CrossRef]
- Tamura, K.; Yamagishi, A.; Kitazawa, T.; Sato, H. Harvesting of Light Energy by Iridium(III) Complexes on a Clay Surface. Phys. Chem. Chem. Phys. 2015, 17, 18288–18293. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Shimada, T.; Ishida, Y.; Fujimura, T.; Masui, D.; Tachibana, H.; Eguchi, M.; Inoue, H. Size-Matching Effect on Inorganic Nanosheets: Control of Distance, Alignment, and Orientation of Molecular Adsorption as a Bottom-Up Methodology for Nanomaterials. Langmuir 2013, 29, 2108–2119. [Google Scholar] [CrossRef] [PubMed]

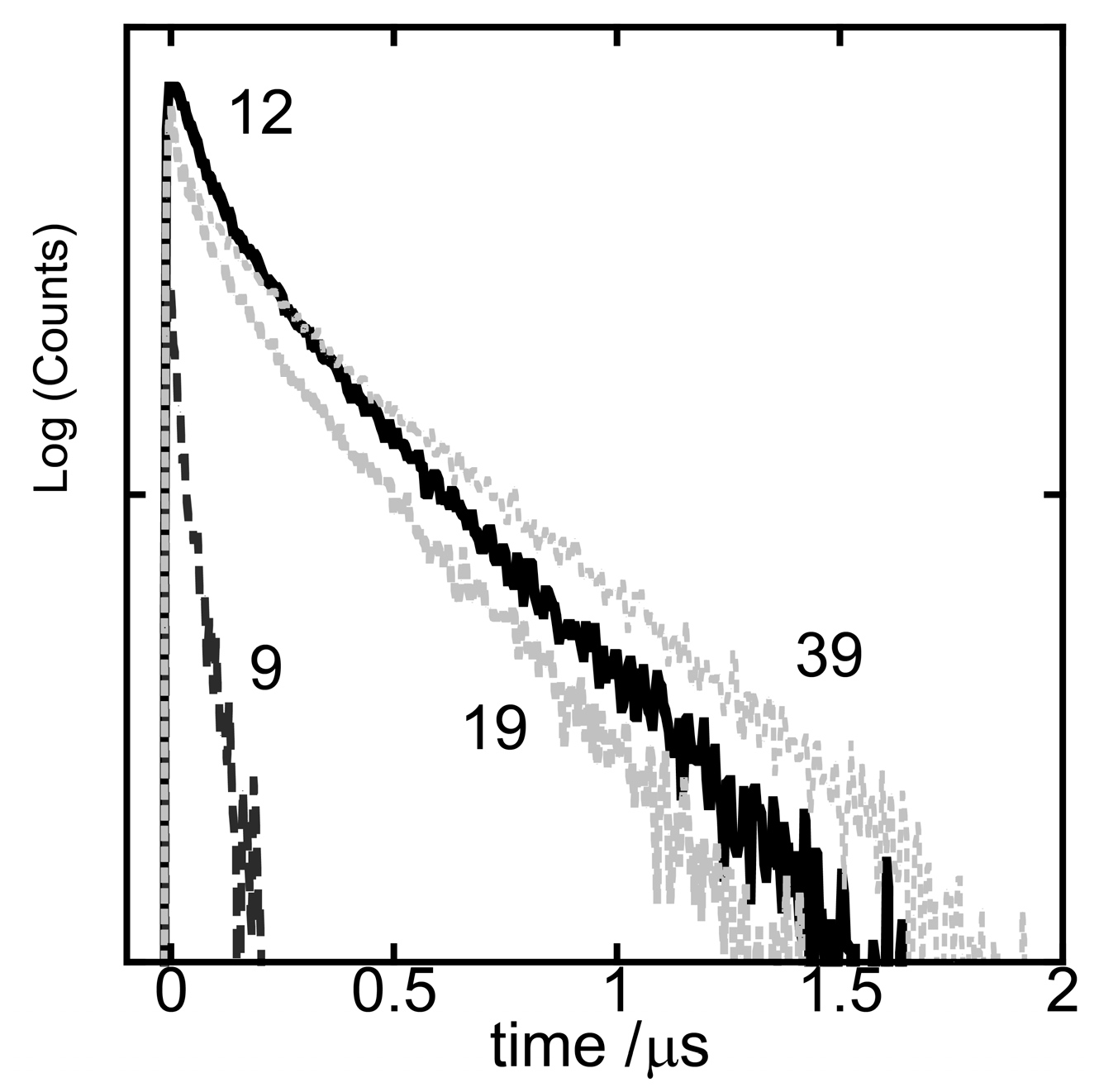

| DFPPY/PIQ | 490 nm τ1/μs | 490 nm τ2/μs | F1 # | F2 # |
| 1 | 0.046 | 0.470 | 0.57 | 0.43 |
| 3 | 0.094 | 0.512 | 0.54 | 0.46 |
| 6 | 0.088 | 0.452 | 0.59 | 0.41 |
| 9 | 0.068 | 0.359 | 0.70 | 0.30 |
| 12 | 0.118 | 0.538 | 0.45 | 0.55 |
| 19 | 0.143 | 0.612 | 0.48 | 0.52 |
| 39 | 0.165 | 0.722 | 0.38 | 0.62 |
| DFPPY/PIQ | 570 nm τ1/μs | 570 nm τ2/μs | F1 # | F2 # |
| 1 | 0.263 | 1.33 | 0.46 | 0.54 |
| 3 | 0.209 | 1.41 | 0.41 | 0.59 |
| 6 | 0.203 | 1.17 | 0.49 | 0.51 |
| 9 | 0.167 | 1.41 | 0.48 | 0.52 |
| 12 | 0.178 | 0.77 | 0.43 | 0.57 |
| 19 | 0.194 | 0.813 | 0.43 | 0.57 |
| 39 | 0.199 | 0.89 | 0.37 | 0.63 |
| DFPPY/PIQ | Ksv1 | Ksv2 | f1 | f2 | Ksvav |
|---|---|---|---|---|---|
| 1 | 0.0041 | 0.165 | 0.58 | 0.42 | 0.069 |
| 3 | 0.0042 | 0.095 | 0.60 | 0.40 | 0.038 |
| 6 | 0.0039 | 0.155 | 0.61 | 0.39 | 0.060 |
| 9 | 0.0030 | 0.077 | 0.62 | 0.38 | 0.029 |
| 12 | 0.0 | 0.018 | 0.48 | 0.51 | 0.009 |
| 19 | 0.0007 | 0.023 | 0.56 | 0.44 | 0.010 |
| 39 | 0.0049 | 0.115 | 0.70 | 0.30 | 0.034 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, H.; Tamura, K.; Yamagishi, A. Oxygen Sensing by the Hybrid Langmuir-Blodgett Films of Iridium(III) Complexes and Synthetic Saponite on the Basis of Energy Transfer. Chemosensors 2017, 5, 27. https://doi.org/10.3390/chemosensors5040027
Sato H, Tamura K, Yamagishi A. Oxygen Sensing by the Hybrid Langmuir-Blodgett Films of Iridium(III) Complexes and Synthetic Saponite on the Basis of Energy Transfer. Chemosensors. 2017; 5(4):27. https://doi.org/10.3390/chemosensors5040027
Chicago/Turabian StyleSato, Hisako, Kenji Tamura, and Akihiko Yamagishi. 2017. "Oxygen Sensing by the Hybrid Langmuir-Blodgett Films of Iridium(III) Complexes and Synthetic Saponite on the Basis of Energy Transfer" Chemosensors 5, no. 4: 27. https://doi.org/10.3390/chemosensors5040027




