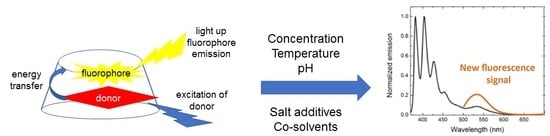
Towards Rational Chemosensor Design through Improved Understanding of Experimental Parameter Variation and Tolerance in Cyclodextrin-Promoted Fluorescence Detection
Abstract
:1. Introduction
2. Materials and Methods
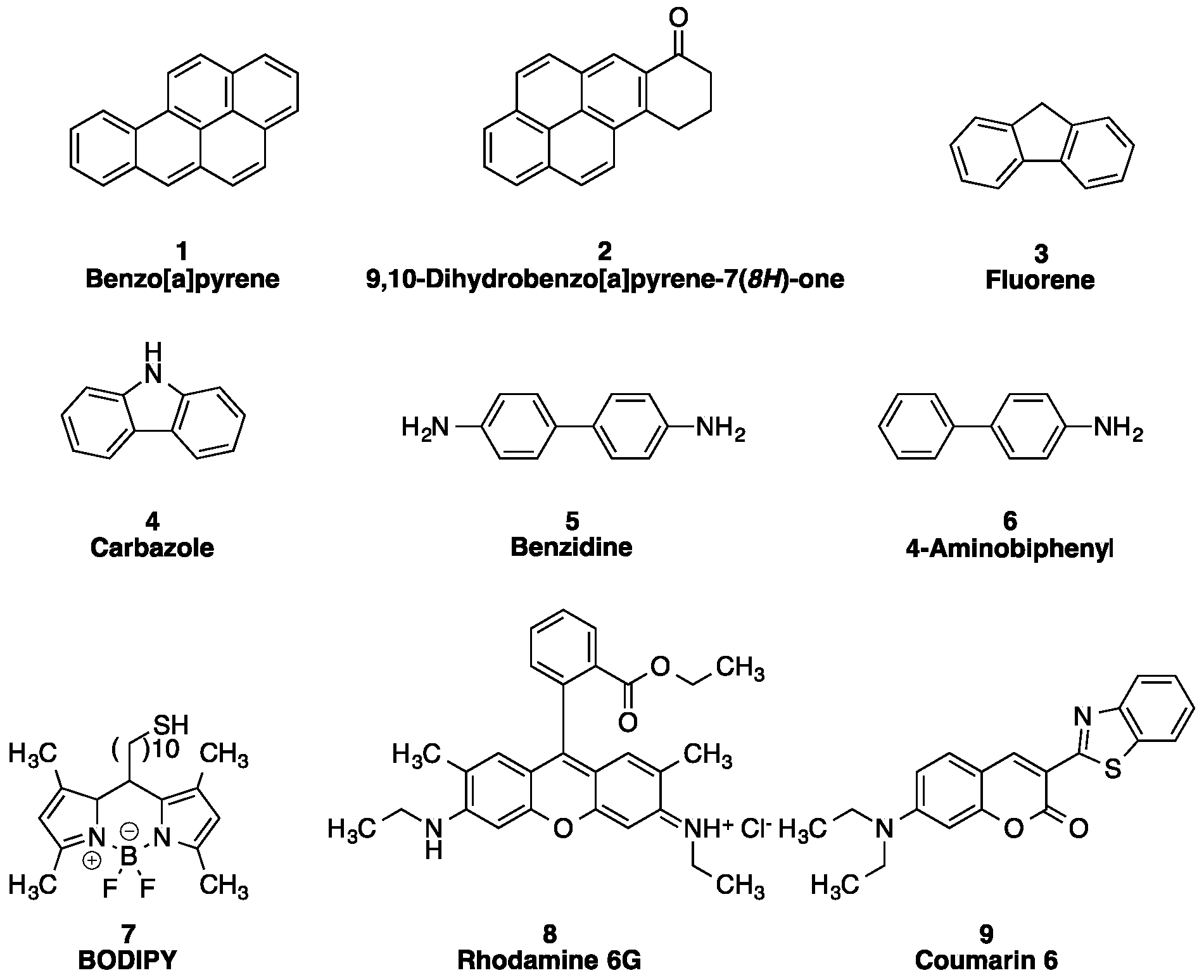
2.1. Analyte Selection
2.2. Cyclodextrin Selection
2.3. General Procedure for Energy Transfer Experiments
- (a)
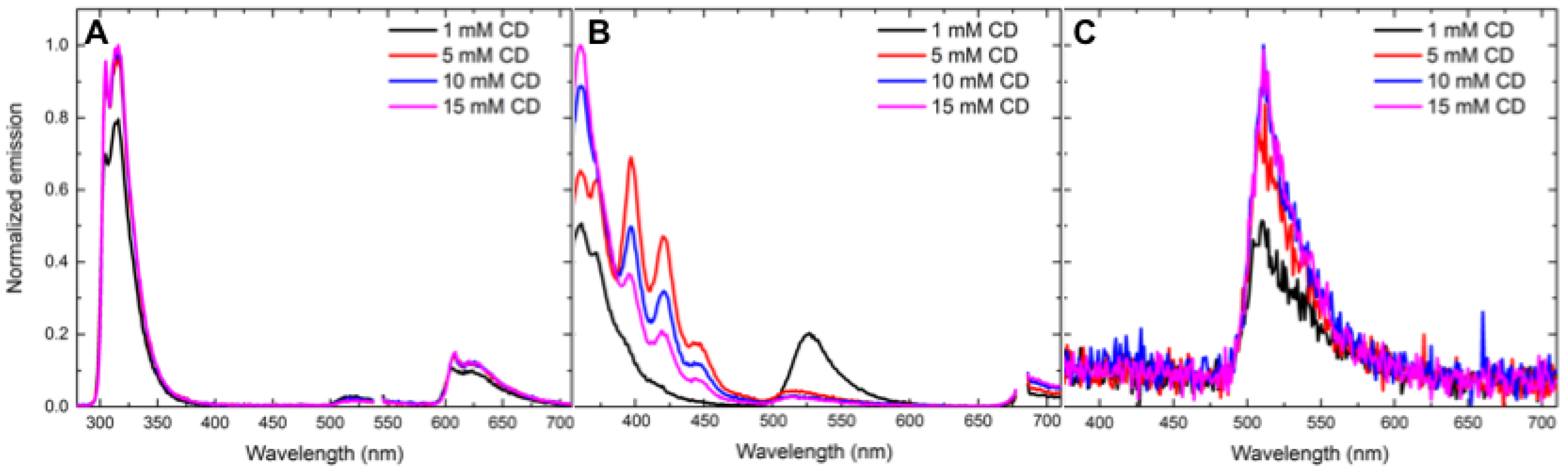
- Concentration effects. Various concentrations of cyclodextrin were used to understand the effect of the host concentration on cyclodextrin-promoted energy transfer. The following concentrations of γ-cyclodextrin were prepared in phosphate buffered saline (PBS): 5 mM, 10 mM and 15 mM. Energy-transfer experiments were conducted using the above procedures.
- (b)
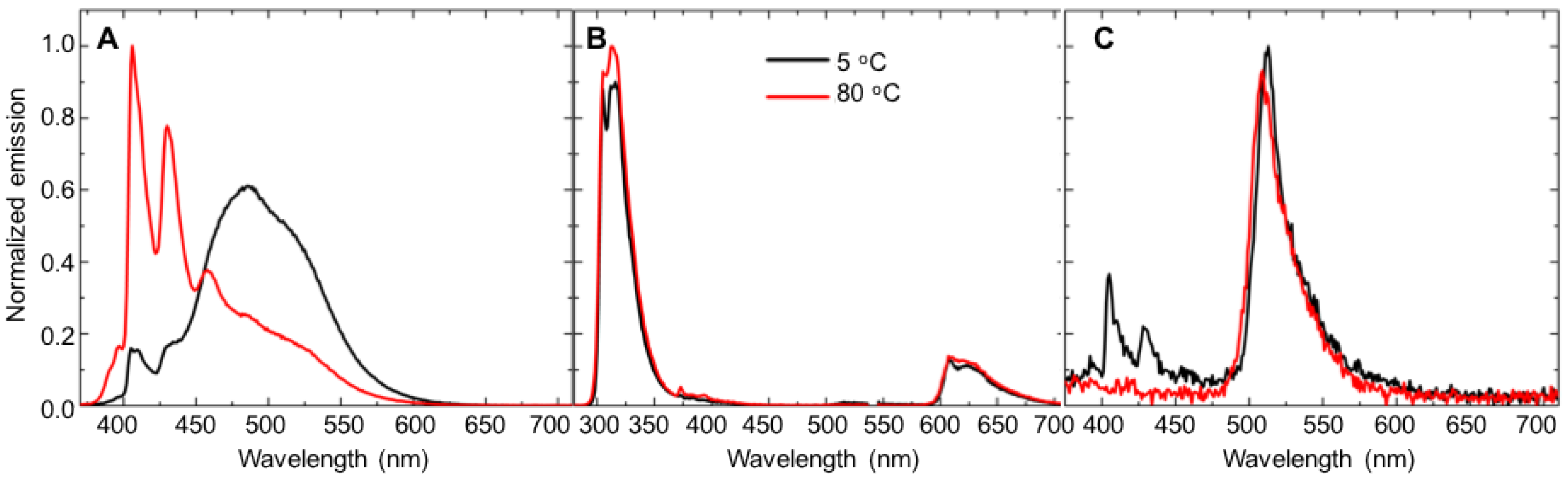
- Temperature effects. A 10 mM γ-cyclodextrin solution was prepared in PBS. Energy-transfer experiments were then conducted using the above procedures at the following temperatures: 5 °C, 20 °C, 35 °C, 50 °C, 65 °C and 80 °C. The temperature control system indicated when the desired temperature was reached, and each sample was allowed to sit in the unit after the desired temperature was reached for 5 min before the fluorescence emission spectrum was collected. Energy-transfer experiments were conducted using the above procedures.
- (c)
- pH effects. 10 mM γ-cyclodextrin solutions were prepared using various concentrations of aqueous HCl and NaOH at the following pH levels: 0, 3, 5, 8, 10, 12. Energy-transfer experiments were conducted using the above procedures.
- (d)
- Salt effects. Sodium chloride and guanidinium hydrochloride were used to investigate the effect of chaotropic and kosmotropic salts, respectively, on the cyclodextrin-promoted energy transfer. 1 M solutions of each salt were prepared in deionized water. A 10 mM γ-cyclodextrin solution was then prepared using these salt solutions. A control experiment was also performed with a 10 mM γ-cyclodextrin solution in deionized water in the absence of salt. Energy-transfer experiments were then conducted using the above procedures.
- (e)
- Solvent effects. The effect of an ethanol co-solvent on cyclodextrin-promoted energy transfer was investigated. A 10 mM γ-cyclodextrin solution was prepared in phosphate buffered saline (PBS). For these experiments, 1.25 mL of γ-cyclodextrin and 1.25 mL of ethanol were used (1:1 v/v). Energy-transfer experiments were then conducted using the above procedures.
2.4. Array-Generation Procedures
- (a)
- Classical discriminant analysis
- (b)
- Grouping variable: analytes
- (c)
- Predictors: γ-cyclodextrin/BODIPY, γ-cyclodextrin/Rhodamine 6G, γ-cyclodextrin/Coumarin 6
- (d)
- Long-range statistics: Mahal
3. Results and Discussion
3.1. Concentration-Dependent Energy-Transfer Experiments
3.2. Temperature-Dependent Energy-Transfer Experiments
3.3. pH-Dependent Energy-Transfer Experiments
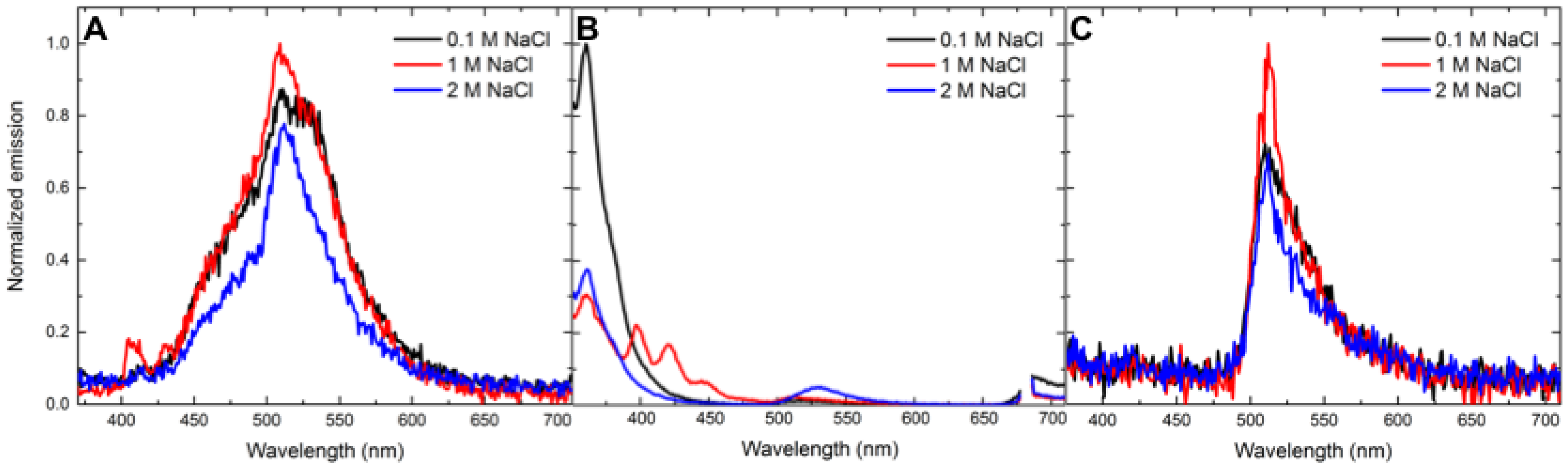
3.4. Salt-Dependent Energy-Transfer Experiments
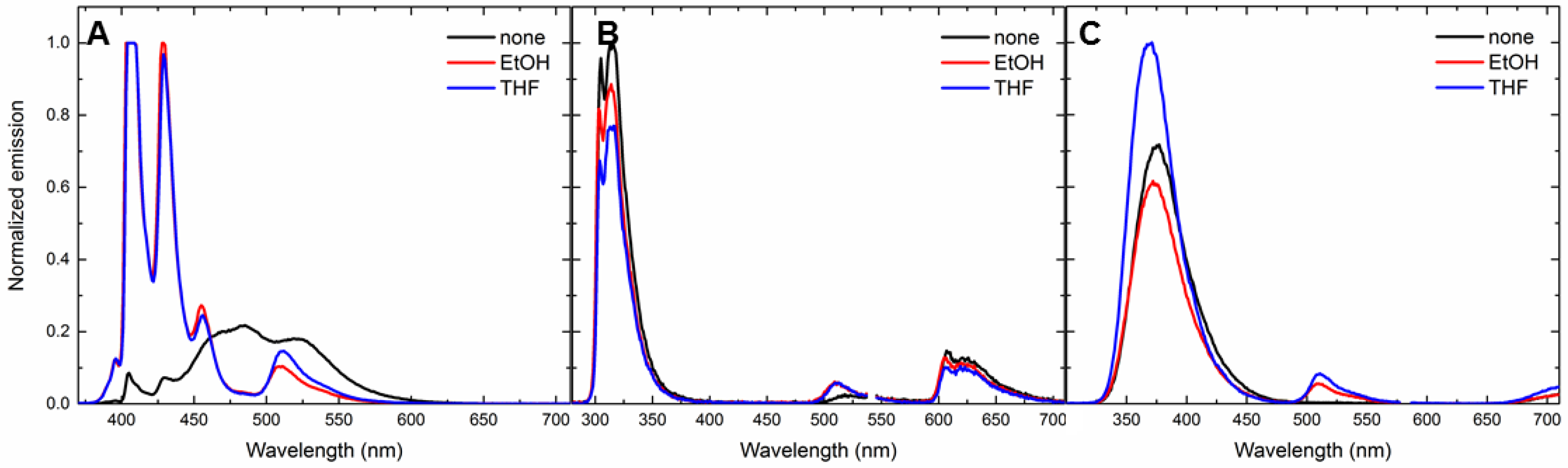
3.5. Solvent-Dependent Energy-Transfer Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric nanocarriers based on cyclodextrins for drug delivery: Host-guest interaction as stimuli responsive linker. Mol. Pharm. 2017, 14, 2475–2486. [Google Scholar] [CrossRef]
- Erdogar, N.; Varan, G.; Bilensoy, E. Amphiphilic cyclodextrin derivatives for targeted drug delivery to tumors. Curr. Top. Med. Chem. 2017, 17, 1521–1528. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefansson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef]
- Cagliero, C.; Sgorbini, B.; Cordero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C. Enantioselective gas chromatography with derivatized cyclodextrins in the flavour and fragrance field. Israel J. Chem. 2016, 56, 925–939. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Ferreira, M.; Leger, B.; Bricout, H.; Tilloy, S.; Monflier, E. Catalysis in cyclodextrin-based unconventional reaction media: Recent developments and future opportunities. ACS Sustain. Chem. Eng. 2017, 5, 3598–3606. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Zaki, H.; Levine, M. Environmentally friendly procedure for the aqueous oxidation of benzyl alcohols to aldehydes with dibromodimethylhydantoin (DBDMH) and cyclodextrin: Scope and mechanistic insights. Synth. Commun. 2016, 46, 636–644. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Phelan, T.; Levine, M. Cyclodextrin-promoted Diels Alder reactions of a polycyclic aromatic hydrocarbon under mild reaction conditions. Tetrahedron Lett. 2015, 56, 1619–1623. [Google Scholar] [CrossRef]
- Xin, X.; Wang, J.; Gong, C.; Xu, H.; Wang, R.; Ji, S.; Dong, H.; Meng, Q.; Zhang, L.; Dai, F.; et al. Cyclodextrin-based metal-organic nanotube as fluorescent probe for selective turn-on detection of hydrogen sulfide in living cells based on H2S-involved coordination mechanism. Sci. Rep. 2016, 6, 21951–21960. [Google Scholar] [CrossRef]
- Teka, S.; Gaied, A.; Jaballah, N.; Xiaonan, S.; Majdoub, M. Thin sensing layer based on semi-conducting β-cyclodextrin rotaxane for toxic metals detection. Mater. Res. Bull. 2016, 74, 248–257. [Google Scholar] [CrossRef]
- Bhopate, S.B.; Dhole, S.N. Inclusion complexation by cyclodextrin: A novel approach to improve solubility and bioavailability of poorly water soluble drug. Int. J. Pharm. 2014, 4, 175–188. [Google Scholar]
- Dardeer, H.M. Importance of cyclodextrins into inclusion complexes. Int. J. Adv. Res. 2014, 2, 414–428. [Google Scholar]
- Park, S. Cyclic glucans enhance solubility of bioavailable flavonoids. Molecules 2016, 21, 556. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, A.; Wang, B. Carvedilol solubility enhancement by inclusion complexation and solid dispersion: Review. J. Drug Deliv. Ther. 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Serio, N.; Moyano, D.F.; Rotello, V.M.; Levine, M. Array-based detection of persistent organic pollutants via cyclodextrin promoted energy transfer. Chem. Commun. 2015, 51, 11615–11618. [Google Scholar] [CrossRef] [PubMed]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Forster resonance energy transfer investigations using quantum-dot fluorophores. ChemPhysChem 2006, 7, 47–57. [Google Scholar] [CrossRef] [PubMed]
- DiScenza, D.J.; Verderame, M.; Levine, M. Detection of benzene and alkylated benzene derivatives in fuel contaminated environments. Clean Soil Air Water 2016, 44, 1621–1627. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Levine, M. Selective detection of non-aromatic pesticides via cyclodextrin-promoted fluorescence modulation. New J. Chem. 2016, 40, 789–793. [Google Scholar] [CrossRef]
- Mako, T.; Marks, P.; Cook, N.; Levine, M. Fluorescent detection of polycyclic aromatic hydrocarbons in ternary cyclodextrin complexes. Supramol. Chem. 2012, 24, 743–747. [Google Scholar] [CrossRef]
- Serio, N.; Prignano, L.; Peters, S.; Levine, M. Detection of medium-sized polycyclic aromatic hydrocarbons via fluorescence energy transfer. Polycycl. Aromat. Compound. 2014, 34, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Serio, N.; Miller, K.; Levine, M. Efficient detection of polycyclic aromatic hydrocarbons and polychlorinated biphenyls via three-component energy transfer. Chem. Commun. 2013, 49, 4821–4823. [Google Scholar] [CrossRef] [PubMed]
- Serio, N.; Roque, J.; Badwal, A.; Levine, M. Rapid and efficient pesticide detection via cyclodextrin-promoted energy transfer. Analyst 2015, 140, 7503–7507. [Google Scholar] [CrossRef] [PubMed]
- DiScenza, D.J.; Levine, M. Sensitive and selective detection of alcohols via fluorescence modulation. Supramol. Chem. 2016, 28, 881–891. [Google Scholar] [CrossRef]
- Serio, N.; Chanthalyma, C.; Prignano, L.; Levine, M. Cyclodextrin-promoted energy transfer for broadly applicable small-molecule detection. Supramol. Chem. 2014, 26, 714–721. [Google Scholar] [CrossRef] [PubMed]
- DiScenza, D.J.; Gareau, L.; Serio, N.; Roque, J.; Prignano, L.; Verderame, M.; Levine, M. Cyclodextrin-promoted detection of aromatic toxicants and toxicant metabolites in urine. Anal. Chem. Lett. 2016, 6, 345–353. [Google Scholar] [CrossRef]
- DiScenza, D.J.; Lynch, J.; Verderame, M.; Serio, N.; Prignano, L.; Gareau, L.; Levine, M. Efficient fluorescence detection of aromatic toxicants and toxicant metabolites in human breast milk. Supramol. Chem. 2017, 1–11. [Google Scholar] [CrossRef]
- Panja, S.; Chowdhury, P.; Chakravorti, S. Modulation of complexation of 4(1H-pyrrole-1-yl)-benzoic acid with β-cyclodextrin in aqueous and non-aqueous environments. Chem. Phys. Lett. 2004, 393, 409–415. [Google Scholar] [CrossRef]
- Thomas, S.W., III; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Perevoshchikova, I.V.; Kotova, E.A.; Antonenko, Y.N. Fluorescence correlation spectroscopy in biology, chemistry, and medicine. Biochemistry 2011, 76, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.C.S.; Bohne, C. Dynamics of guest binding to supramolecular systems: Techniques and selected examples. Adv. Phys. Org. Chem. 2008, 42, 167–223. [Google Scholar] [CrossRef]
- Wagner, B.D. Recent applications of host-guest inclusion in fluorescence-based trace analysis. Curr. Anal. Chem. 2007, 3, 183–195. [Google Scholar] [CrossRef]
- Hamai, S. Complex formation of tetrakis(4-sulfonatophenyl)porphyrin with γ-cyclodextrin, phenylalanine, and tryptophan in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 471–481. [Google Scholar] [CrossRef]
- Hamai, S. Ternary inclusion complexes of γ-cyclodextrin with sodium 1-pyrenesulfonate and cationic and anionic organic compounds having an alkyl chain in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 77–86. [Google Scholar] [CrossRef]
- Rakkaew, P.; Suksiriworapong, J.; Chantasart, D. β-Cyclodextrin-based ternary complexes of haloperidol and organic acids: The effect of organic acids on the drug solubility enhancement. Pharm. Dev. Technol. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.L.; Kell, A.; Chung, E.; Sinclar, C.W.; Workentin, M.S.; Bizzotto, D. Selective reductive desorption of a SAM-coated gold electrode revealed using fluorescence microscopy. J. Am. Chem. Soc. 2004, 126, 8329–8335. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Yamamoto, A.; Matsunaga, A.; Toriba, A.; Hayakawa, K. Micellar electrokinetic chromatography of monohydroxybenzo[a]pyrene positional isomers using γ-cyclodextrin. Analyst 2000, 125, 1555–1559. [Google Scholar] [CrossRef]
- Carrillo, I.; Quintana, C.; Esteva, A.M.; Hernandez, L.; Hernandez, P. Self-assembled submonolayer of β-cyclodextrins on gold electrode for the selective determination of 4-aminobiphenyl. Electroanalysis 2011, 23, 2862–2869. [Google Scholar] [CrossRef]
- Dyck, A.S.M.; Kisiel, U.; Bohne, C. Dynamics for the assembly of pyrene-γ-cyclodextrin host-guest complexes. J. Phys. Chem. B 2003, 107, 11652–11659. [Google Scholar] [CrossRef]
- Hamdi, H.; Abderrahim, R.; Meganem, F. Spectroscopic studies of inclusion complex of β-cyclodextrin and benzidine diammonium dipicrate. Spectrochim. Acta Part A 2010, 75, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.B.; Gopidas, K.R. Observation of supramolecular chirality in a hierarchically self-assembled mixed-stack charge-transfer complex. Chem. Eur. J. 2017, 23, 9600–9606. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Salmeron, R.; Chaab, I.; Carn, F.; Djabourov, M.; Bouchemal, K. Pickering emulsions with α-cyclodextrin inclusions: Structure and thermal stability. J. Colloid Interface Sci. 2016, 482, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lemli, B.; Peles, J.; Kollar, L.; Nagy, G.; Kunsagi-Mate, S. The rate of host-guest complex formation of some calixarene derivatives towards neutral aromatic guests. Supramol. Chem. 2006, 18, 251–256. [Google Scholar] [CrossRef]
- Cervero, M.; Mendicuti, F. Inclusion complexes of dimethyl 2,6-naphthalenedicarboxylate with α- and β-cyclodextrins in aqueous medium: Thermodynamics and molecular mechanics studies. J. Phys. Chem. B 2000, 104, 1572–1580. [Google Scholar] [CrossRef]
- Hiroshiba, N.; Morimoto, K.; Hayakawa, R.; Chikyow, T.; Wakayama, Y.; Matsuishi, K. Study of the exciton relaxation and recombination processes of a heteromolecular interface fabricated by a molecular superlattice growth technique. Chem. Phys. Lett. 2011, 512, 227–230. [Google Scholar] [CrossRef]
- Macanita, A.L.; Zachariasse, K.A. Viscosity dependence of intramolecular excimer formation with 1,5-Bis(1-pyrenylcarboxy)pentane in alkane solvents as a function of temperature. J. Phys. Chem. A 2011, 115, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Baiardi, A.; Bloino, J.; Barone, V. Vibronic effects on rates of excitation energy transfer and their temperature dependence. J. Chem. Theory Comput. 2016, 12, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Eersels, K.; Lieberzeit, P.; Wagner, P. A review on synthetic receptors for bioparticle detection created by surface-imprinting techniques-from principles to applications. ACS Sens. 2016, 1, 1171–1187. [Google Scholar] [CrossRef]
- Aki, H.; Niiya, T.; Iwase, Y.; Yamamoto, M. Multimodal inclusion complexes between barbiturates and 2-hydroxypropyl-β-cyclodextrin in aqueous solution: Isothermal titration microcalorimetry, 13C NMR spectrometry, and molecular dynamics simulation. J. Pharm. Sci. 2001, 90, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Strokopytov, B.; Penninga, D.; Rozeboom, H.J.; Kalk, K.H.; Dijkhuizen, L.; Dijkstra, B.W. X-ray structure of cyclodextrin glycosyltransferase complexed with acarbose. implications for the catalytic mechanism of glycosidases. Biochemistry 1995, 34, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Janzen, E.G. Effect of pH and salt concentration on bimodal inclusion of a nitroxide by cyclodextrins. J. Am. Chem. Soc. 1989, 111, 7319–7323. [Google Scholar] [CrossRef]
- Rossi, B.; Venuti, V.; D’Amico, F.; Gessini, A.; Mele, A.; Punta, C.; Melone, L.; Crupi, V.; Majolino, D.; Masciovecchio, C. Guest-matrix interactions affect the solvation of cyclodextrin-based polymeric hydrogels: A UV Raman scattering study. Soft Matter 2016, 12, 8861–8868. [Google Scholar] [CrossRef] [PubMed]
- Junquera, E.; Ruiz, D.; Aicart, E. Role of hydrophobic effect on the noncovalent interactions between salicylic acid and a series of β-cyclodextrins. J. Colloid Interface Sci. 1999, 216, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Tagiuri, A.; Mohamedali, M.; Henni, A. Dissociation constant (pKa) and thermodynamic properties of some tertiary and cyclic amines from (298 to 333) K. J. Chem. Eng. Data 2016, 61, 247–254. [Google Scholar] [CrossRef]
- Costero, A.M.; Gavina, P.; Rodriguez-Muniz, G.M.; Gil, S. N-biphenyl thioureas as carboxylate receptors. Effect of the ligand substituents on the geometry of the complexes. Tetrahedron 2006, 62, 8571–8577. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.A.; Nazim, S.; Khan, T. A recent trends in enhancement of solubility and dissolution rate of poorly soluble hydrophobic drugs by using physical and chemical modifications. J. Drug Discov. Ther. 2013, 1, 13–24. [Google Scholar]
- Parve, B.; Shinde, P.; Rawat, S.; Rathod, S.; Waghmode, G. Solubility enhancement techniques: A review. World J. Pharm. Pharm. Sci. 2014, 3, 400–422. [Google Scholar]
- Curtis, R.A.; Prausnitz, J.M.; Blanch, H.W. Protein-protein and protein-salt interactions in aqueous protein solutions containing concentrated electrolytes. Biotechnol. Bioeng. 1998, 57, 11–21. [Google Scholar] [CrossRef]
- Parui, S.; Manna, R.N.; Jana, B. Destabilization of hydrophobic core of chicken villin headpiece in guanidinium chloride induced denaturation: hint of π-cation interaction. J. Phys. Chem. B 2016, 120, 9599–9607. [Google Scholar] [CrossRef] [PubMed]
- Huan, J.; Catena, G.C.; Bright, F.V. Fluorescence-based investigations of alcohol co-solvents on the nature of cyclodextrin inclusion complexation. Appl. Spectrosc. 1992, 46, 606–614. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, C.; van der Spoel, D.; Feng, W.; Tan, T. Insight into the structural deformations of β-cyclodextrin caused by alcohol cosolvents and guest molecules. J. Phys. Chem. B 2012, 116, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.D.; Kim, J.H.; Kim, T.K.; Kim, S.H.; Lee, Y.H. Esterification of hydrophobic substrates by lipase in the cyclodextrin induced emulsion reaction system. Enzyme Microb. Technol. 2002, 30, 835–842. [Google Scholar] [CrossRef]
- Donze, C.; Coleman, A.W. Solvent effects in competition between guest molecules for β-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 23, 11–21. [Google Scholar] [CrossRef]
- Patonay, G.; Fowler, K.; Shapira, A.; Nelson, G.; Warner, I.M. Cyclodextrin complexes of polyaromatic hydrocarbons in the presence of aliphatic alcohols. J Incl. Phenom. 1987, 5, 717–723. [Google Scholar] [CrossRef]
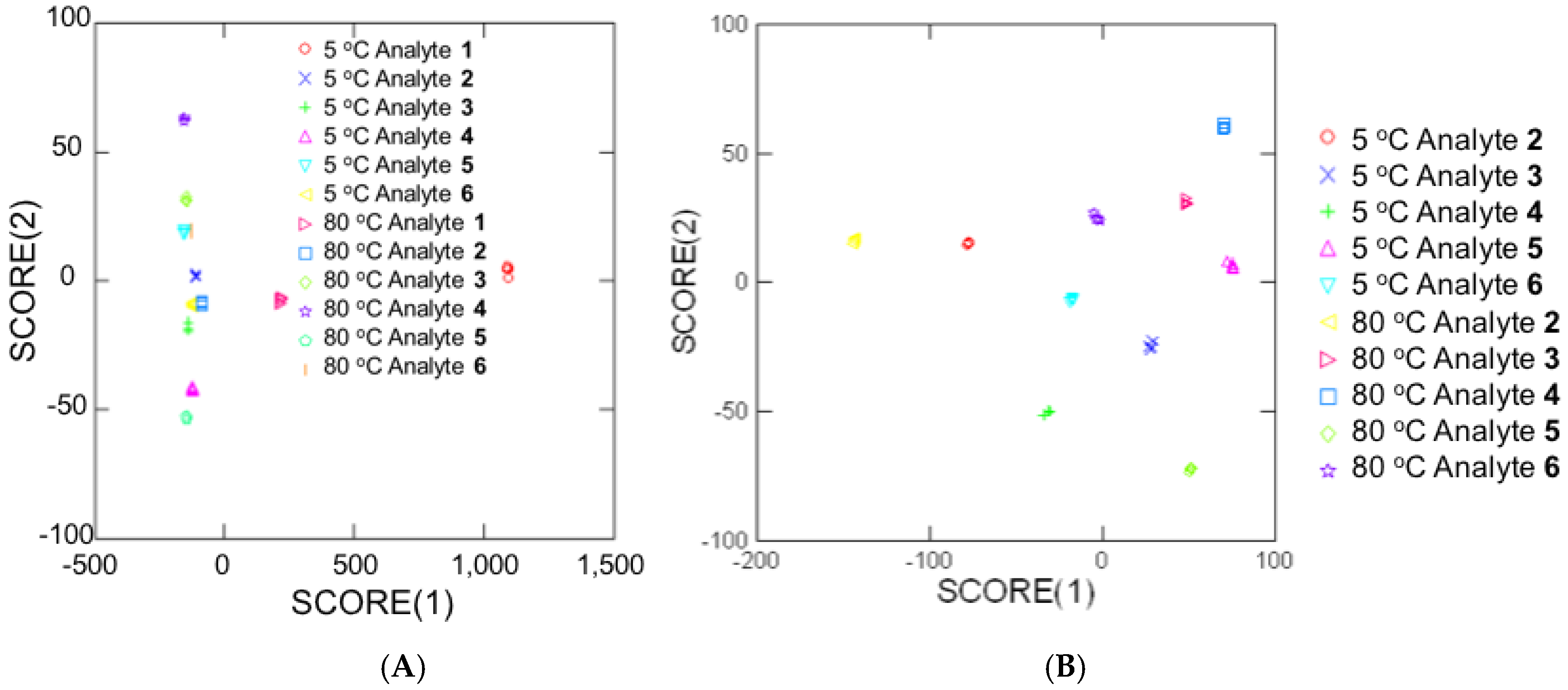


| Analyte | Concentration (mM) | Energy Transfer % (Control Ratio) |
|---|---|---|
| 3 | 1 5 10 15 | 75.8 ± 0.06 (0.32 ± 0.02) 44.1 ± 0.01 (0.71 ± 0.01) 40.8 ± 0.02 (0.74 ± 0.03) 40.5 ± 0.02 (0.79 ± 0.03) |
| 4 | 1 5 10 15 | 134.2 ± 0.25 (0.24 ± 0.05) 65.3 ± 0.00 (0.55 ± 0.03) 50.0 ± 0.02 (0.73 ± 0.03) 47.7 ± 0.03 (0.83 ± 0.03) |
| Analyte | Temperature (°C) | Fluorophore 7 | Fluorophore 8 | Fluorophore 9 |
|---|---|---|---|---|
| 1 | 5 | 501.1 ± 0.21 (0.16 ± 0.01) | 36.4 ± 0.00 (0.19 ± 0.00) | 463.4 ± 0.01 (0.02 ± 0.00) |
| 80 | 290.5 ± 0.02 (0.08 ± 0.00) | 10.5 ± 0.00 (0.34 ± 0.00) | 171.0 ± 0.01 (0.06 ± 0.00) | |
| 2 | 5 | 102.1 ± 0.00 (0.33 ± 0.00) | 15.5 ± 0.00 (0.56 ± 0.00) | 88.5 ± 0.00 (0.22 ± 0.00) |
| 80 | 62.2 ± 0.00 (0.33 ± 0.00) | 8.3 ± 0.00 (0.97 ± 0.00) | 45.5 ± 0.00 (0.28 ± 0.00) | |
| 3 | 5 | 34.3 ± 0.01 (0.86 ± 0.02) | 13.0 ± 0.00 (0.54 ± 0.00) | 52.9 ± 0.00 (0.61 ± 0.01) |
| 80 | 21.4 ± 0.00 (1.03 ± 0.02) | 8.9 ± 0.07 (0.43 ± 0.00) | 21.1 ± 0.00 (0.64 ± 0.01) | |
| 4 | 5 | 87.3 ± 0.02 (0.35 ± 0.01) | 11.8 ± 0.00 (1.05 ± 0.00) | 71.1 ± 0.01 (0.37 ± 0.00) |
| 80 | 26.3 ± 0.00 (0.94 ± 0.01) | 10.0 ± 0.00 (1.07 ± 0.00) | 17.5 ± 0.00 (0.93 ± 0.01) | |
| 5 | 5 | 33.9 ± 0.00 (1.02 ± 0.00) | 6.2 ± 0.00 (1.09 ± 0.00) | 33.6 ± 0.01 (0.70 ± 0.03) |
| 80 | 27.2 ± 0.00 (0.99 ± 0.01) | 5.5 ± 0.00 (1.00 ± 0.00) | 15.5 ± 0.00 (1.02 ± 0.01) | |
| 6 | 5 | 57.9 ± 0.00 (0.95 ± 0.00) | 14.3 ± 0.00 (2.09 ± 0.01) | 81.4 ± 0.02 (0.96 ± 0.00) |
| 80 | 37.98 ± 0.01 (1.02 ± 0.00) | 7.3 ± 0.00 (2.83 ± 0.01) | 37.1 ± 0.01 (1.50 ± 0.00) |
| Analyte | pH | Energy Transfer % (Control Ratio) |
|---|---|---|
| 5 | 0 | 35.4 ± 0.00 (1.09 ± 0.00) |
| 3 | 39.7 ± 0.00 (1.19 ± 0.01) | |
| 5 | 38.4 ± 0.00 (1.18 ± 0.00) | |
| 8 | 42.4 ± 0.00 (0.26 ± 0.01) | |
| 10 | 40.1 ± 0.00 (1.08 ± 0.01) | |
| 12 | 36.3 ± 0.01 (1.10 ± 0.03) | |
| 6 | 0 | 56.1 ± 0.00 (0.70 ± 0.00) |
| 3 | 60.0 ± 0.00 (0.64 ± 0.01) | |
| 5 | 73.3 ± 0.01 (0.54 ± 0.00) | |
| 8 | 80.9 ± 0.02 (0.13 ± 0.00) | |
| 10 | 79.4 ± 0.01 (0.53 ± 0.00) | |
| 12 | 71.9 ± 0.01 (0.58 ± 0.01) |
| Analyte | 0.1 M NaCl | 1 M NaCl | 2 M NaCl | 0.1 M GuHCl | 1 M GuHCl | 2 M GuHCl |
|---|---|---|---|---|---|---|
| 2 | 71.3 ± 0.00 (0.47 ± 0.00) | 69.8 ± 0.03 (0.49 ± 0.00) | 69.6 ± 0.01 (0.55 ± 0.01) | 75.6 ± 0.00 (0.49 ± 0.00) | 67.1 ± 0.01 (0.49 ± 0.01) | 66.3 ± 0.01 (0.51 ± 0.01) |
| 4 | 40.5 ± 0.00 (0.92 ± 0.01) | 51.4 ± 0.01 (0.78 ± 0.01) | 84.3 ± 0.08 (0.49 ± 0.04) | 40.1 ± 0.01 (0.90 0.01) | 41.4 ± 0.01 (0.89 ± 0.02) | 50.2 ± 0.08 (0.71 ± 0.10) |
| 5 | 37.6 ± 0.01 (1.03 ± 0.01) | 40.0 ± 0.00 (1.14 ± 0.00) | 43.3 ± 0.01 (1.01 ± 0.01) | 38.1 ± 0.00 (1.03 0.00) | 37.7 ± 0.00(1.07 ± 0.01) | 33.6 ± 0.00 (1.02 ± 0.00) |
| Analyte | GCD | GCD + Ethanol | GCD + THF |
|---|---|---|---|
| 1 | 1056.7 ± 0.10 (0.03 ± 0.00) | 12.7 ± 0.00 (1.51 ± 0.01) | 15.8 ± 0.00 (1.23 ± 0.02) |
| 3 | 40.8 ± 0.02 (0.74 ± 0.03) | 2.5 ± 0.00 (7.70 ± 0.04) | 5.10 ± 0.00 (3.73 ± 0.07) |
| 6 | 69.4 ± 0.00 (0.57 ± 0.01) | 5.6 ± 0.00 (3.37 ± 0.01) | 7.84 ± 0.00 (2.63 ± 0.03) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiScenza, D.J.; Culton, E.; Verderame, M.; Lynch, J.; Serio, N.; Levine, M. Towards Rational Chemosensor Design through Improved Understanding of Experimental Parameter Variation and Tolerance in Cyclodextrin-Promoted Fluorescence Detection. Chemosensors 2017, 5, 34. https://doi.org/10.3390/chemosensors5040034
DiScenza DJ, Culton E, Verderame M, Lynch J, Serio N, Levine M. Towards Rational Chemosensor Design through Improved Understanding of Experimental Parameter Variation and Tolerance in Cyclodextrin-Promoted Fluorescence Detection. Chemosensors. 2017; 5(4):34. https://doi.org/10.3390/chemosensors5040034
Chicago/Turabian StyleDiScenza, Dana J., Ella Culton, Molly Verderame, Julie Lynch, Nicole Serio, and Mindy Levine. 2017. "Towards Rational Chemosensor Design through Improved Understanding of Experimental Parameter Variation and Tolerance in Cyclodextrin-Promoted Fluorescence Detection" Chemosensors 5, no. 4: 34. https://doi.org/10.3390/chemosensors5040034