Miniaturized Single Chip Arrangement of a Wheatstone Bridge Based Calorimetric Gas Sensor
Abstract
:1. Introduction
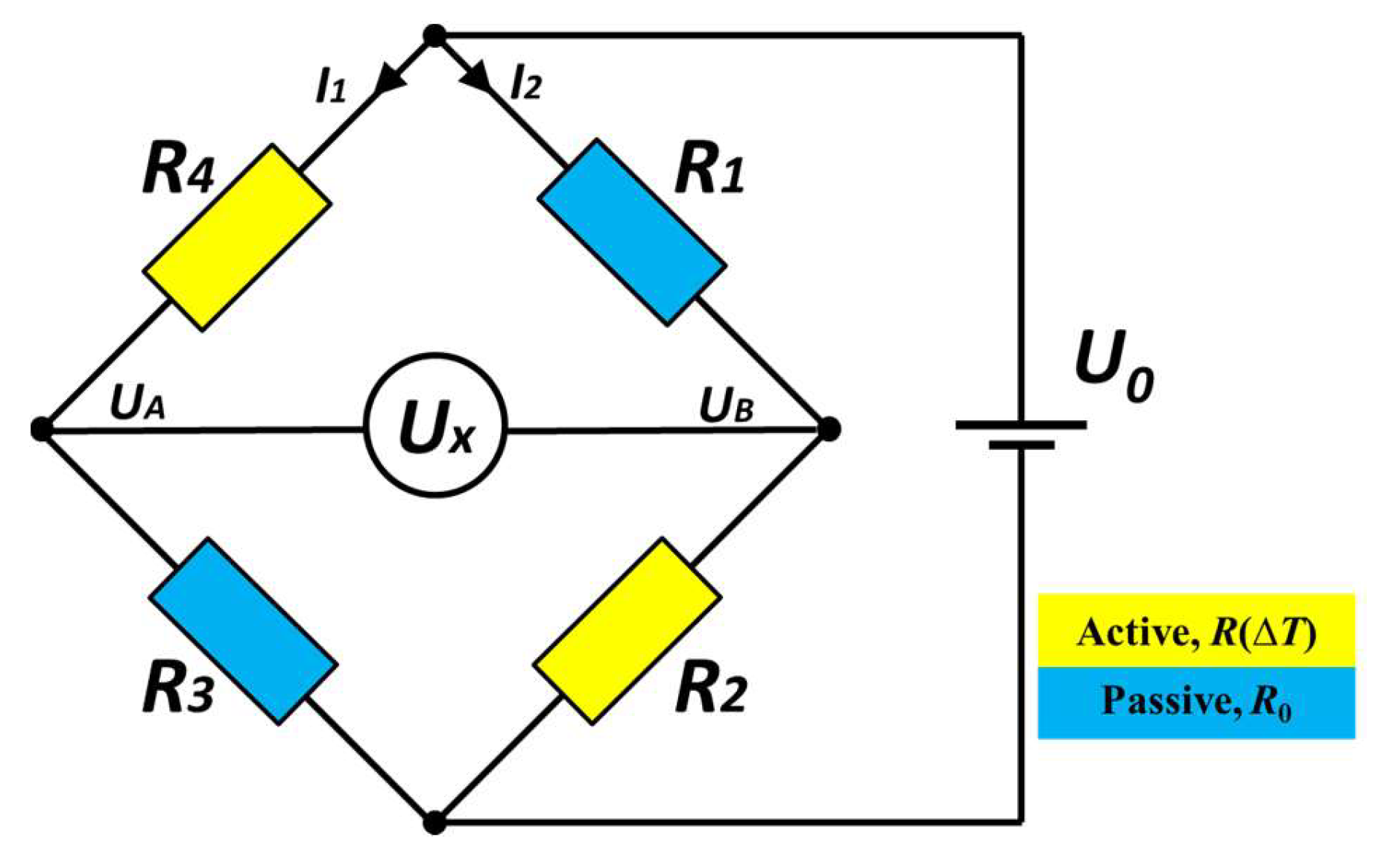
2. Sensor Concept
3. Materials and Methods
4. Results & Discussion
5. Conclusions and Outlook
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Halsey, M. SERVOTOUGH Flue Gas Exact (2700) Product Overview. Thick Film Catalytic Calorimeter. Servomex Product Information. 2016. Available online: http://servomex.net/servomex/web/web.nsf/en/thick-film-catalytic-calorimeter (accessed on 18 May 2018).
- Dabill, D.W.; Gentry, S.J.; Walsh, P.T. A fast-response catalytic sensor for flammable gases. Sens. Actuators 1987, 11, 135–143. [Google Scholar] [CrossRef]
- Park, N.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Calorimetric Thermoelectric Gas Sensor for the Detection of Hydrogen, Methane and Mixed Gases. Sensors 2014, 14, 8350–8362. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagina, E.; Tiggelaar, R.M.; Sanders, R.G.P.; Wolters, R.A.M.; Gardeniers, J.G.E. Low power micro-calorimetric sensors for analysis of gaseous samples. Sens. Actuators B Chem. 2015, 206, 772–787. [Google Scholar] [CrossRef]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-B.; Hwang, I.-S.; Cha, J.-H.; Lee, H.-J.; Lee, W.-B.; Pak, J.J.; Lee, J.-H.; Lu, B.-K. Micromachined catalytic combustible hydrogen gas sensor. Sens. Actuators B Chem. 2011, 153, 392–397. [Google Scholar] [CrossRef]
- Kirchner, P.; Oberländer, J.; Friedrich, P.; Berger, J.; Rysstad, G.; Kreusgen, M.; Schöning, M.J. Realisation of a calorimetric gas sensor on polyimide foil for applications in aseptic food industry. Sens. Actuators B Chem. 2012, 170, 60–66. [Google Scholar] [CrossRef]
- Wiegärtner, S.; Hagen, G.; Kita, J.; Reitmeier, W.; Hien, M.; Moos, R. Thermoelectric hydrocarbon sensor in thick-film technology for on-board diagnotics of a diesel oxidation catalyst. Sens. Actuators B Chem. 2015, 214, 234–240. [Google Scholar] [CrossRef]
- Kozlov, A.G. Optimization of structure and power supply conditions of catalytic gas sensor. Sens. Actuators B Chem. 2002, 82, 24–33. [Google Scholar] [CrossRef]
- Kita, J.; Engelbrecht, A.; Schubert, F.; Groß, A.; Rettig, F.; Moos, R. Some practical points to consider with respect to thermal conductivity and electrical resistivity of ceramic substrates for high-temperature gas sensors. Sens. Actuators B Chem. 2015, 213, 541–546. [Google Scholar] [CrossRef]
- Illyaskutty, N.; Knoblauch, J.; Schwotzer, M.; Kohler, H. Thermally modulated multi sensor arrays of SnO2/additive/electrode combinations for enhanced gas identification. Sens. Actuators B Chem. 2013, 217, 2–12. [Google Scholar] [CrossRef]
- Jerger, A.; Kohler, H.; Becker, F.; Keller, H.B.; Seifert, R. New applications of tin oxide gas sensors: II. Intelligent sensor system for reliable monitoring of ammonia leakages. Sens. Actuators B Chem. 2002, 81, 301–307. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illyaskutty, N.; Kansizoglu, O.; Akdag, O.; Ojha, B.; Knoblauch, J.; Kohler, H. Miniaturized Single Chip Arrangement of a Wheatstone Bridge Based Calorimetric Gas Sensor. Chemosensors 2018, 6, 22. https://doi.org/10.3390/chemosensors6020022
Illyaskutty N, Kansizoglu O, Akdag O, Ojha B, Knoblauch J, Kohler H. Miniaturized Single Chip Arrangement of a Wheatstone Bridge Based Calorimetric Gas Sensor. Chemosensors. 2018; 6(2):22. https://doi.org/10.3390/chemosensors6020022
Chicago/Turabian StyleIllyaskutty, Navas, Onur Kansizoglu, Oguzhan Akdag, Binayak Ojha, Jens Knoblauch, and Heinz Kohler. 2018. "Miniaturized Single Chip Arrangement of a Wheatstone Bridge Based Calorimetric Gas Sensor" Chemosensors 6, no. 2: 22. https://doi.org/10.3390/chemosensors6020022




