Targeting Autophagy for Oncolytic Immunotherapy
Abstract
:1. Introduction
2. Autophagy, Immunity, and Cancer
2.1. Autophagy Machinery
2.2. Autophagy and Cancer
2.3. Autophagy, Immunity, and Cancer Immunotherapy
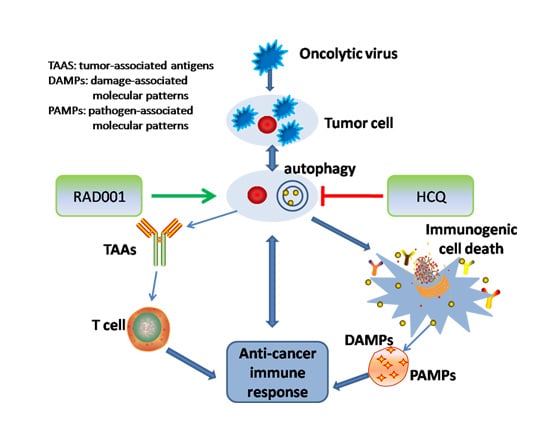
3. Oncolytic Viruses (OV)-Modulated Autophagy in Oncolytic Immunotherapy
4. Combination Strategies Using Oncolytic Virus and Chemical Agents for Stimulating and Inhibiting Autophagy
5. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tesniere, A.; Apetoh, L.; Ghiringhelli, F.; Joza, N.; Panaretakis, T.; Kepp, O.; Schlemmer, F.; Zitvogel, L.; Kroemer, G. Immunogenic cancer cell death: A key-lock paradigm. Curr. Opin. Immunol. 2008, 20, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.; Todryk, S.; Hardwick, N.; Ford, M.; Jacobson, M.; Vile, R.G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998, 4, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Hogan, B.L. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu. Rev. Cell. Dev. Biol. 2011, 27, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.; Nolte, M.A.; Sporri, R.; Reis e Sousa, C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009, 227, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Aurelian, L. Oncolytic viruses as immunotherapy: Progress and remaining challenges. Onco Targets Ther. 2016, 9, 2627–2637. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic immunotherapy: Dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.L.; Samir, P.; Howard, L.M.; Niu, X.; Prasad, N.; Galassie, A.; Liu, Q.; Allos, T.M.; Floyd, K.A.; Guo, Y.; et al. A cell-based systems biology assessment of human blood to monitor immune responses after influenza vaccination. PLoS ONE 2015, 10, e0118528. [Google Scholar] [CrossRef] [PubMed]
- Tsun, A.; Miao, X.N.; Wang, C.M.; Yu, D.C. Oncolytic immunotherapy for treatment of cancer. Adv. Exp. Med. Biol. 2016, 909, 241–283. [Google Scholar]
- Allan, K.J.; Stojdl, D.F.; Swift, S.L. High-throughput screening to enhance oncolytic virus immunotherapy. Oncolytic Virother. 2016, 5, 15–25. [Google Scholar]
- Schmid, D.; Dengjel, J.; Schoor, O.; Stevanovic, S.; Munz, C. Autophagy in innate and adaptive immunity against intracellular pathogens. J. Mol. Med. 2006, 84, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, R., Jr.; Levine, B. Selective autophagy and viruses. Autophagy 2011, 7, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Levine, B. Autophagy and viruses: Adversaries or allies? J. Innate Immun. 2013, 5, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Beljanski, V.; Chiang, C.; Hiscott, J. The intersection between viral oncolysis, drug resistance, and autophagy. Biol. Chem. 2015, 396, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, G.; Hattingh, S.M.; Engelbrecht, A.M. Enhanced therapeutic efficacy in cancer patients by short-term fasting: The autophagy connection. Front. Oncol. 2016, 6, 242. [Google Scholar] [CrossRef] [PubMed]
- Dengjel, J.; Schoor, O.; Fischer, R.; Reich, M.; Kraus, M.; Muller, M.; Kreymborg, K.; Altenberend, F.; Brandenburg, J.; Kalbacher, H.; et al. Autophagy promotes mhc class ii presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 7922–7927. [Google Scholar] [CrossRef] [PubMed]
- Gauvrit, A.; Brandler, S.; Sapede-Peroz, C.; Boisgerault, N.; Tangy, F.; Gregoire, M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008, 68, 4882–4892. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Breckenridge, C.A.; Choi, B.D.; Suryadevara, C.M.; Chiocca, E.A. Potentiating oncolytic viral therapy through an understanding of the initial immune responses to oncolytic viral infection. Curr. Opin. Virol. 2015, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, W.; Gu, L.B.; Tu, Y.; Liu, H. molecular mechanism of emodin on inhibiting autophagy induced by HBSS in renal tubular cells. Zhongguo Zhong Yao Za Zhi 2015, 40, 1965–1970. (In Chinese) [Google Scholar] [PubMed]
- Coffin, R.S. From virotherapy to oncolytic immunotherapy: Where are we now? Curr. Opin. Virol. 2015, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, J.F.; Achard, C.; Zaupa, C.; Foloppe, J.; Erbs, P. Oncolytic immunotherapy: The new clinical outbreak. Oncoimmunology 2016, 5, e1066961. [Google Scholar] [CrossRef] [PubMed]
- Workenhe, S.T.; Verschoor, M.L.; Mossman, K.L. The role of oncolytic virus immunotherapies to subvert cancer immune evasion. Future Oncol. 2015, 11, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E.; Chiocca, E.A. Oncolytic virus-mediated immunotherapy: A combinatorial approach for cancer treatment. J. Clin. Oncol. 2015, 33, 2812–2814. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, T.D.; Janssen, A.B.; van Beusechem, V.W. Arming oncolytic viruses to leverage antitumor immunity. Expert Opin. Biol. Ther. 2015, 15, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Hemminki, A. A century of oncolysis evolves into oncolytic immunotherapy. Oncoimmunology 2016, 5, e1074377. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Klionsky, D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007, 27, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klionsky, D.J. The regulation of autophagy—Unanswered questions. J. Cell Sci. 2011, 124, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.; Martens, S. Mechanisms and regulation of autophagosome formation. Curr. Opin. Cell Biol. 2012, 24, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, M.; di Rienzo, M.; Piacentini, M.; Fimia, G.M. Emerging mechanisms in initiating and terminating autophagy. Trends Biochem. Sci. 2016, 42, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Bauvy, C.; Tonelli, G.; Yue, W.; Delomenie, C.; Nicolas, V.; Zhu, Y.; Domergue, V.; Marin-Esteban, V.; Tharinger, H.; et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 2013, 32, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [PubMed]
- Saito, H.; Inazawa, J.; Saito, S.; Kasumi, F.; Koi, S.; Sagae, S.; Kudo, R.; Saito, J.; Noda, K.; Nakamura, Y. Detailed deletion mapping of chromosome 17q in ovarian and breast cancers: 2-cm region on 17q21.3 often and commonly deleted in tumors. Cancer Res. 1993, 53, 3382–3385. [Google Scholar] [PubMed]
- Amaravadi, R.K.; Lippincott-Schwartz, J.; Yin, X.M.; Weiss, W.A.; Takebe, N.; Timmer, W.; DiPaola, R.S.; Lotze, M.T.; White, E. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011, 17, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. The challenge of developing autophagy inhibition as a therapeutic strategy. Cancer Res. 2016, 76, 5610–5614. [Google Scholar] [CrossRef] [PubMed]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, B.; Eng, C.H. Revisiting autophagy addiction of tumor cells. Autophagy 2016, 12, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Crotzer, V.L.; Blum, J.S. Autophagy and adaptive immunity. Immunology 2010, 131, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, inflammation, and immunity: A troika governing cancer and its treatment. Cell 2016, 166, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy as an innate immunity paradigm: Expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 2012, 24, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, L.; Xu, Y.; Han, W.; Lou, F.; Fei, W.; Liu, S.; Jing, Z.; Sui, X. Autophagy-associated immune responses and cancer immunotherapy. Oncotarget 2016, 7, 21235–21246. [Google Scholar] [PubMed]
- Zhong, Z.; Sanchez-Lopez, E.; Karin, M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin. Exp. Rheumatol. 2016, 34, 12–16. [Google Scholar] [PubMed]
- Rey-Jurado, E.; Riedel, C.A.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. Contribution of autophagy to antiviral immunity. FEBS Lett. 2015, 589, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Z.; Simon, H.U. Targeting autophagy as a potential therapeutic approach for melanoma therapy. Semin. Cancer Biol. 2013, 23, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Gomes-de-Silva, L.C.; Dewitte, H.; Breckpot, K.; Fucikova, J.; Spisek, R.; Galluzzi, L.; Kepp, O.; Kroemer, G. Combinatorial strategies for the induction of immunogenic cell death. Front. Immunol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Aoki, H.; Kuhnel, F.; Kondo, Y.; Kubicka, S.; Wirth, T.; Iwado, E.; Iwamaru, A.; Fujiwara, K.; Hess, K.R.; et al. Autophagic cell death of malignant glioma cells induced by a conditionally replicating adenovirus. J. Natl. Cancer Inst. 2006, 98, 625–636. [Google Scholar] [CrossRef]
- Meng, S.; Xu, J.; Wu, Y.; Ding, C. Targeting autophagy to enhance oncolytic virus-based cancer therapy. Expert Opin. Biol. Ther. 2013, 13, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Aurelian, L.; Bollino, D.; Colunga, A. The oncolytic virus ΔPK has multimodal anti-tumor activity. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- Bollino, D.; Colunga, A.; Li, B.; Aurelian, L. Deltapk oncolytic activity includes modulation of the tumour cell milieu. J. Gen. Virol. 2016, 97, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Jiang, H.; Hossain, M.B.; Fan, X.; Gumin, J.; Dong, A.; Alonso, M.M.; Gomez-Manzano, C.; Fueyo, J. Critical role of autophagy in the processing of adenovirus capsid-incorporated cancer-specific antigens. PLoS ONE 2016, 11, e0153814. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Sakai, R.; Ouchi, M.; Onimatsu, H.; Hioki, M.; Kagawa, S.; Uno, F.; Watanabe, Y.; Urata, Y.; Tanaka, N.; et al. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene 2008, 27, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Koks, C.A.; Garg, A.D.; Ehrhardt, M.; Riva, M.; Vandenberk, L.; Boon, L.; De Vleeschouwer, S.; Agostinis, P.; Graf, N.; van Gool, S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer 2015, 136, E313–E325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Li, Y.; Zhu, Q.; Xu, J.; Wang, Y.; Deng, W.; Liu, Q.; Zhang, G.; Meng, S. Pharmacological modulation of autophagy enhances newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cells. BMC Cancer 2014, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Gonzalez, P.; Li, C.; Meng, G.; Jiang, A.; Wang, H.; Gao, Q.; Debatin, K.M.; Beltinger, C.; Wei, J. Mitophagy enhances oncolytic measles virus replication by mitigating DDX58/RIG-I-like receptor signaling. J. Virol. 2014, 88, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Leib, D.A.; Alexander, D.E.; Cox, D.; Yin, J.; Ferguson, T.A. Interaction of icp34.5 with beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J. Virol. 2009, 83, 12164–12171. [Google Scholar] [CrossRef] [PubMed]
- Beug, S.T.; Tang, V.A.; LaCasse, E.C.; Cheung, H.H.; Beauregard, C.E.; Brun, J.; Nuyens, J.P.; Earl, N.; St-Jean, M.; Holbrook, J.; et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat. Biotechnol. 2014, 32, 182–190. [Google Scholar] [PubMed]
- Kanai, R.; Wakimoto, H.; Martuza, R.L.; Rabkin, S.D. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin. Cancer Res. 2011, 17, 3686–3696. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Liu, H.; Deng, Y.; Xie, Y.; Shen, G. Effects of high mobility group protein box 1 and toll like receptor 4 pathway on warts caused by human papillomavirus. Mol. Med. Rep. 2014, 10, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Ellerhoff, T.P.; Berchtold, S.; Venturelli, S.; Burkard, M.; Smirnow, I.; Wulff, T.; Lauer, U.M. Novel EPI-virotherapeutic treatment of pancreatic cancer combining the oral histone deacetylase inhibitor resminostat with oncolytic measles vaccine virus. Int. J. Oncol. 2016, 49, 1931–1944. [Google Scholar] [CrossRef]
- Shulak, L.; Beljanski, V.; Chiang, C.; Dutta, S.M.; van Grevenynghe, J.; Belgnaoui, S.M.; Nguyen, T.L.; di Lenardo, T.; Semmes, O.J.; Lin, R.; et al. Histone deacetylase inhibitors potentiate vesicular stomatitis virus oncolysis in prostate cancer cells by modulating NF-κB-dependent autophagy. J. Virol. 2014, 88, 2927–2940. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, Y.; Zhao, X.; Lv, C.; Yan, J. Oncolytic vaccinia virus inhibits human hepatocellular carcinoma MHCC97-H cell proliferation via endoplasmic reticulum stress, autophagy and wnt pathways. J. Gene Med. 2016, 18, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fang, X.; Xu, Y.; Ni, A.; Liu, X.Y.; Chu, L. An oncolytic adenovirus that expresses the HAB18 and interleukin 24 genes exhibits enhanced antitumor activity in hepatocellular carcinoma cells. Oncotarget 2016, 7, 60491–60502. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef]
- Workenhe, S.T.; Mossman, K.L. Oncolytic virotherapy and immunogenic cancer cell death: Sharpening the sword for improved cancer treatment strategies. Mol. Ther. 2014, 22, 251–256. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Iwado, E.; Kondo, Y.; Aoki, H.; Hayashi, Y.; Georgescu, M.M.; Sawaya, R.; Hess, K.R.; Mills, G.B.; Kawamura, H.; et al. Autophagy-inducing agents augment the antitumor effect of telerase-selve oncolytic adenovirus OBP-405 on glioblastoma cells. Gene Ther. 2008, 15, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.M.; Jiang, H.; Yokoyama, T.; Xu, J.; Bekele, N.B.; Lang, F.F.; Kondo, S.; Gomez-Manzano, C.; Fueyo, J. Δ-24-RGD in combination with rad001 induces enhanced anti-glioma effect via autophagic cell death. Mol. Ther. 2008, 16, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tao, L.; Rivera, A.; Zhang, X. Rapamycin enhances the activity of oncolytic herpes simplex virus against tumor cells that are resistant to virus replication. Int. J. Cancer 2011, 129, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.Q.; Jang, J.H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 2009, 15, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Lambert, L.; Herrod, J.; Conteh, S.; Orr-Gonzalez, S.; Carter, D.; Duffy, P.E. Chloroquine neither eliminates liver stage parasites nor delays their development in a murine chemoprophylaxis vaccination model. Front. Microbiol. 2015, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.K.; Aerts, J.L.; Eddaoudi, A.; Lockley, M.; Lemoine, N.R.; McNeish, I.A. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene 2008, 27, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Colunga, A.G.; Laing, J.M.; Aurelian, L. The HSV-2 mutant ΔpK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 2010, 17, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Botta, G.; Passaro, C.; Libertini, S.; Abagnale, A.; Barbato, S.; Maione, A.S.; Hallden, G.; Beguinot, F.; Formisano, P.; Portella, G. Inhibition of autophagy enhances the effects of E1A-defective oncolytic adenovirus dl922–947 against glioma cells in vitro and in vivo. Hum. Gene Ther. 2012, 23, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Alkassar, M.; Gartner, B.; Roemer, K.; Graesser, F.; Rommelaere, J.; Kaestner, L.; Haeckel, I.; Graf, N. The combined effects of oncolytic reovirus plus newcastle disease virus and reovirus plus parvovirus on U87 and U373 cells in vitro and in vivo. J. Neurooncol. 2011, 104, 715–727. [Google Scholar] [CrossRef] [PubMed]
| Oncolytic Virus (OV) | Impact of OV on Autophagy | Impact of Autophagy on Oncolytic Immunotherapy | Key References |
|---|---|---|---|
| Herpes simplex virus HSV-2 (ΔPK) | Modulates the tumor microenvironment through autophagy-dependent pathways. | Decreased tumor cell secretion of the type 2 immunosuppressive and pro-cancerous cytokines, IL-10, and IL-18 and concomitant increased secretion of the pro-inflammatory cytokines TNF-α, GM-CSF, IL-6, and IL-1β. Upregulates the NKG2D ligand, MICA, expressed by cytotoxic NK and T cells, and downregulates the negative immune checkpoint regulator cytotoxic T-lymphocyte antigen-4 (CTLA-4) | [53] |
| Herpes simplex virus type 2 (ΔPK) | Induces autophagy by upregulating inflammatory cytokines through autophagy-dependent activation of TLR-2 signaling. | Upregulates the secretion of inflammatory cytokines TNF-α, granulocyte macrophage colony-stimulating factor and IL-1β through autophagy-mediated activation of Toll-like receptor 2 pathways | [54] |
| Ad(Δ24FvIII) | Induces autophagy through JNK activation. | Autophagy inducers may enhance the processing and presentation of cancer-specific antigens | [55] |
| Ad (5/3-D24-GMCSF) | Increases tumor cell autophagy. | Releases HMGB1 into serum in patients and elicits anti-tumor immune responses. | [56] |
| Ad (OBP-301) | Induces autophagy-associated cell death. | Produces the endogenous danger signaling molecule, uric acid which stimulates DCs to produce interferon-γ (IFN-γ) and interleukin 12 (IL-12). | [57] |
| Newcastle disease virus (NDV) | Induces autophagy in ICD. | Primes adaptive anti-tumor immunity. | [58] |
| NDV/FMW | Pharmacological modulation of autophagy; enhances the oncolytic effects of NDV/FMW. | Releases HMGB1. | [59] |
| Measles virus (MV-Edm) | Triggers SQSTM1/p62-mediated mitophagy. | Mitigates DDX58/RIG-I-like receptor signaling and the innate immune response. | [60] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Jiang, K.; Ding, C.; Meng, S. Targeting Autophagy for Oncolytic Immunotherapy. Biomedicines 2017, 5, 5. https://doi.org/10.3390/biomedicines5010005
Hu L, Jiang K, Ding C, Meng S. Targeting Autophagy for Oncolytic Immunotherapy. Biomedicines. 2017; 5(1):5. https://doi.org/10.3390/biomedicines5010005
Chicago/Turabian StyleHu, Lulu, Ke Jiang, Chan Ding, and Songshu Meng. 2017. "Targeting Autophagy for Oncolytic Immunotherapy" Biomedicines 5, no. 1: 5. https://doi.org/10.3390/biomedicines5010005