Novel Computerized Method for Measurement of Retinal Vessel Diameters
Abstract
:1. Introduction
2. Material and Methods
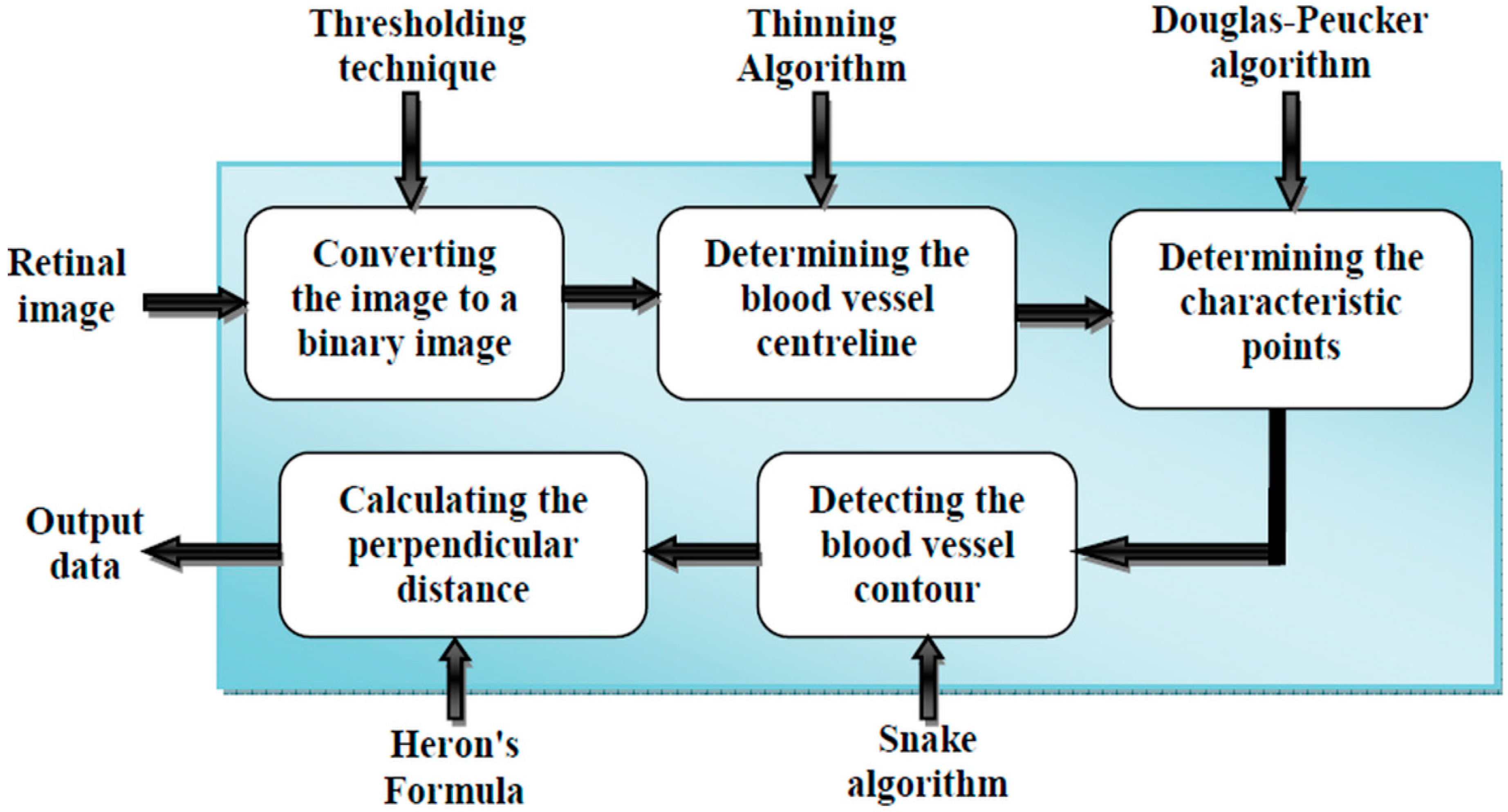
2.1. Proposed Method
- (i)
- First, we will give the image source and introduce its features.
- (ii)
- Then, we will present pre-processing of the 2D image.
- (iii)
- Subsequently, the determination of the control point using the Douglas-Peucker algorithm will be explained.
- (iv)
- Then, we will detect the blood vessel contour using the active contour technique.
- (v)
- Finally, the Heron’s formula will be presented to determine the blood vessels diameter.
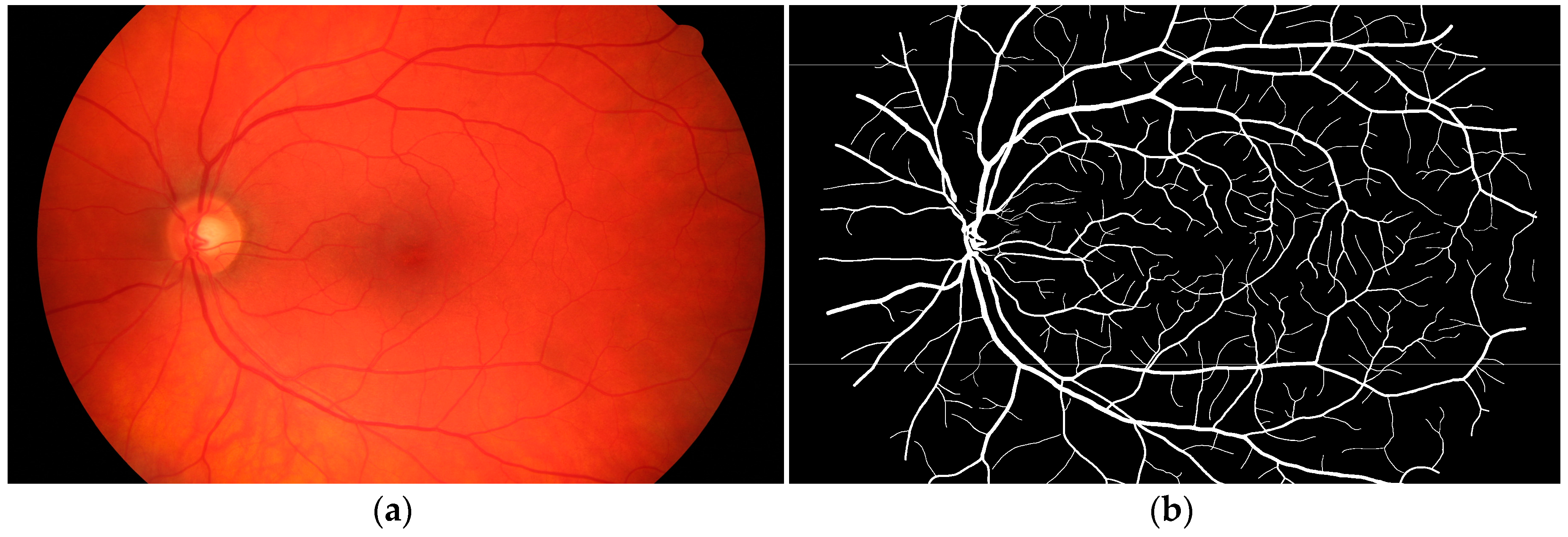
2.2. Retinal Image Segmentation
2.2.1. Image Source
STARE Database
High-Resolution Fundus (HRF) Image Database
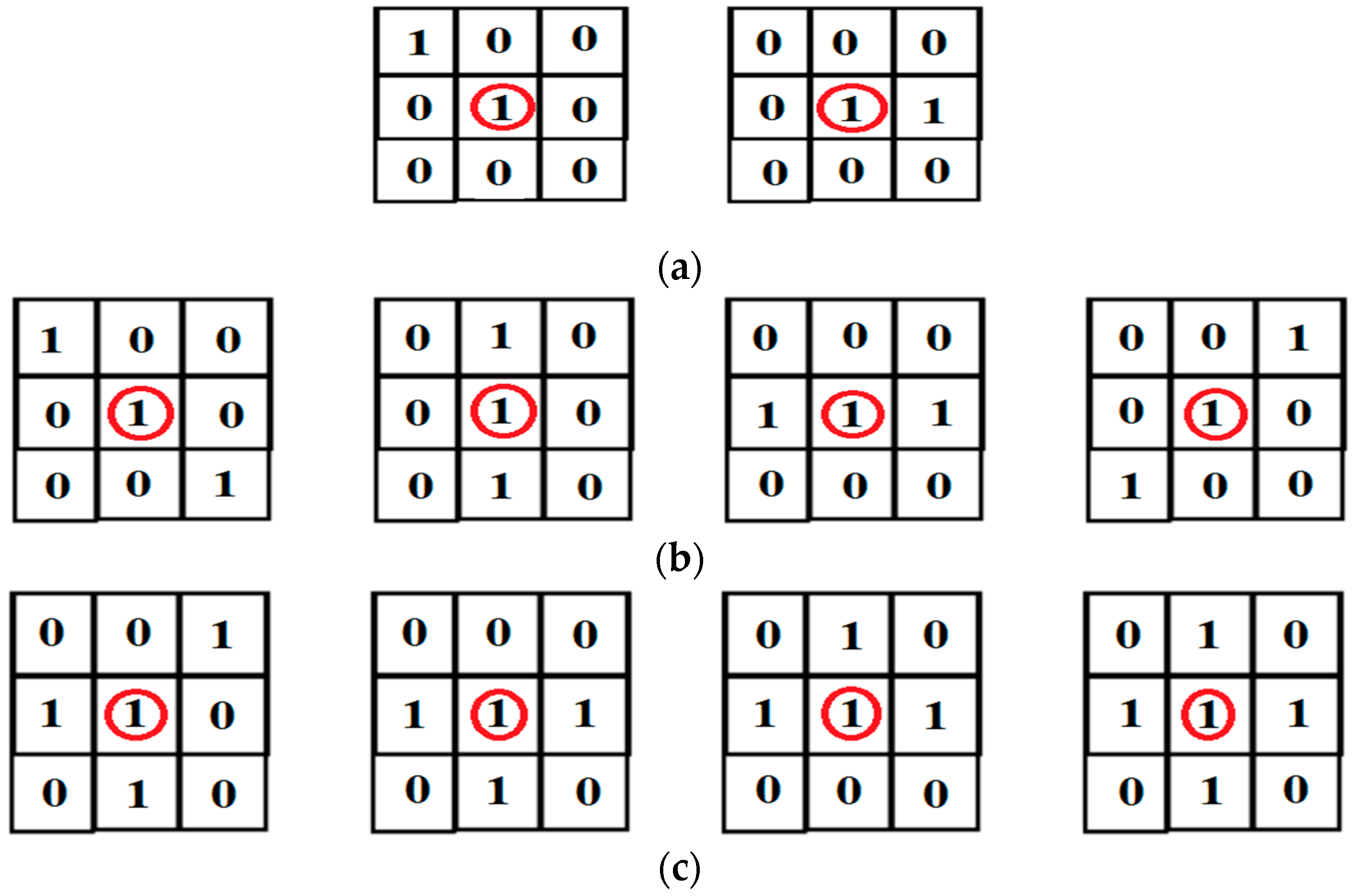
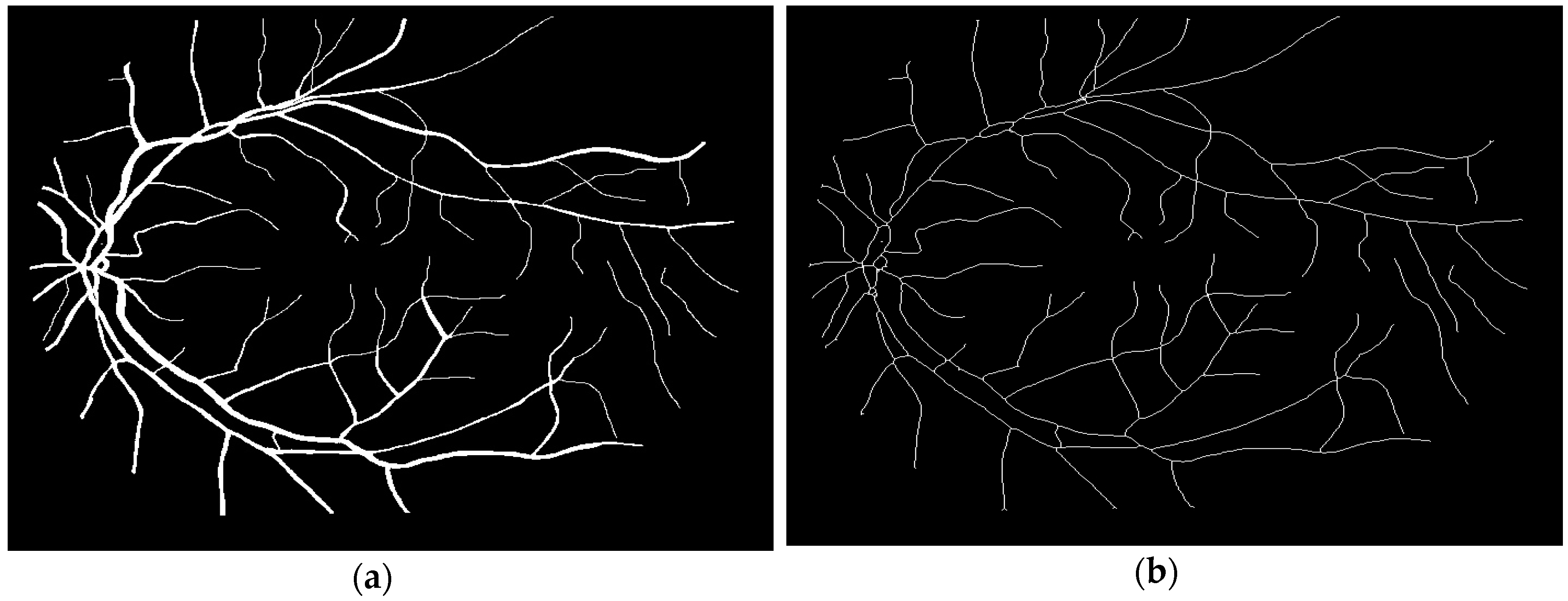
2.2.2. Binary and Skeletonization Image
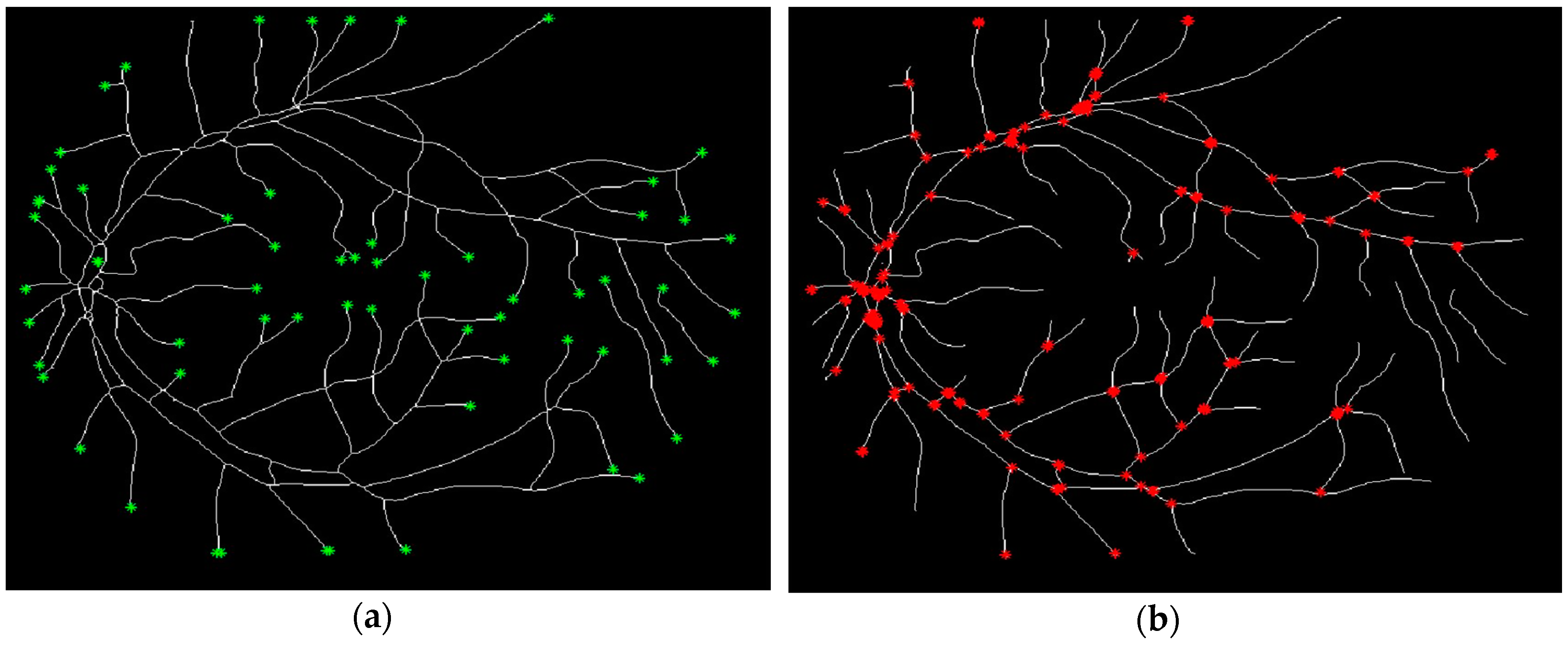
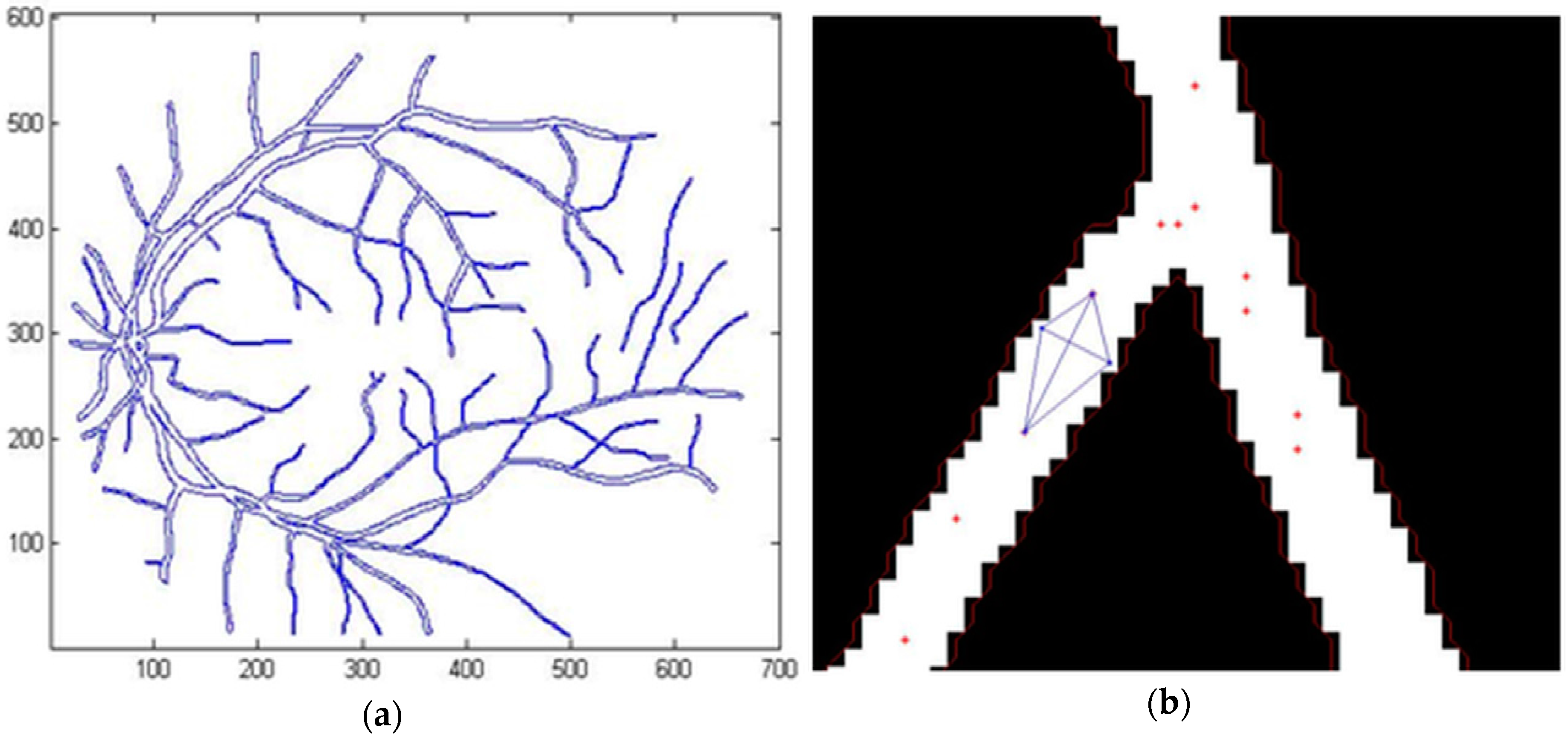
2.2.3. Point Detection
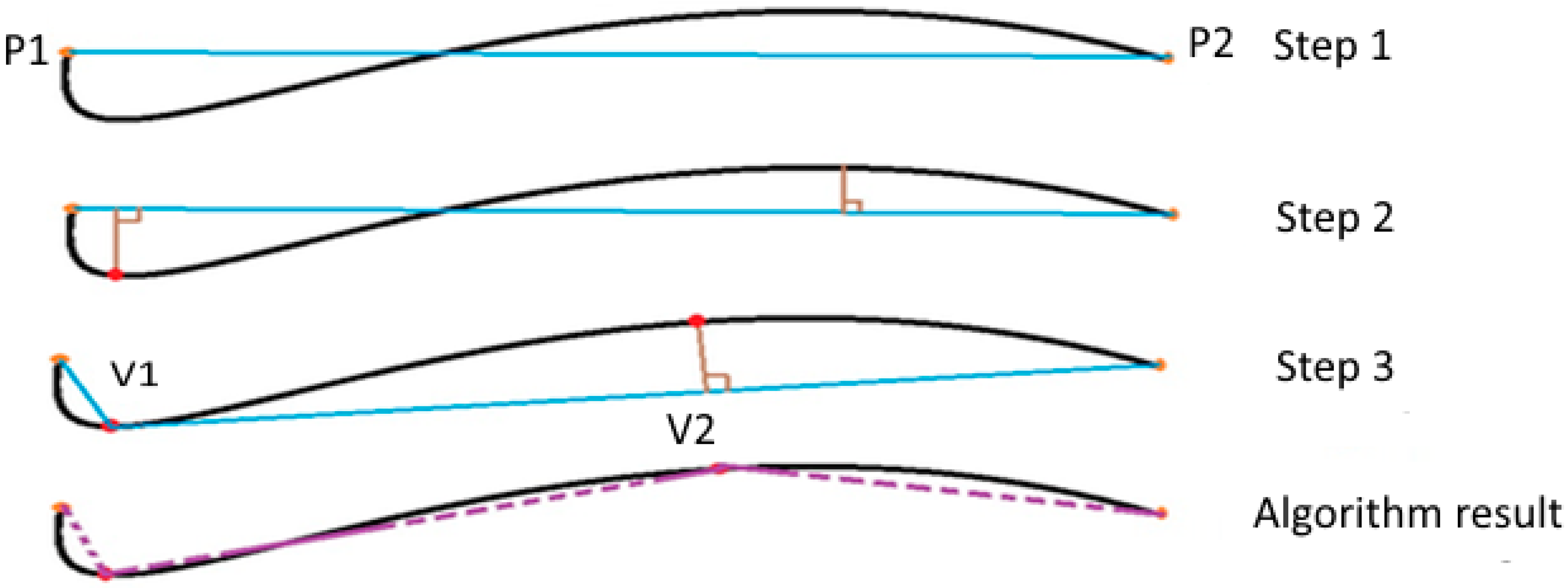
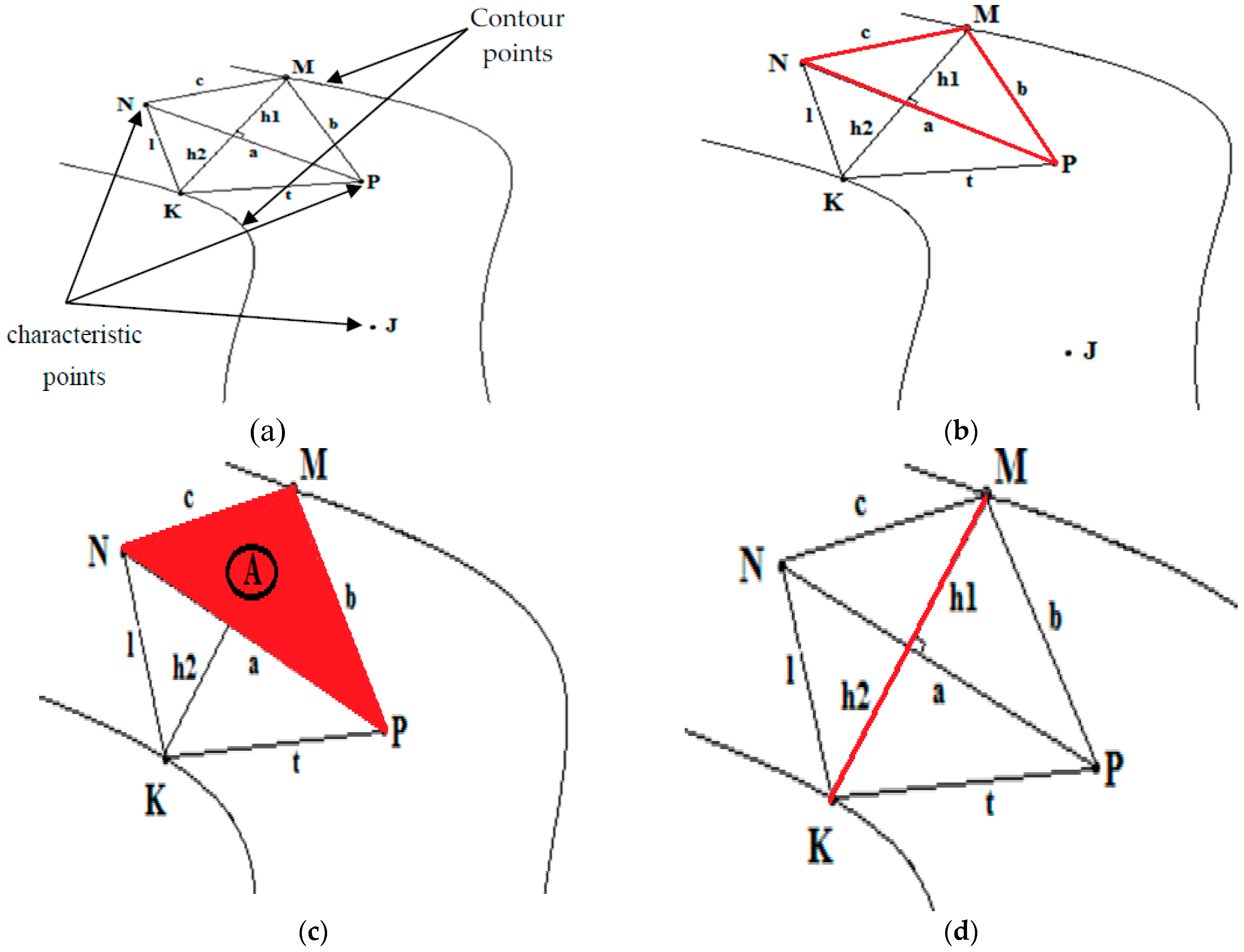
2.2.4. Determination of the Characteristic Points from the Blood Vessel Curve
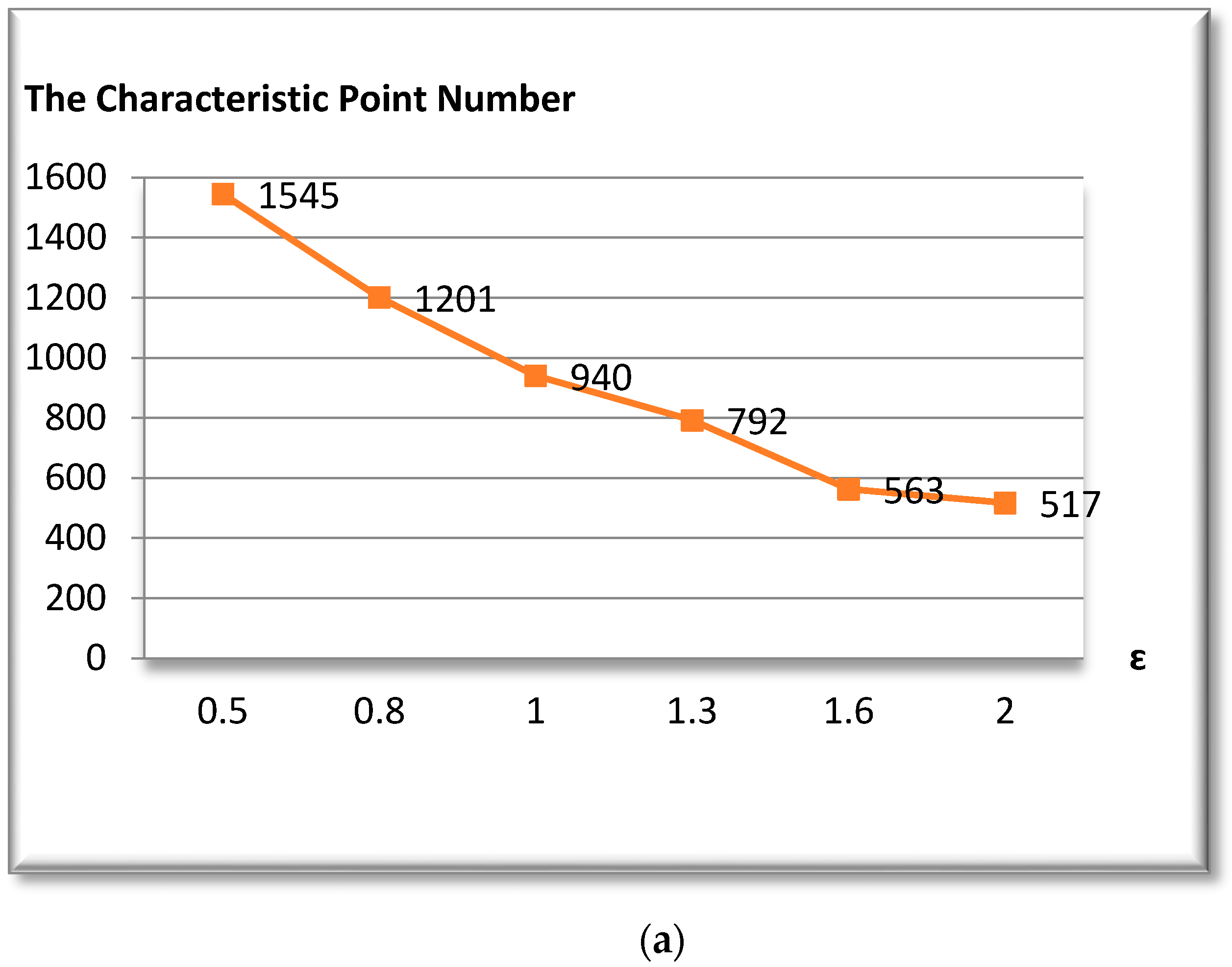
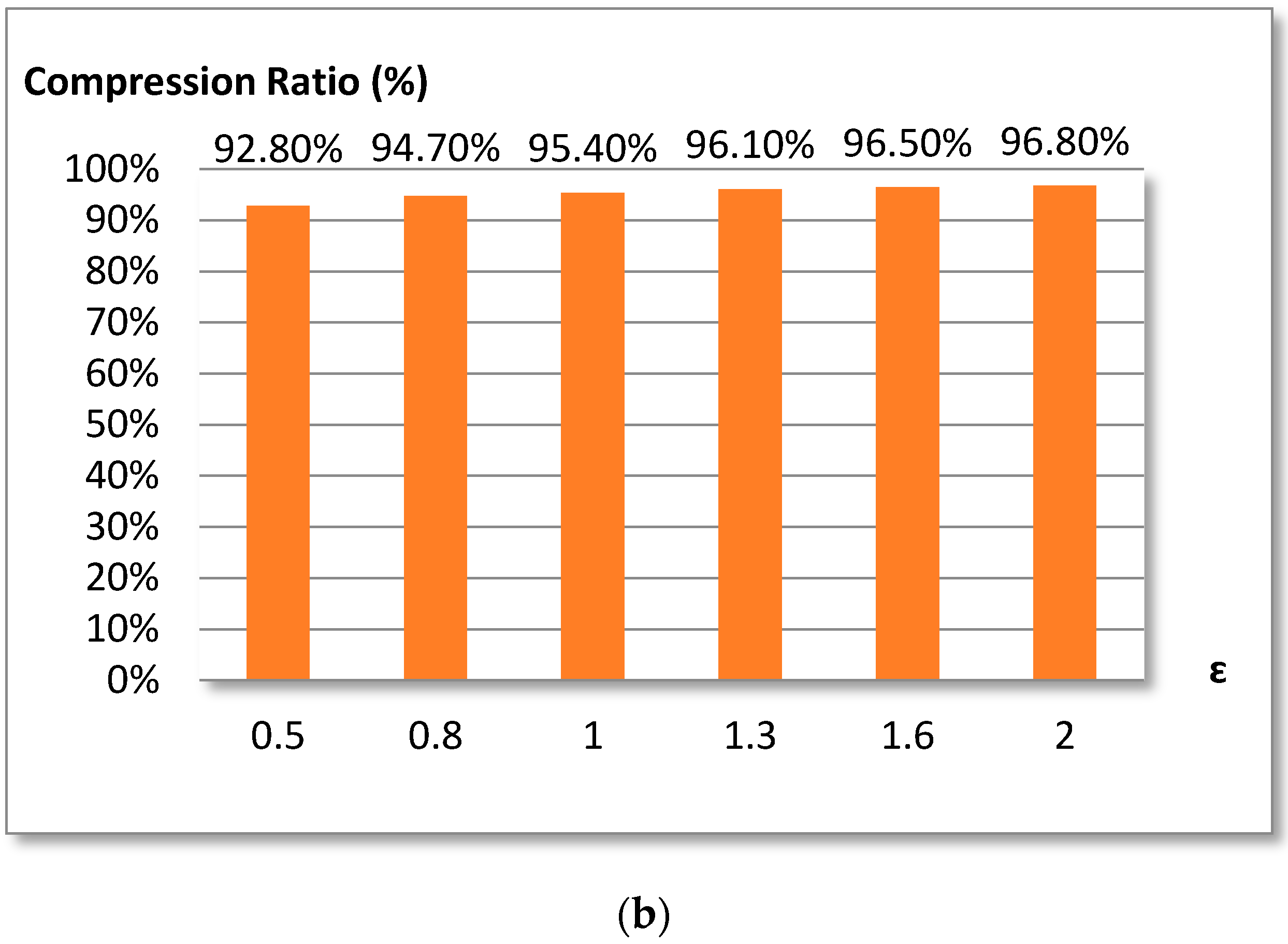
The Douglas-Peucker algorithm
2.3. Edge Detection with Snake Algorithm
2.3.1. The Snake Algorithm
2.3.2. The Internal Energy
- Econt: is the continuity energy, it ensures that the parameterization of the points remains equidistant from each other.
- Ecurv: is the curvature energy, it maintains the rigidity of the snake.
- vs(s): is the first derivative of screws with respect to s.
- vss(s): is the second derivative of v(s) with respect to s.
- α(s): is the contour elasticity.
- β(s): is the contour rigidity.
2.3.3. The External Energy
- γ: is a constant used to control the importance of the Eext.
- Gσ(x,y): is a Gaussian kernel with a scale σ.
2.4. Methods
2.4.1. Initialization.
2.4.2. Determination of Vessel Diameters
3. Results
3.1. Segmentation of the Human Retina Image
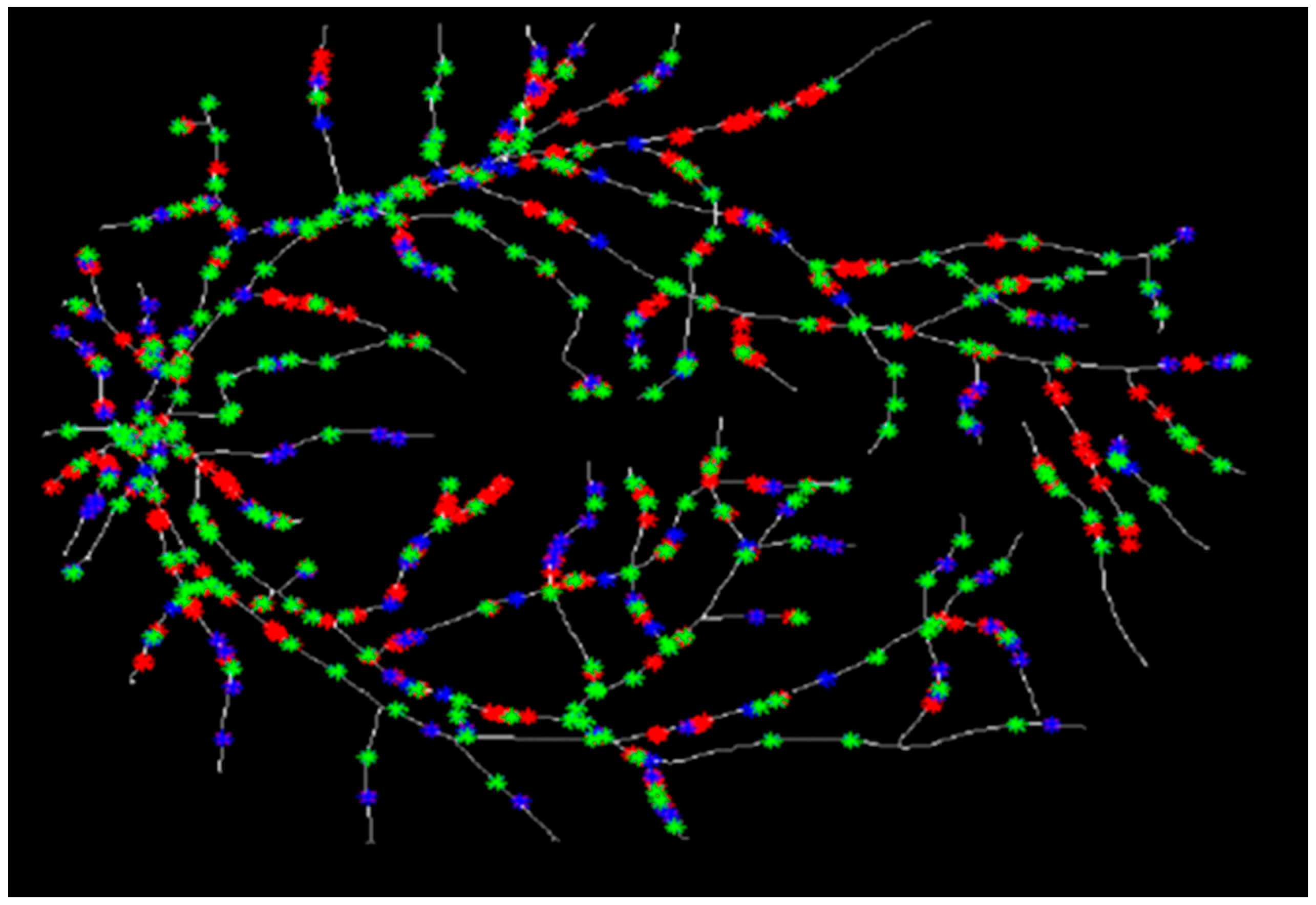
3.2. Douglas-Peucker Algorithm
3.3. Diameter Measurement
- -
- x is the manual measurement value.
- -
- is the measured value obtained using the proposed method.
- -
- n is the number of test measurements.
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Vidal, F.P.; Bello, F.; Brodlie, K.W.; John, N.W.; Gould, D.; Phillips, R.; Avis, N.J. Principles and applications of computer graphics in medicine. Comput. Graph. Fourm 2006, 25, 113–137. [Google Scholar] [CrossRef]
- Wernick, M.N.; Bouman, C.A.; Leahy, R.M.; Duncan, J.S. The Roles of Signal Processing in Medical Imaging. IEEE Signal Process. Mag. 2010, 27, 12–140. [Google Scholar] [CrossRef]
- Quistgaard, J. Signal Acquisition and Processing in Medical Diagnostic Ultrasound. IEEE Signal Process. Mag. 1997, 14, 67–74. [Google Scholar] [CrossRef]
- McCloy, R.; Stone, R. Virtual reality in surgery. Br. Med. J. 2001, 323, 912–915. [Google Scholar] [CrossRef]
- Evans, A.C.; Marret, S.; Collins, L.; Peters, T.M. Anatomical functional correlative analysis of the human brain using 3d imaging systems. Med. Imaging III Image Process. 1989, 1092, 264–275. [Google Scholar]
- Nguyen, T.T.; Wang, J.; Islam, F.; Mitchell, P.; Tapp, R.J.; Zimmet, P.Z.; Simpson, R.; Shaw, J.; Wong, T.Y. Retinal Arteriolar Narrowing Predicts Incidence of Diabetes: The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes 2008, 57, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Schmidt, M.I.; Pankow, J.S.; Couper, D.J.; Klein, B.E.; Hubbard, L.D.; Duncan, B.B.; ARIC Investigators. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. J. Am. Med. Assoc. 2002, 57, 2528–2533. [Google Scholar] [CrossRef]
- Li, H.; Hsu, W.; Lee, M.L.; Wang, H. A piecewise Gaussian model for profiling and differentiating retinal vessels. In Proceedings of the International Conference on Image Processing (ICIP ’03), Catalonia, Spain, 14–18 September 2003; Volume 1, pp. 1069–1072. [Google Scholar]
- Xu, X.; Reinhardt, J.M.; Hu, Q.; Bakall, B.; Tlucek, P.S.; Bertelsen, G.; Abràmoff, D.M. Retinal Vessel Width Measurement at Branchings Using an Improved Electric Field Theory-Based Graph Approach. PLoS ONE 2012, 7, e49668. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Scholfield, C.N.; McGeown, J.G.; Curtis, T.M. Fast Retinal Vessel Detection and Measurement Using Wavelets and Edge Location Refinement. PLoS ONE 2012, 7, e32435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.K.; Aliahmad, B.; Hao, H. Retinal vessel diameter measurement using unsupervised linear discriminant analysis. ISRN Ophthalmol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.; Nath, B.; Chua, J.; Kotagiri, R. Vessel cross-sectional diameter measurement on color retinal image. In Proceedings of the International Joint Conference, BIOSTEC 2008 Funchal, Madeira, Portugal, 28–31 January 2008; pp. 214–227. [Google Scholar]
- El Abbadi, N.; Al Saadi, E. Bood vessel diameter measurement on retinal image. J. Comput. Sci. 2014, 10, 879–883. [Google Scholar] [CrossRef]
- Gao, X.W.; Bharath, A.; Stanton, A.; Hughes, A.; Chapman, N.; Thom, S. Measurement of vessel diameters on retinal for cardiovascular studies. In Proceedings of the Medical Image Understanding and Analysis, Birmingham, UK, 16–17 July 2001; pp. 1–4. [Google Scholar]
- Gao, X.W.; Bharath, A.; Stanton, A.; Hughes, A.; Chapman, N.; Thom, S. Quantification and characterisation of arteries in retinal images. Comput. Methods Programs Biomed. 2000, 63, 133–146. [Google Scholar] [CrossRef]
- Tizon, A.; Courtney, J. Blood Vessel Diameter Estimation System Using Active Contours. In Proceedings of the 2011 Irish Machine Vision and Image, Dublin, Ireland, 8–9 September 2011. [Google Scholar]
- Lowell, J.; Hunter, A.; Steel, D.; Basu, A.; Ryder, R.; Kennedy, R.L. Measurement of Retinal Vessel Widths From Fundus Images Based on 2-D Modeling. IEEE Trans. Med. Imaging 2004, 23, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Chapman, N.; Witt, N.; Gao, X.; Bharath, A.; Stanton, A.; Thom, S.; Hughes, A. Computer algorithms for the automated measurement of retinal arteriolar diameters. Br. J. Ophthalmol. 2001, 85, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Rzeszotarski, M.S.; Singerman, L.J.; Chokreff, J.M. The detection and quantification of retinopathy using digital angiograms. IEEE Trans. Med. Imaging 1994, 13, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Gang, L.; Chutatape, O.; Krishnan, S.M. Detection and measurement of retinal vessels in fundus images using amplitude modified second-order Gaussian filter. IEEE Trans. Biomed. Eng. 2002, 49, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Duan, W.; Lin, T.; Li, B. A method of retinal vessel width measurement. In Proceedings of the 2nd International Conference on Computer and Automation Engineering (ICCAE’10) 2010, Singapore, 26–28 February 2010; pp. 443–446. [Google Scholar]
- Aliahmad, B.; Kumar, D.K.; Janghorban, S.; Azemin, M.Z.C.; Hao, H.; Kawasaki, R. Retinal vessel diameter measurement using multi-step regression method. In Proceedings of the Biosignals and Biorobotics Conference (BRC ’12), Manaus, Brazil, 9–11 January 2012; pp. 1–4. [Google Scholar]
- STARE (STructured Analysis of the Retina) Project Website. Available online: http://www.ces.clemson.edu/~ahoover/stare (accessed on 10 December 2016).
- High-Resolution Fundus (HRF) Image Database Website. Available online: http://www5.cs.fau.de/research/data/fundus-images (accessed on 15 December 2016).
- Hoover, A.; Kouznetsova, V.; Goldbaum, M. Locating Blood Vessels in Retinal Images by Piece-wise Threhsold Probing of a Matched Filter Response. IEEE Trans. Med. Imaging 2000, 19, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Greig, D.M.; Porteous, B.T.; Seheult, A.H. Exact maximum a posteriori estimation for binary images. J. R. Stat. Soc. Ser. B 1989, 51, 271–279. [Google Scholar]
- Akram, M.U.; Tariq, A.; Khan, S.A. Retinal image blood vessel segmentation. In Proceedings of the International Conference on Information and Communication Technologies, ICICT’09, Karachi, Pakistan, 15–16 August 2009; pp. 181–192. [Google Scholar]
- El Abbadi, N.K.; Al Saadi, E.H. Blood vessels extraction using mathematical morphology. J. Comput. Sci. 2013, 9, 1389–1395. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shapiro, L.G. Computer and Robot Vision; Addison-Wesley: Boston, MA, USA, 1992; Volume 1. [Google Scholar]
- Kong, T.Y.; Rosenfeld, A. Topological Algorithms for Digital Image Processing; Elsevier Science, Inc.: North Holland, The Netherlands, 1996. [Google Scholar]
- Lam, L.; Seong-Whan, L.; Ching, Y.S. Thinning Methodologies-A Comprehensive Survey. IEEE Trans. Pattern Anal. Mach. Intell. 1992, 14, 869–885. [Google Scholar] [CrossRef]
- Bouix, S.; Siddiqi, K.; Tannenbaum, A. Flux driven automatic centerline extraction. Med. Image Anal. 2005, 14, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Dikici, E.; O’Donnell, T.; Grady, L.; White, R. Coronary Artery Centerline Tracking Using Axial Symmetries. Available online: http://www.midasjournal.org/browse/publication/586 (accessed on 1 December 2016).
- Zhou, Y.; Toga, A.W. Efficient Skeletonization of Volumetric Objects. IEEE Trans. Vis. Comput. Graph. 1999, 5, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Tang, X. Preprocessing and postprocessing for skeleton-based fingerprint minutiae extraction. Pattern Recognit. 2007, 40, 1270–1281. [Google Scholar] [CrossRef]
- Guedri, H.; Malek, J.; Belmabrouk, H. Reconstruction of the human retinal blood vessel by fractal interpolation. J. Theor. Appl. Inf. Technol. 2016, 83, 227–233. [Google Scholar]
- Guedri, H.; Chouchene, F.; Belmabrouk, H. 3D Model Reconstruction of Blood Vessels in The Retina with Tubular Structure. Int. J. Electr. Eng. Inform. 2015, 7, 724–734. [Google Scholar]
- White, E.R. Assessment of line-generalization algorithms using characteristic points. Am. Cartogr. 1985, 12, 17–28. [Google Scholar] [CrossRef]
- Marino, J.S. Identification of characteristic points along naturally occurring lines. Cartogr. Int. J. Geogr. Inf. Geovisualization 1979, 16, 70–80. [Google Scholar] [CrossRef]
- Douglas, D.H.; Peucker, T.K. Algorithms for the reduction of the number of points required to represent a line or its caricature. Can. Cartogr. 1973, 10, 112–122. [Google Scholar] [CrossRef]
- Hershberger, J.; Snoeyink, J. Speeding up the Douglas–Peucker line-simplification algorithm. In Proceedings of the Fifth International Symposium on Spatial Data Handling, Charleston, SC, USA, 3–7 August 1992; Volume 1, pp. 134–143. [Google Scholar]
- Kass, M.; Witkin, A.; Terzopoulos, D. Snakes: Active Contour Models. Int. J. Comput. Vis. 1988, 1, 321–331. [Google Scholar] [CrossRef]
- Caselles, V. Geodesic Active Contours. Int. J. Comput. Vis. 1997, 22, 61–79. [Google Scholar] [CrossRef]
- Tajik, H.R.; Rahebi, J. Diffuse Objects extraction in Coronal Holes Using Active Contour Means Model. ACSIJ Adv. Comput. Sci. 2013, 2, 55–61. [Google Scholar]
- Derraz, F.; Beladgham, M.; Khelif, M. Application of active contour models in medical image segmentation. In Proceedings of the Information Technology: Coding and Computing, Las Vegas, NV, USA, 5–7 April 2004; Volume 2, pp. 675–681. [Google Scholar]
- Webster, C.S.; Merry, A.F. The standard deviation and the standard error of the mean. Anaesthesia 1997, 52, 183. [Google Scholar] [PubMed]
- Altman, D.G.; Bland, J.M. Standard deviations and standard errors. BMJ 2005, 52331. [Google Scholar] [CrossRef] [PubMed]

| Image | Vessel No | Diameter (Manual) | Diameter (Proposed Method) | Difference | Standard Deviation (SD) | Average (Eavg) |
|---|---|---|---|---|---|---|
| im0002.ah.jpg | 1 | 6.6212 | 6.6436 | −0.0224 | 0.0162 | 0.0211 |
| 2 | 5.3101 | 5.3099 | 0.0002 | |||
| 3 | 4.4721 | 4.4721 | 0 | |||
| 4 | 9.2540 | 9.2487 | 0.0053 | |||
| 5 | 13.6255 | 13.7002 | −0.0777 | |||
| im0139.ah.jpg | 1 | 4.0000 | 3.752 | 0.2480 | 0.1094 | 0.2390 |
| 2 | 4.1231 | 4.3872 | −0.2641 | |||
| 3 | 5.8310 | 5.642 | 0.189 | |||
| 4 | 8.0000 | 8.176 | −0.176 | |||
| 5 | 3.0000 | 2.682 | 0.318 | |||
| im0077.ah.jpg | 1 | 7.8102 | 7.9394 | −0.1292 | 0.1401 | 0.2752 |
| 2 | 4.2426 | 4.4792 | −0.2366 | |||
| 3 | 2.8284 | 2.674 | 0.1544 | |||
| 4 | 3.1623 | 2.6152 | 0.5471 | |||
| 5 | 8.9443 | 9.2528 | −0.3085 |
| Image | Vessel No | Diameter (Manual) | Diameter (Proposed Method) | Difference | Standard Deviation (SD) | Average (Eavg) |
|---|---|---|---|---|---|---|
| 10_h.tif | 1 | 18.3848 | 18.3564 | 0.0284 | 0.0234 | 0.0307 |
| 2 | 5.000 | 4.887 | 0.1130 | |||
| 3 | 10.0499 | 10.0535 | −0.0036 | |||
| 4 | 2.8284 | 2.8301 | −0.0017 | |||
| 5 | 2.5381 | 2.5312 | 0.0069 | |||
| 02_h.tif | 1 | 3.0000 | 2.9654 | 0.0346 | 0.0092 | 0.0161 |
| 2 | 15.0000 | 15.028 | −0.0280 | |||
| 3 | 28.4253 | 28.4198 | 0.0055 | |||
| 4 | 20.0000 | 20.0035 | −0.0035 | |||
| 5 | 9.2195 | 9.2105 | 0.0090 | |||
| 15_h.tif | 1 | 22.6274 | 22.6248 | 0.0026 | 0.0063 | 0.0090 |
| 2 | 12.0000 | 12.0056 | −0.0056 | |||
| 3 | 21.0950 | 21.1256 | −0.0306 | |||
| 4 | 5.8310 | 5.8296 | 0.0014 | |||
| 5 | 7.6158 | 7.6205 | −0.0047 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guedri, H.; Ben Abdallah, M.; Echouchene, F.; Belmabrouk, H. Novel Computerized Method for Measurement of Retinal Vessel Diameters. Biomedicines 2017, 5, 12. https://doi.org/10.3390/biomedicines5020012
Guedri H, Ben Abdallah M, Echouchene F, Belmabrouk H. Novel Computerized Method for Measurement of Retinal Vessel Diameters. Biomedicines. 2017; 5(2):12. https://doi.org/10.3390/biomedicines5020012
Chicago/Turabian StyleGuedri, Hichem, Mariem Ben Abdallah, Fraj Echouchene, and Hafedh Belmabrouk. 2017. "Novel Computerized Method for Measurement of Retinal Vessel Diameters" Biomedicines 5, no. 2: 12. https://doi.org/10.3390/biomedicines5020012






