CO-Sensing Properties of Diode-Type Gas Sensors Employing Anodized Titania and Noble-Metal Electrodes under Hydrogen Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
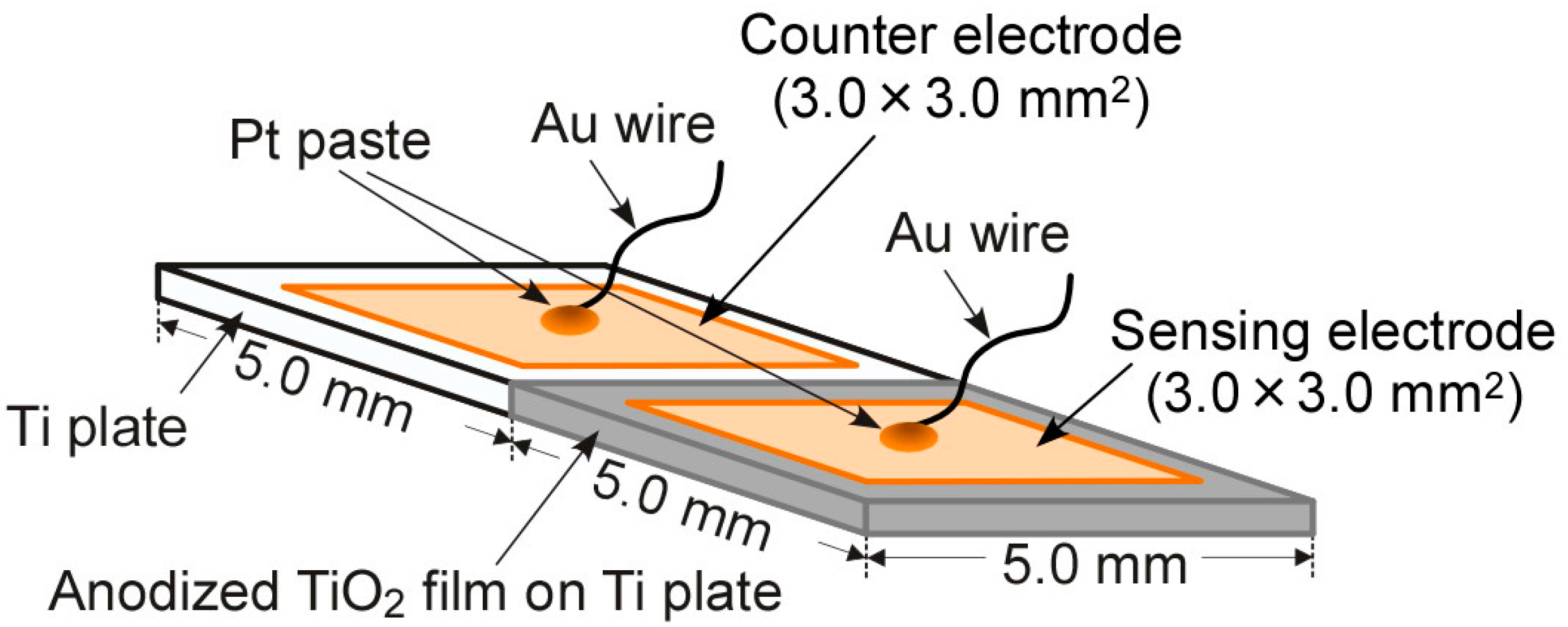
2.1. Fabrication of Diode-Type Gas Sensors
2.2. Measurements of Gas-Sensing Properties
3. Results and Discussion
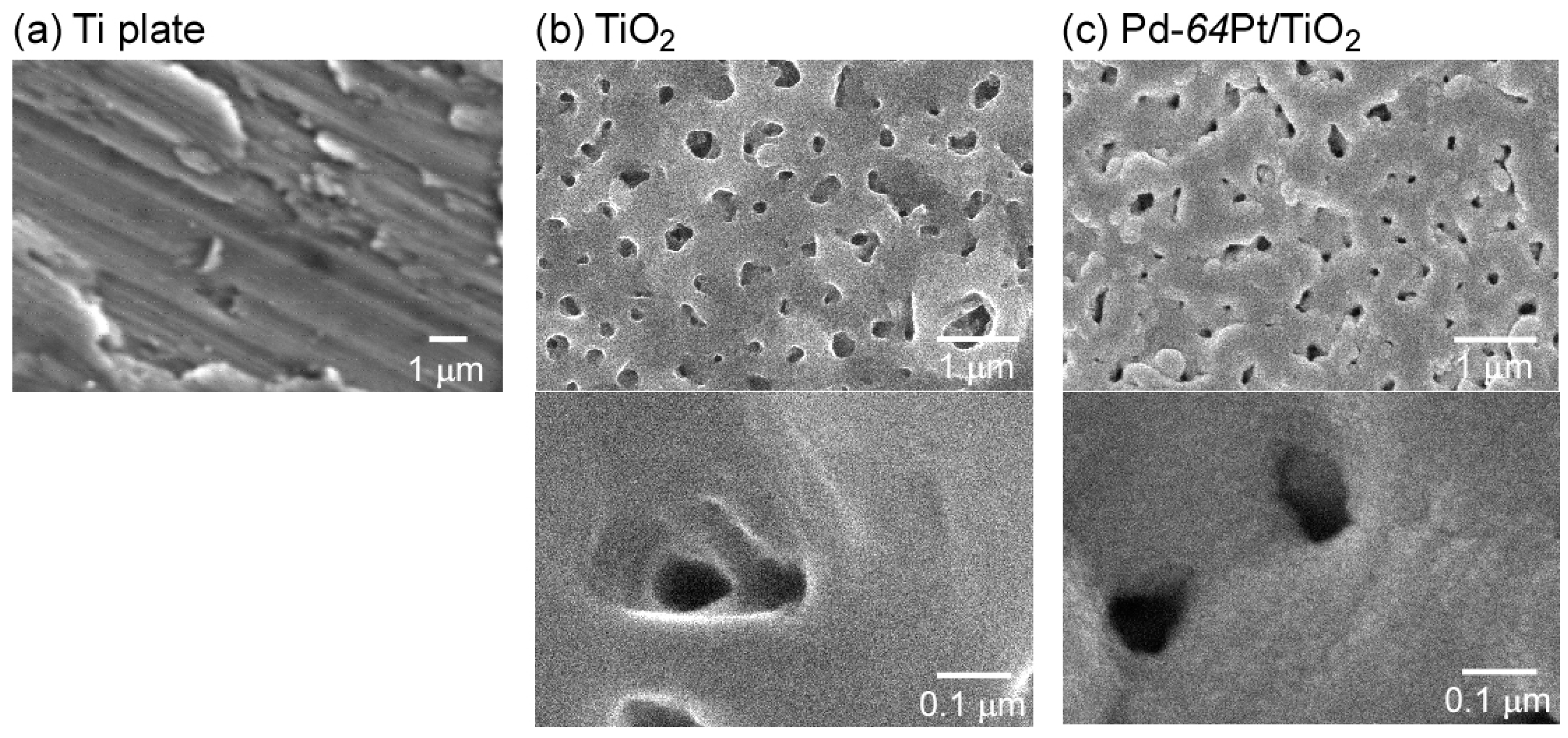
3.1. Microstructure
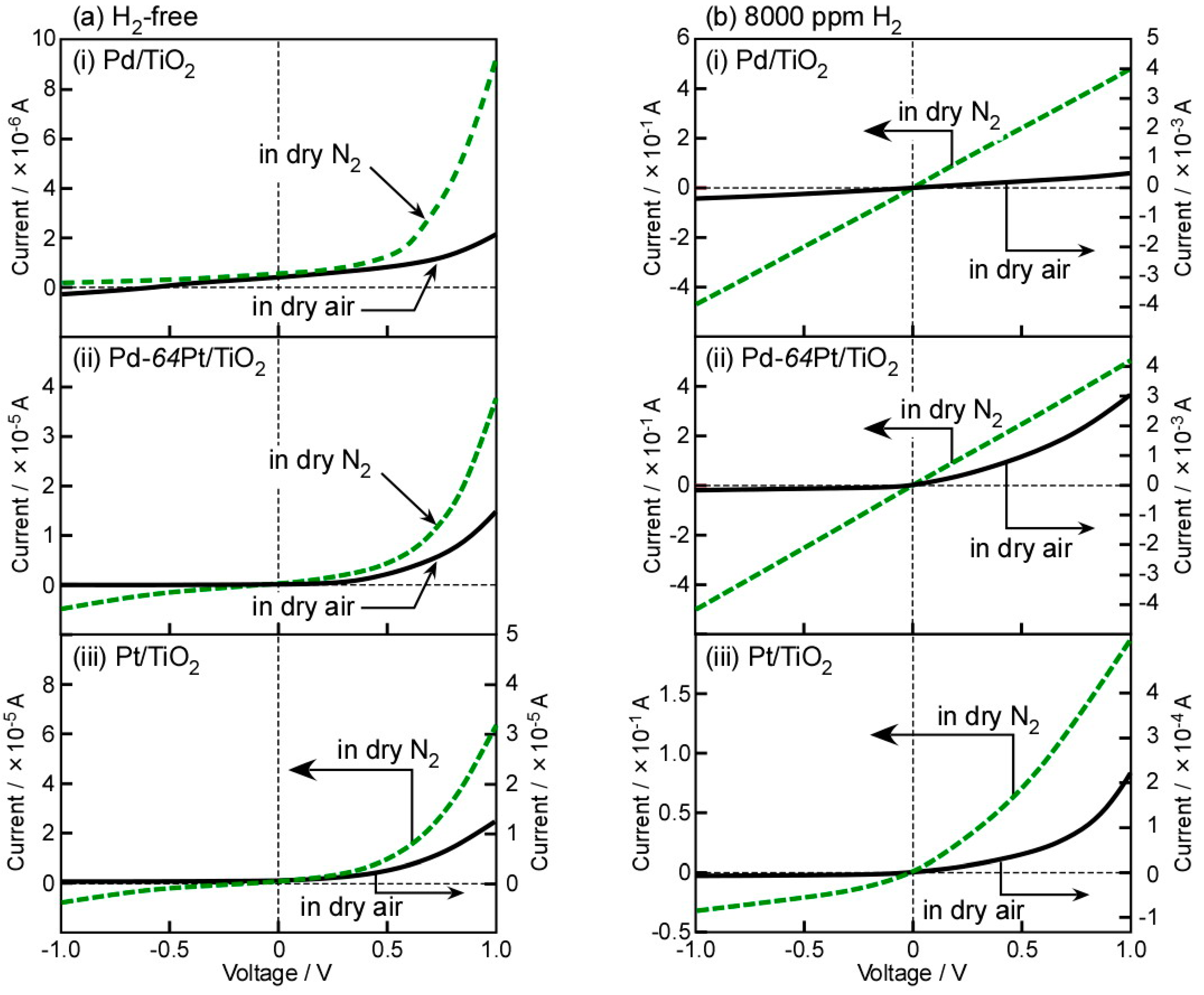
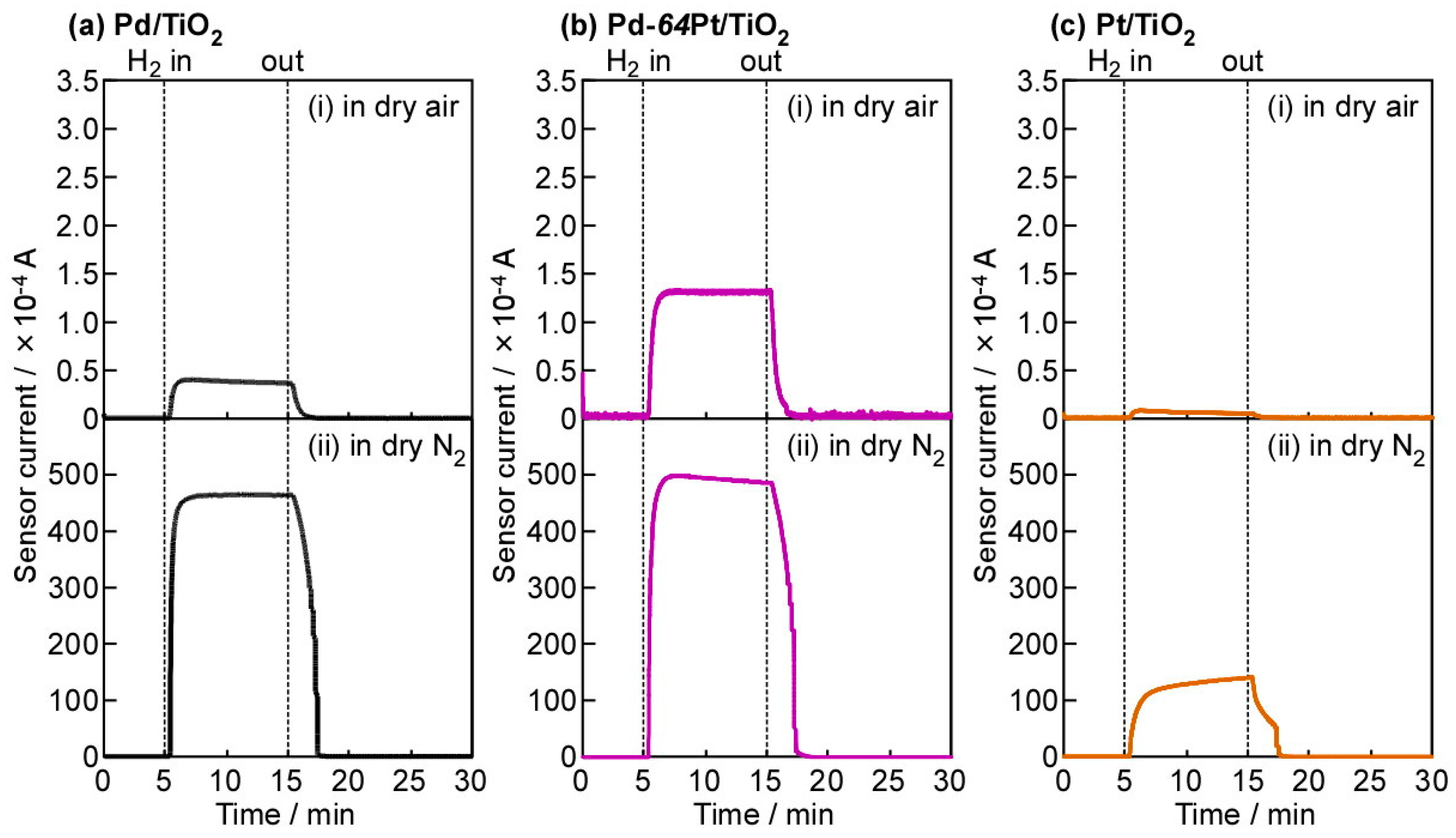
3.2. Basic Diode Characteristics and H2-Sensing Properties in Dry Air and N2
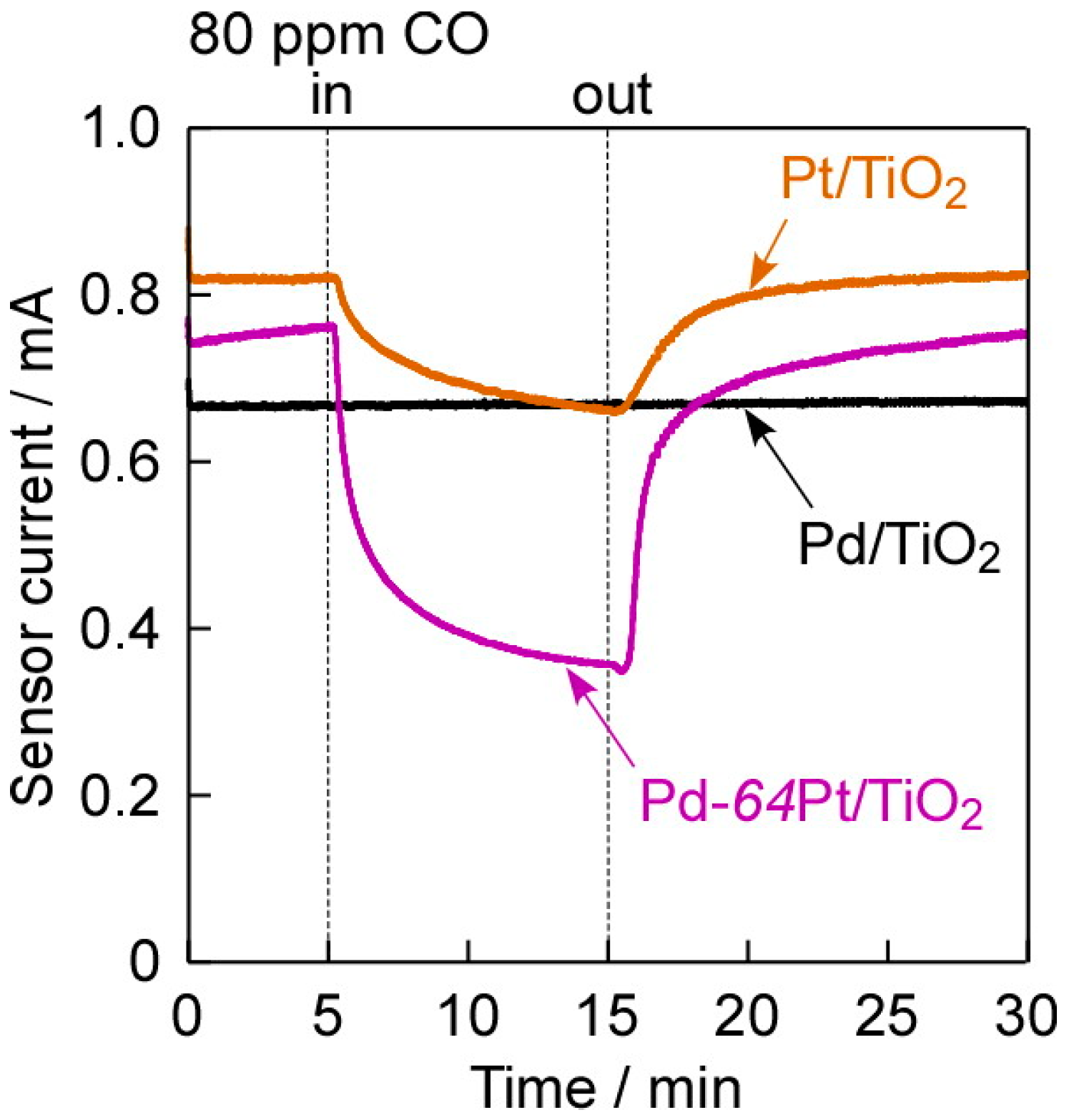
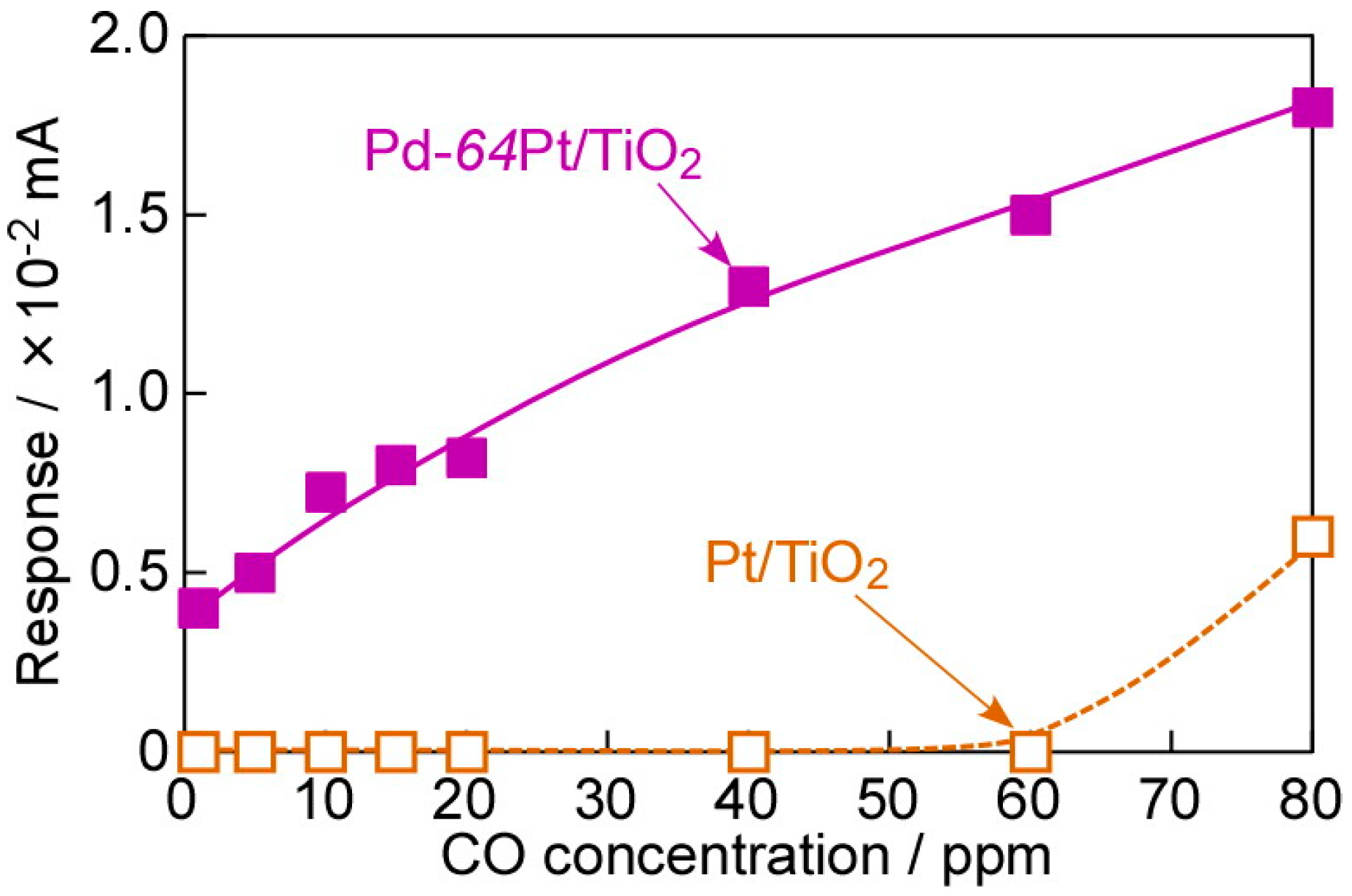
3.3. Typical I–V Characteristics and CO-Sensing Properties in H2
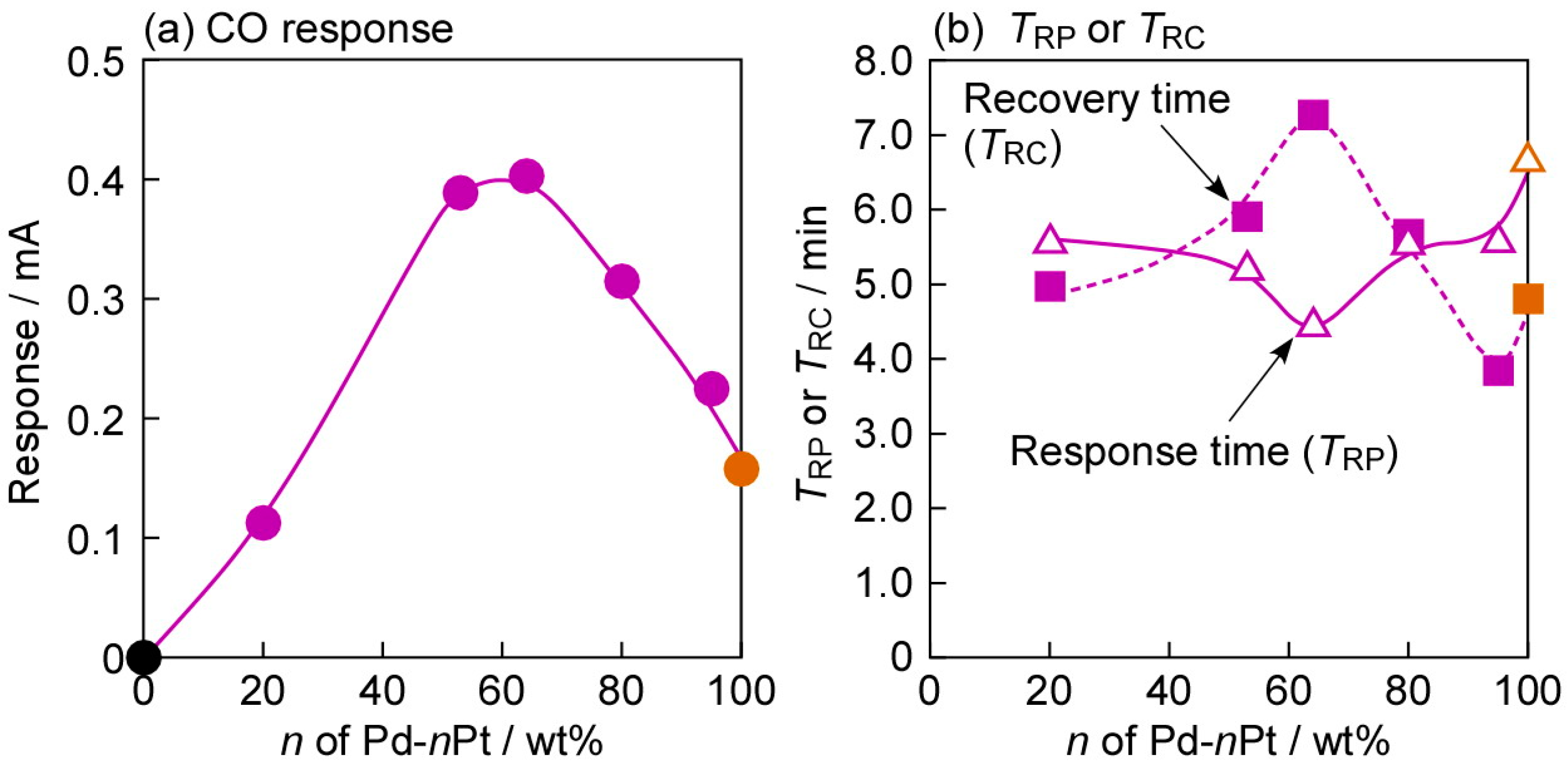
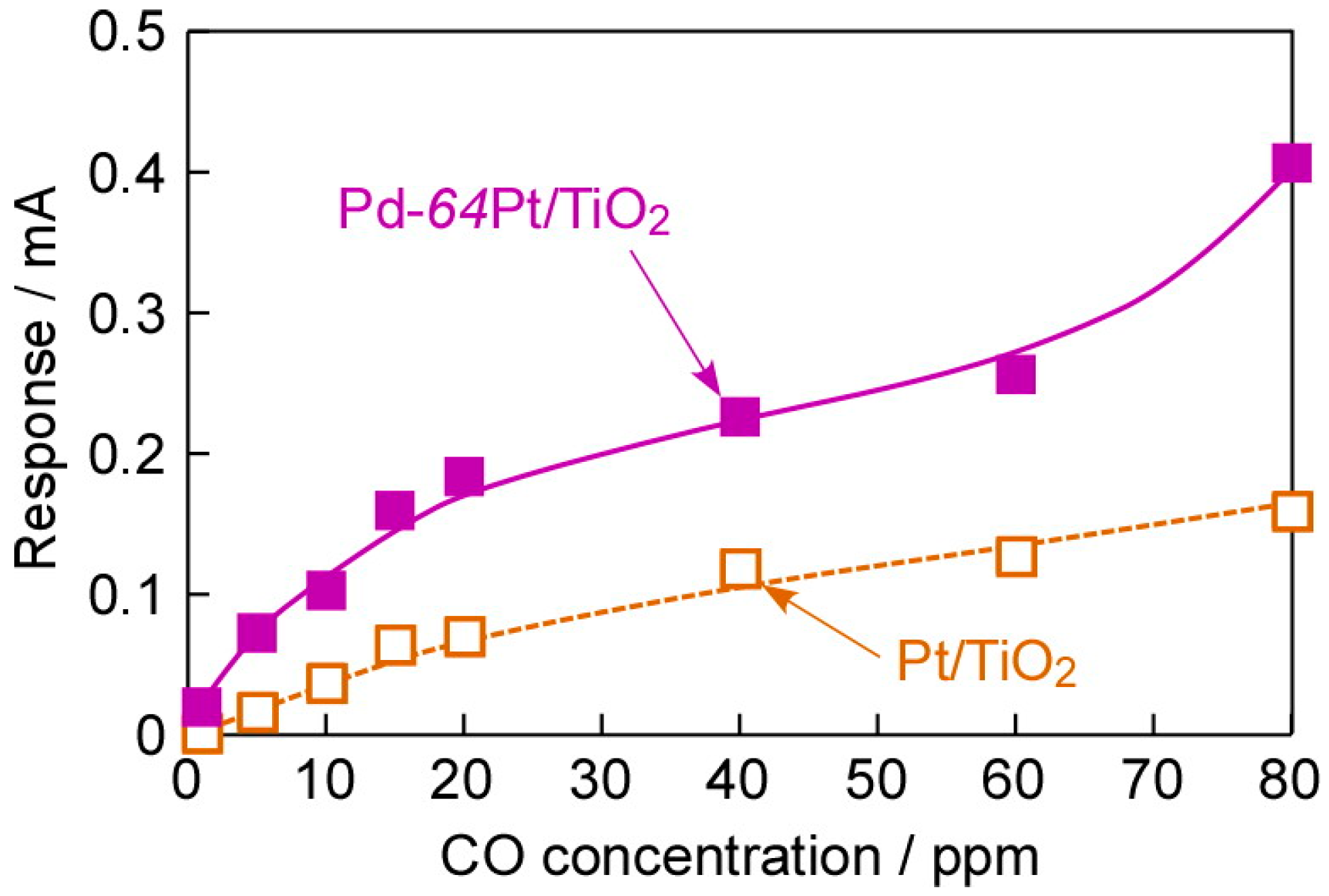
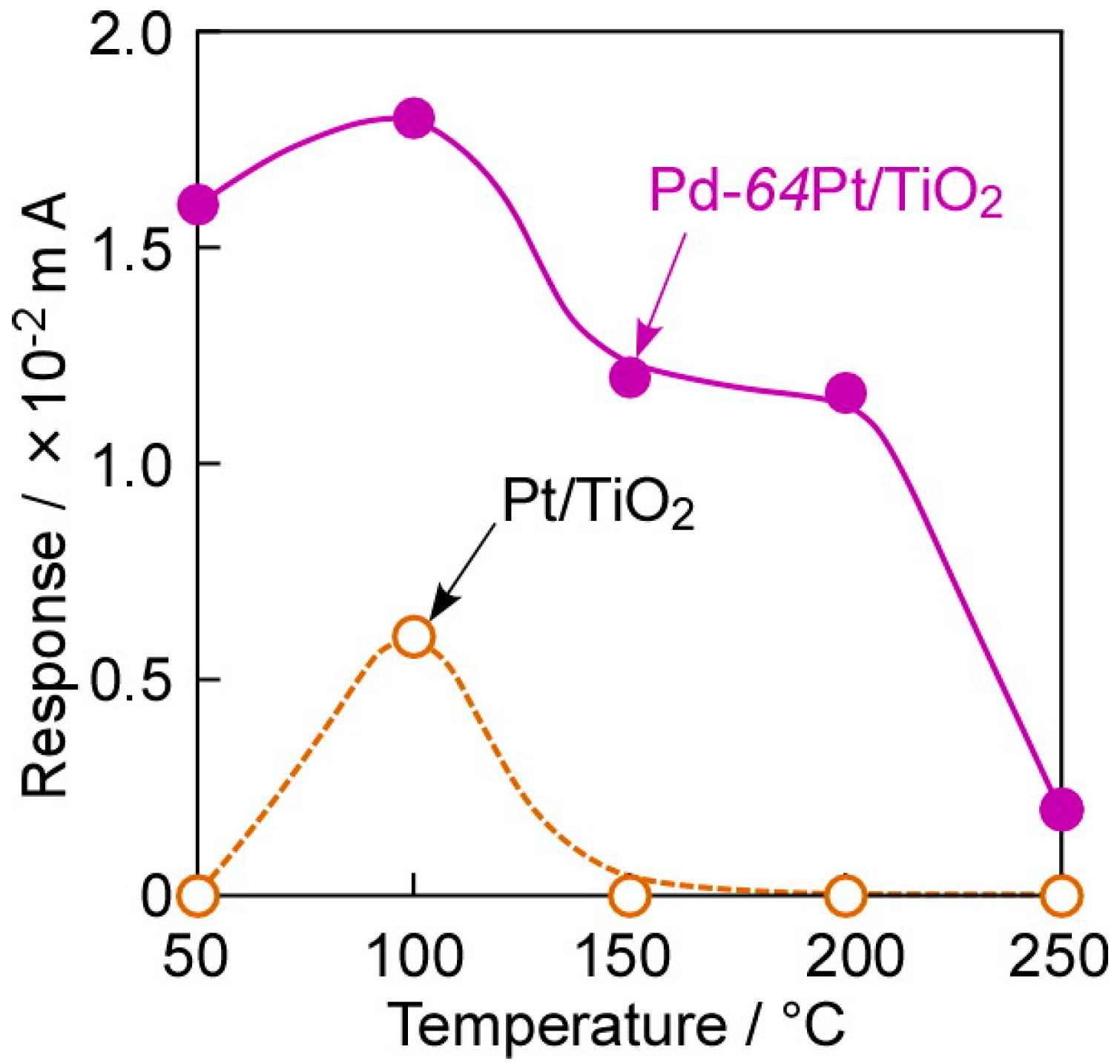
3.4. Impacts of Various Factors on CO-Sensing Properties in H2
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Pérez, L.C.; Koski, P.; Ihonen, J.; Sousa, J.M.; Mendes, A. Effect of fuel utilization on the carbon monoxide poisoning dynamics of polymer electrolyte membrane fuel cells. J. Power Sources 2014, 258, 122–128. [Google Scholar] [CrossRef]
- Yamaura, H.; Iwasaki, Y.; Hirao, S.; Yahiro, H. CuO/SnO2–In2O3 sensor for monitoring CO concentration in a reducing atmosphere. Sens. Actuators B Chem. 2011, 153, 465–467. [Google Scholar] [CrossRef]
- Prasad, R.M.; Gurlo, A.; Riedel, R.; Hübner, M.; Barsan, N.; Weimar, U. Microporous ceramic coated SnO2 sensors for hydrogen and carbon monoxide sensing in harsh reducing conditions. Sens. Actuators B Chem. 2010, 149, 105–109. [Google Scholar] [CrossRef]
- Yamaura, H.; Nakaoka, M.; Hirao, S.; Fujiwara, A.; Yahiro, H. CO sensing property of transition metal oxide-loaded SnO2 in a reducing atmosphere. Mater. Manuf. Process. 2010, 25, 350–353. [Google Scholar] [CrossRef]
- Yamaura, H.; Nakaoka, M.; Yahiro, H. Effect of supported transition metal on CO sensing performance using SnO2 in reducing atmosphere. Adv. Mater. Res. 2008, 47–50, 1518–1521. [Google Scholar] [CrossRef]
- Wurzinger, O.; Reinhardt, G. CO-sensing properties of doped SnO2 sensors in H2-rich gases. Sens. Actuators B Chem. 2004, 103, 104–110. [Google Scholar] [CrossRef]
- Liu, C.; Noda, Z.; Sasaki, K.; Hayashi, K. Development of a polyaniline nanofiber-based carbon monoxide sensor for hydrogen fuel cell application. Int. J. Hydrogen Energy 2012, 37, 13529–13535. [Google Scholar] [CrossRef]
- Gopal Reddy, C.V.; Dutta, P.K.; Akbar, S.A. Detection of CO in a reducing, hydrous environment using CuBr as electrolyte. Sens. Actuators B Chem. 2003, 92, 351–355. [Google Scholar] [CrossRef]
- Holt, C.T.; Azad, A.-M.; Swartz, S.L.; Rao, R.R.; Dutta, P.K. Carbon monoxide sensor for PEM fuel cell systems. Sens. Actuators B Chem. 2002, 87, 414–420. [Google Scholar] [CrossRef]
- Dutta, P.K.; Rao, R.R.; Swartz, S.L.; Holt, C.T. Sensing of carbon monoxide gas in reducing environments. Sens. Actuators B Chem. 2002, 84, 189–193. [Google Scholar] [CrossRef]
- Pijolat, C.; Tournier, G.; Viricelle, P. CO detection in H2 reducing atmosphere with mini fuel cell. Sens. Actuators B Chem. 2011, 156, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Mukundan, R.; Brosha, E.L.; Garzon, F.H. A low temperature sensor for the detection of carbon monoxide in hydrogen. Solid State Ion. 2004, 175, 497–501. [Google Scholar] [CrossRef]
- Kirby, K.W.; Chu, A.C.; Fuller, K.C. Detection of low level carbon monoxide in hydrogen-rich gas streams. Sens. Actuators B Chem. 2003, 95, 224–231. [Google Scholar] [CrossRef]
- Pijolat, C.; Tournier, G.; Viricelle, P. Detection of CO in H2-rich gases with a samarium doped ceria (SDC) sensor for fuel cell applications. Sens. Actuators B Chem. 2009, 141, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, A.; Hibino, T.; Sano, M. Solid oxide fuel cells that enable the detection of CO in reformed gases. Sens. Actuators B Chem. 2002, 86, 12–19. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kuwano, N.; Hyodo, T.; Egashira, M. High H2 sensing performance of anodically oxidized TiO2 film contacted with Pd. Sens. Actuators B Chem. 2002, 83, 195–201. [Google Scholar] [CrossRef]
- Iwanaga, T.; Hyodo, T.; Shimizu, Y.; Egashira, M. H2 sensing properties and mechanism of anodically oxidized TiO2 film contacted with Pd Electrode. Sens. Actuators B Chem. 2003, 93, 519–525. [Google Scholar] [CrossRef]
- Miyazaki, H.; Hyodo, T.; Shimizu, Y.; Egashira, M. Hydrogen-sensing properties of anodically oxidized TiO2 film sensors: Effects of preparation and pretreatment conditions. Sens. Actuators B Chem. 2003, 93, 519–525. [Google Scholar] [CrossRef]
- Hyodo, T.; Nakaoka, M.; Shimizu, Y.; Egashira, M. Diode-type H2 sensors using anodized TiO2 films-structural and compositional controls of noble metal sensing electrodes. Sens. Lett. 2011, 9, 641–645. [Google Scholar] [CrossRef]
- Yamamoto, G.; Yamashita, T.; Matsuo, K.; Hyodo, T.; Shimizu, Y. Effects of polytetrafluoroethylene or polyimide coating on H2 sensing properties of anodized TiO2 films equipped with Pd–Pt electrodes. Sens. Actuators B Chem. 2013, 183, 253–264. [Google Scholar] [CrossRef]
- Hyodo, T.; Yamashita, T.; Shimizu, Y. Effects of surface modification of noble-metal sensing electrodes with Au on the hydrogen-sensing properties of diode-type gas sensors employing an anodized titania film. Sens. Actuators B Chem. 2015, 207, 105–116. [Google Scholar] [CrossRef]
- Hyodo, T.; Ohoka, J.; Shimizu, Y. Design of anodically oxidized Nb2O5 films as a diode-type H2 sensing material. Sens. Actuators B Chem. 2006, 117, 359–366. [Google Scholar] [CrossRef]
- Hyodo, T.; Shibata, H.; Shimizu, Y. H2 sensing properties of diode-type gas sensors fabricated with Ti- and/or Nb-based materials. Sens. Actuators B Chem. 2009, 142, 92–104. [Google Scholar] [CrossRef]
- Singh-Miller, N.E.; Marzari, N. Surface energies, work functions, and surface relaxations of low index metallic surfaces from first-principles. Phys. Rev. B 2009, 80, 235407. [Google Scholar] [CrossRef]
- Chen, H.; Li, P.; Umezawa, N.; Abe, H.; Ye, J.; Shiraishi, K.; Ohta, A.; Miyazaki, S. Bonding and electron energy-level alignment at metal/TiO2 interfaces: A density functional theory study. J. Phys. Chem. C 2016, 120, 5549–5556. [Google Scholar] [CrossRef]
- Matsushima, S.; Maekawa, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Dispersion and electric interaction of palladium particles supported on tin oxide. Nippon Kagaku Kaishi 1991, 1991, 1677–1683. [Google Scholar] [CrossRef]
- Kirner, U.K.; Schierbaum, K.D.; Göpel, W. Interface-reactions of Pt/TiO2: Comparative electrical, XPS-, and AES-depth profile investigations. Fresenius J. Anal. Chem. 1991, 341, 416–420. [Google Scholar] [CrossRef]
- Dittrich, T.; Zinchuk, V.; Shryshevskyy, V.; Urban, I.; Hilt, O. Electrical transport in passivated Pt/TiO2/Ti Schottky diodes. J. Appl. Phys. 2005, 98, 104501. [Google Scholar] [CrossRef]
- Lewis, F.A.; Kandasamy, K.; Tong, X.Q. Platinum and palladium-hydrogen. In Hydrogen in Metal Systems II; Lewis, F.A., Aladjem, A., Eds.; Scitec Publications Ltd.: Uetikon, Switzerland, 2000; pp. 207–501. ISBN 3-908450-55-1. [Google Scholar]
- Zhou, C.; Wu, J.; Nie, A.; Forrey, R.C.; Tachibana, A.; Cheng, H. On the sequential hydrogen dissociative chemisorption on small platinum clusters: A density functional theory study. J. Phys. Chem. C 2007, 111, 12773–12778. [Google Scholar] [CrossRef]
- Rioux, R.M.; Hoefelmeyer, J.D.; Grass, M.; Song, H.; Niesz, K.; Yang, P.; Somorjai, G.A. Adsorption and co-adsorption of ethylene and carbon monoxide on silica-supported monodisperse Pt nanoparticles: Volumetric adsorption and infrared spectroscopy studies. Langmuir 2008, 24, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Arai, H. Hydrogenation of carbon monoxide with solid catalysts. J. Jpn. Oil Chem. Soc. 1978, 27, 565–571. [Google Scholar] [CrossRef]
- Thinon, O.; Rachedi, K.; Diehl, F.; Avenier, P.; Schuurman, Y. Kinetics and mechanism of the water-gas shift reaction over platinum supported catalysts. Top. Catal. 2009, 52, 1940. [Google Scholar] [CrossRef]


| Electrode | Power to Target/W | Thickness/nm | ||
|---|---|---|---|---|
| Material | n * | Pd | Pt | |
| Pd | 300 | ca. 160 | ||
| Pt | 300 | ca. 200 | ||
| Pd-nPt | 20 | 300 | 50 | ca. 193 ** |
| 53 | 300 | 150 | ca. 260 ** | |
| 64 | 300 | 200 | ca. 293 ** | |
| 80 | 200 | 300 | ca. 307 ** | |
| 95 | 30 | 300 | ca. 216 ** | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyodo, T.; Morinaga, N.; Shimizu, Y. CO-Sensing Properties of Diode-Type Gas Sensors Employing Anodized Titania and Noble-Metal Electrodes under Hydrogen Atmosphere. Chemosensors 2018, 6, 7. https://doi.org/10.3390/chemosensors6010007
Hyodo T, Morinaga N, Shimizu Y. CO-Sensing Properties of Diode-Type Gas Sensors Employing Anodized Titania and Noble-Metal Electrodes under Hydrogen Atmosphere. Chemosensors. 2018; 6(1):7. https://doi.org/10.3390/chemosensors6010007
Chicago/Turabian StyleHyodo, Takeo, Naoki Morinaga, and Yasuhiro Shimizu. 2018. "CO-Sensing Properties of Diode-Type Gas Sensors Employing Anodized Titania and Noble-Metal Electrodes under Hydrogen Atmosphere" Chemosensors 6, no. 1: 7. https://doi.org/10.3390/chemosensors6010007





