Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment
Abstract
:1. Introduction
2. Types of Impurities
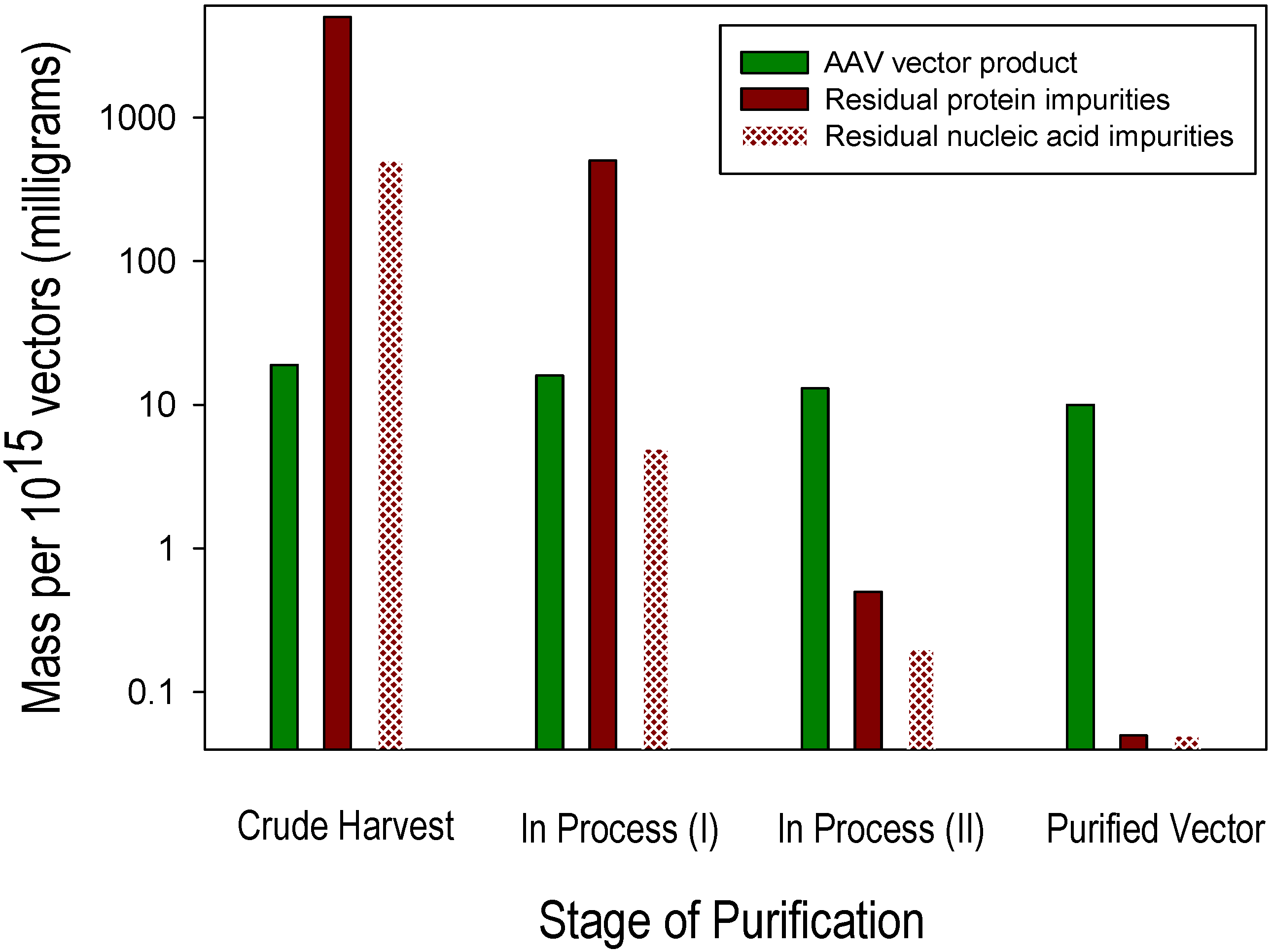
3. Process-Related Impurities in AAV Vectors
| Process-related impurity | Method of measurement | Potential toxicity |
|---|---|---|
| Residual host cell DNA/RNA (nuclease-sensitive) | qPCR using amplicons to generic host cell genome (e.g., 18SRNA gene) | Genotoxicity |
| qPCR using amplicons for sequences of specific concern (e.g., AdE1) | ||
| Residual host cell protein | ELISA using polyclonal antibodies detecting representative proteins [30] | Immunotoxicity |
| Residual plasmid DNA (nuclease-sensitive) | qPCR using amplicons for non-vector genome sequences | Genotoxicity |
| Residual helper viruses (nucleic acids and proteins) | qPCR using amplicons for helper virus sequences | Immunotoxicity, Genotoxicity, Infectious risk |
| Infectious titer of helper viruses; | ||
| ELISA or Western blotting for helper virus proteins | ||
| Residual cell culture medium components, antibiotics, supplements, inducers, etc. | Various, depending on component | Various |
| Residual purification buffers, chromatography media ligands, centrifugation media, detergents, enzymes, inorganic salts, etc. | Various, depending on component | Various |
4. Product-Related Impurities in AAV Vectors
| Product related impurity | Method for measurement | Potential toxicity |
|---|---|---|
| AAV empty capsids | Ultracentrifugation; electron microscopy; spectrophotometry [32]; ion exchange chromatography [33] | Immunotoxicity |
| Encapsidated host cell nucleic acids (nuclease-resistant) | qPCR using amplicons to generic host cell genome sequences | Genotoxicity, Immunotoxicity |
| qPCR using amplicons to specific sequences of concern (e.g., E1A) | ||
| Encapsidated helper component DNA (nuclease-resistant) | qPCR using amplicons for helper backbone sequences | Genotoxicity, Immunotoxicity |
| Replication-competent AAV | Ad-dependent amplification | Immunotoxicity |
| Noninfectious AAV particles | Ad-dependent infectivity in susceptible cells | Immunotoxicity |
| Other, including aggregated, degraded, and oxidized AAV vectors | Various, including size exclusion chromatography; dynamic light scattering; electrophoresis, etc. | Immunotoxicity |
4.1. AAV Empty Capsids
4.1.1. Description
4.1.2. Risk Assessment
4.1.3. Process Optimization
4.2. Residual Host Cell Nucleic Acids Packaged within AAV Capsids
4.2.1. Description
4.2.2. Risk Assessment
4.2.3. Process Optimization
4.3. Residual Helper DNA Sequences Packaged in AAV Capsids
4.3.1. Description
4.3.2. Risk Assessment
4.3.3. Process Optimization
4.4. Replication-Competent AAV Species
4.4.1. Description
4.4.2. Risk Assessment
4.4.3. Process Optimization
4.5. Non-Infectious AAV Vector Particles
4.5.1. Description
4.5.2. Risk Assessment
4.5.3. Process Optimization
4.6. Aggregated, Degraded, Oxidized AAV Vectors
5. Conclusions
Conflicts of Interest
References
- Carter, B.J. Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 2005, 16, 541–550. [Google Scholar] [CrossRef]
- Warrington, K.H.; Herzog, R.W. Treatment of human disease by adeno-associated viral gene transfer. Hum. Genet. 2006, 119, 571–603. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat. Rev. Genet. 2011, 12, 341–355. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605. [Google Scholar] [CrossRef]
- Simonelli, F.; Maguire, A.M.; Testa, F.; Pierce, E.A.; Mingozzi, F.; Bennicelli, J.L.; Rossi, S.; Marshall, K.; Banfi, S.; Surace, E.M.; et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2010, 18, 643–650. [Google Scholar] [CrossRef]
- Bennett, J.; Ashtari, M.; Wellman, J.; Marshall, K.A.; Cyckowski, L.L.; Chung, D.C.; McCague, S.; Pierce, E.A.; Chen, Y.; Bennicelli, J.L.; et al. AAV2 gene therapy re-administration in three adults with congenital blindness. Sci. Transl. Med. 2012, 4, 120ra15. [Google Scholar]
- Testa, F.; Maguire, A.M.; Rossi, S.; Pierce, E.A.; Melillo, P.; Marshall, K.; Banfi, S.; Surace, E.M.; Sun, J.; Acerra, C.; et al. Three year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis Type 2. Ophthalmology 2013, 120, 1283–1291. [Google Scholar]
- Nathwani, A.C.; Tuddenham, E.G.D.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Jaquilmac Pie, A.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- Xie, Q.; Bu, W.; Bhatia, S.; Hare, J.; Somasundarm, T.; Azzi, A.; Chapman, M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 6, 10405–10410. [Google Scholar]
- European Medicines Agency. Assessment Report: Glybera. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002145/WC500135476.pdf (accessed on 25 February 2014).
- ICH harmonised tripartite guideline. Specification: Test procedures and acceptance criteria for biotechnological/biological product. Q6B. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002824.pdf (accessed on 25 February 2014).
- US Department of Health Human Services, Food and Drug Administration. International conference on harmonisation; Guidance on viral safety evaluation of biotechnology products derived from cell lines of human or animal origin; availablility—FDA. Notice. Fed. Regist. 1998, 63, 51074–51084. [Google Scholar]
- PDA. Points to consider for aseptic processing. PDA J. Pharm. Sci. Technol. 2003, 57, 1–72. [Google Scholar]
- US Department of Health Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Office of Regulatory Affairs. Guidance for Industry: Sterile drug products produced by aseptic processing—Current good manufacturing practice. Available online: http://www.fda.gov/cber/guidelines.htm (accessed on 25 February 2014).
- US Dept Health Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for Industry: INDs—Approaches to complying with CGMP during Phase 1. Available online: http://www.fda.gov/ohrms/dockets/98fr/05d-0286-gdl0001.pdf (accessed on 25 February 2014).
- Vaccines and related biological product advisory committee meeting. FDA Briefing Document. Cell lines derived from human tumors for vaccine manufacture. Available online: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm319554.htm (accessed on 25 February 2014).
- Gombold, J.; Peden, K.; Gavin, D.; Wei, Z.; Baradaran, K.; Mire-Sluis, A.; Schenerman, M. Lot release and characterization testing of live-virus-based vaccines and gene therapy products, Part 1: Factors influencing assay choices. BioProcess Int. 2006, 4, 46–56. [Google Scholar]
- Clark, K.R.; Voulgaropoulou, F.; Fraley, D.M.; Johnson, P.R. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995, 6, 1329–1341. [Google Scholar] [CrossRef]
- Conway, J.E.; Rhys, C.M.J.; Zolotukhin, I.; Zolotukhin, S.; Muzyczka, N.; Hayward, G.S.; Byrne, B.J. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type 1 vector expressing AAV-2 Rep and Cap. Gene Ther. 1999, 6, 986–993. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Samulski, R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998, 72, 2224–2232. [Google Scholar]
- Matsushita, T.; Elliger, S.; Elliger, C.; Podsakoff, G.; Villarreal, L.; Kurtzman, G.J.; Iwaki, Y.; Colosi, P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998, 5, 938–945. [Google Scholar]
- Urabe, M.; Ding, C.; Kotin, R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002, 13, 1935–1943. [Google Scholar] [CrossRef]
- Smith, R.H.; Ding, C.; Kotin, R.M. Serum-free production and column purification of adeno-asssociated virus type 5. J. Virol. Meth. 2003, 114, 115–124. [Google Scholar] [CrossRef]
- Negrete, A.; Yang, L.C.; Mendez, A.F.; Levy, J.R.; Kotin, R.M. Economized large-scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. J. Gene Med. 2007, 9, 938–948. [Google Scholar] [CrossRef]
- Belter, P.A.; Cussler, C.L.; Hu, W.S. Bioseparations; John Wiley and Sons: New York, NY, USA, 1988. [Google Scholar]
- Sadana, A. Bioseparation of Proteins; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Wang, X.; Hunter, A.K.; Mozier, N.M. Host cell proteins in biologics development: Identification, quantitation, and risk assessment. Biotechnol. Bioeng. 2009, 103, 446–458. [Google Scholar] [CrossRef]
- Kapranov, P.; Chen, L.; Dederich, D.; Dong, B.; He, J.; Steinmann, K.E.; Moore, A.R.; Thompson, J.F.; Milos, P.M.; Xiao, W. Native molecular state of adeno-associated viral vectors revealed by singe-molecule sequencing. Hum. Gene Ther. 2012, 23, 46–56. [Google Scholar] [CrossRef]
- Sommer, J.M.; Smith, P.H.; Parthasarathy, S.; Isaacs, J.; Vijay, S.; Kieran, J.; Powell, S.K.; McClelland, A.; Wright, J.F. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003, 7, 122–128. [Google Scholar] [CrossRef]
- Lock, M.; Alvira, M.R.; Wilson, J.M. Analysis of particle content of recombinant adeno-associated virus serotype 8 vectors by ion-exchange chromatography. Hum. Gene Ther. Meth. 2012, 23, 56–64. [Google Scholar] [CrossRef]
- Allay, J.M.; Sleep, S.; Long, S.; Tillman, D.M.; Clark, R.; Carney, G.; Fagone, P.; McIntosh, J.H.; Nienhuis, A.W.; Davidoff, A.M.; et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a haemophilia B clinical trial. Hum. Gene Ther. 2011, 22, 595–604. [Google Scholar] [CrossRef]
- Manno, C.S.; Pierce, G.F.; Arruda, V.R.; Glader, B.; Ragni, M.; Rasko, J.J.; Ozelo, M.C.; Hoots, K.; Blatt, P.; Konkle, B.; et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006, 12, 342–347. [Google Scholar] [CrossRef]
- Mingozzi, F.; Maus, M.V.; Hui, D.J.; Sabatino, D.E.; Murphy, S.L.; Rasko, J.E.; Ragni, M.V.; Manno, C.S.; Sommer, J.; Jiang, H.; et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007, 13, 419–422. [Google Scholar] [CrossRef]
- Pien, G.C.; Hasbrouck, N.C.; Maus, M.V.; Mingozzi, F.; High, K.A. Quantifying capsid peptide:MHC I complexes following adeno-associated virus (AAV) transduction. Blood 2007, 110, 1090A. [Google Scholar] [CrossRef]
- Parker, A.; Nagy, D.; Vargas, J.; Anand, V.; Qu, G.; Wright, J.F.; Couto, L. In vivo performance of AAV2 vectors purified by CsCl gradient centrifugation or column chromatography. Mol. Ther. 2003, 7, S390. [Google Scholar]
- Mingozzi, F.; Anguela, X.M.; Pavani, G.; Chen, Y.; Davidson, R.J.; Hui, D.J.; Yazicioglu, M.; Elkouby, L.; Hinderer, C.J.; Faella, A.; et al. Overcoming pre-existing humoral immunity to AAV using capsid decoys. Science Trans. Med. 2013, 5, 194ra92. [Google Scholar]
- Ayuso, E.; Mingozzi, F.; Montane, J.; Leon, X.; Anguela, X.M.; Haurigot, V.; Edmonson, S.A.; Africa, L.; Zhou, S.; High, K.A.; et al. High AAV vector purity results in serotype- and tissue- independent enhancement of transduction efficiency. Gene Ther. 2010, 17, 503–510. [Google Scholar] [CrossRef]
- Wright, J.F.; Wellman, J.; High, K.A. Manufacturing and regulatory strategies for clinical AAV2-hRPE65. Curr. Gene Ther. 2011, 12, 341–349. [Google Scholar]
- Zolotukhin, S.; Byrne, B.J.; Mason, E.; Zolotukhin, E.; Potter, M.; Chesnut, K.; Summerford, C.; Samulski, R.J.; Muzyczka, N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999, 6, 973–985. [Google Scholar] [CrossRef]
- Qu, G.; Bahr-Davidson, J.; Prado, J.; Tai, A.; Cataniag, F.; McDonnell, J.; Zhou, J.; Hauck, B.; Luna, J.; Sommer, J.M.; et al. Separation of adeno-associated virus type 2 empty particles from genome containing vector by anion-exchange column chromatography. J. Virol. Meth. 2007, 140, 183–192. [Google Scholar] [CrossRef]
- Brument, N.; Morenweiser, R.; Blouin, V.; Toublance, E.; Raimbaud, I.; Chérel, Y.; Folliot, S.; Gaden, F.; Boulanger, P.; Kroner-Lux, G.; et al. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol. Ther. 2002, 6, 678–686. [Google Scholar] [CrossRef]
- Hauck, B.; Murphy, S.L.; Smith, P.H.; Liu, X.; Zelenaia, O.; Mingozzi, F.; Sommer, J.M.; High, K.A.; Wright, J.F. Undetectable transcription of cap in a clinical AAV vector: implications for pre-formed capsids in immune responses. Mol. Ther. 2009, 17, 144–152. [Google Scholar] [CrossRef]
- Wistuba, A.; Kern, A.; Weger, S.; Grimm, D.; Kleinschmidt, J.A. Subcellular compartmentalization of adeno-associated virus type 2 assembly. J. Virol. 1997, 71, 1341–1352. [Google Scholar]
- Sonntag, F.; Schmidt, K.; Kleinschmidt, J.A. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. USA 2010, 107, 10220–10225. [Google Scholar] [CrossRef]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.G., 2nd; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA Vaccines: Investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.H.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Sheng, L.; Cai, F.; Zhu, Y.; Pal, A.; Athanasiou, M.; Orrison, B.; Blair, D.G.; Hughes, S.H.; Coffin, J.M.; Lewis, A.M.; et al. Oncogenicity of DNA in vivo: Tumor induction with expression plasmids for activated H-ras and c-myc. Biologicals 2008, 36, 184–197. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Requirements for the Use of Animal Cells as in Vitro Substrates for the Production of Biologicals (Requirement for Biological Substance No. 50); WHO Technical Report Series, No. 878; WHO Expert Committee on Biological Standardization: Geneva, Swizerland, 1998; Annex 1. [Google Scholar]
- European Medicines Agency. Reflection paper on quality, non-clinical and clinical issues related to the development of recombinant adeno-associated viral vectors. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/07/WC500094345.pdf (accessed on 25 February 2014).
- Chadeuf, G.; Ciron, C.; Moullier, P.; Salvetti, A. Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol. Ther. 2005, 12, 744–753. [Google Scholar] [CrossRef]
- Dolgin, E. Gene therapies advance, but some see manufacturing challenges. Nat. Med. 2012, 18, 1718–1719. [Google Scholar] [CrossRef]
- Samulski, R.J.; Shang, L.-S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 61, 3096–3101. [Google Scholar]
- Allen, J.M.; Debelak, D.J.; Reynolds, T.C.; Miller, A.D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by non-homologous recombination during AAV vector production. J. Virol. 1997, 71, 6816–6822. [Google Scholar]
- Clark, K.R.; Liu, X.; McGrath, J.P.; Johnson, P.R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type virus. Hum. Gene Ther. 1999, 10, 1031–1039. [Google Scholar] [CrossRef]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) Types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Maecker, H.T.; Ghanekar, S.A.; Suni, M.A.; He, X.S.; Picker, L.J.; Maino, V.C. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J. Immunol. 2001, 166, 7268–7275. [Google Scholar]
- Grimm, D.; Kern, A.; Rittner, K.; Kleinschmidt, J.A. Novel tools for production and purification of recombinant adeno associated virus vectors. Hum. Gene Ther. 1998, 9, 2745–2760. [Google Scholar] [CrossRef]
- Lu, H.; Qu, G.; Yang, X.; Xu, R.; Xiao, W. Systemic elimination of de novo capsid protein synthesis from replication-competent AAV contamination in the liver. Hum. Gene Ther. 2011, 22, 625–632. [Google Scholar] [CrossRef]
- Dong, B.; Moore, A.R.; Dai, J.; Roberts, S.; Chu, K.; Kapranov, P.; Moss, B.; Xiao, W. A concept of eliminating nonhomologous recombinant for scalabe and safet AAV vector generation for human gene therapy. Nucleic Acids Res. 2013, 41, 6609–6617. [Google Scholar] [CrossRef]
- Salvetti, A.; Oreve, S.; Chadeuf, G.; Favre, D.; Cherel, Y.; Champion-Arnaud, P.; David-Ameline, J.; Moullier, P. Factors influencing recombinant adeno-associated virus production. Hum. Gene Ther. 1998, 9, 695–706. [Google Scholar] [CrossRef]
- Zhen, Z.; Espinoza, Y.; Bleu, T.; Sommer, J.M.; Wright, J.F. Infectious titer assay for adeno-associated virus vectors with sensitivity sufficient to detect single infectious events. Hum. Gene Ther. 2004, 15, 709–715. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Loiler, S.; Zolotukhin, I.; Byrne, B.J.; Flotte, T.R.; Snyder, R.O. Herpesvirus-based infectious titering of recombinant adeno-associated viral vectors. Mol. Ther. 2005, 11, 320–326. [Google Scholar]
© 2014 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wright, J.F. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines 2014, 2, 80-97. https://doi.org/10.3390/biomedicines2010080
Wright JF. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines. 2014; 2(1):80-97. https://doi.org/10.3390/biomedicines2010080
Chicago/Turabian StyleWright, J. Fraser. 2014. "Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment" Biomedicines 2, no. 1: 80-97. https://doi.org/10.3390/biomedicines2010080




