Glycosylated Triterpenoids as Endosomal Escape Enhancers in Targeted Tumor Therapies
Abstract
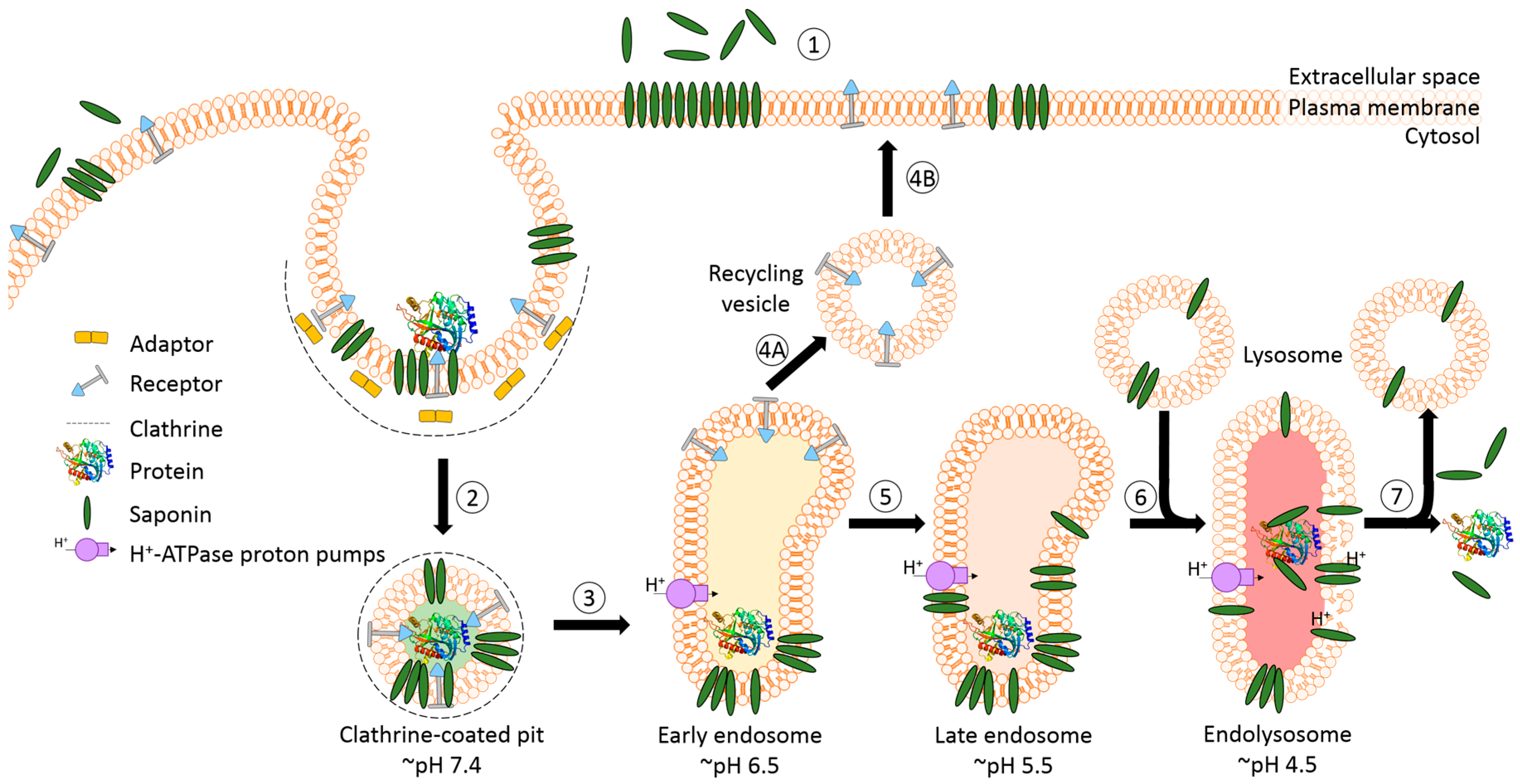
:1. Introduction
2. Glycosylated Triterpenoids
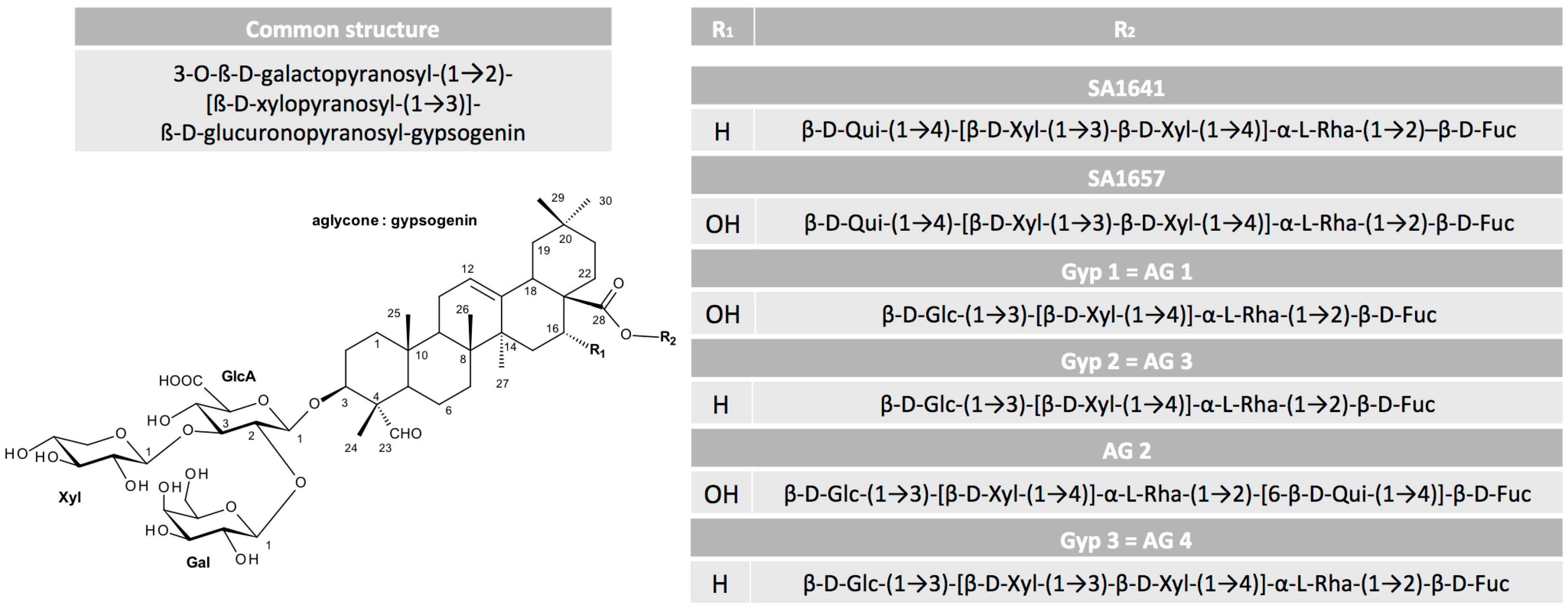
2.1. Origin and Structure
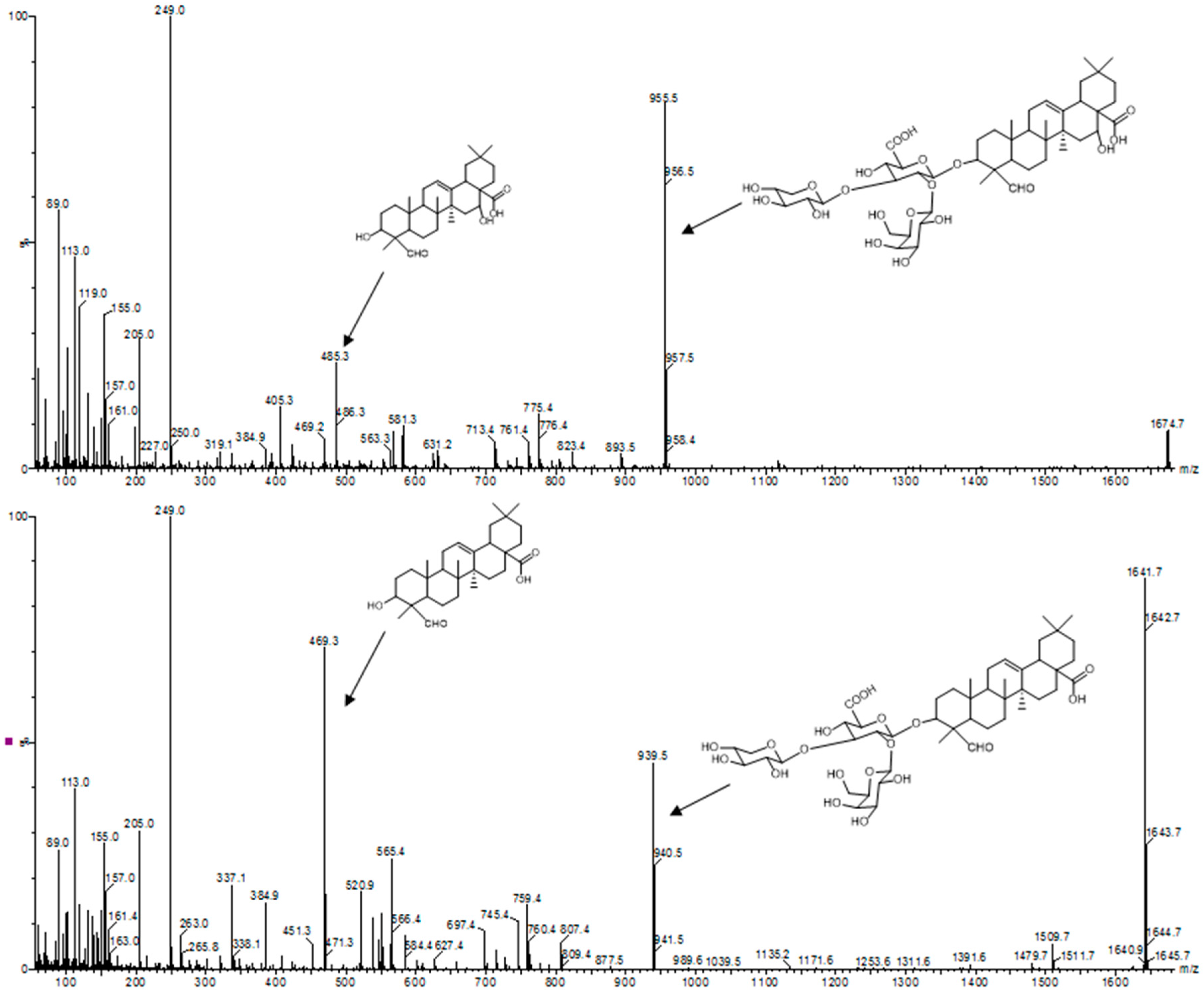
2.2. Purification
2.3. Molecular Interactions
2.3.1. Hemolytic Activity of Saponins
2.3.2. Interaction of Saponins with Cell Membranes and Their Components
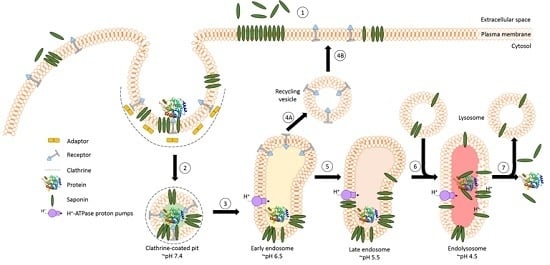
3. Endosomal Escape in Cell Culture Models
3.1. Cellular Interactions
3.2. Endosomal Release of Proteins
3.3. Synergistic Effects with Targeted Toxins
4. Endosomal Escape in Animal Models
4.1. Biodistribution and Toxicity
4.2. Efficacy
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Polito, L.; Djemil, A.; Bortolotti, M. Plant toxin-based immunotoxins for cancer therapy: A short overview. Biomedicines 2016, 4, 12. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Kohne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pinter, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Al-Taei, S.; Penning, N.A.; Simpson, J.C.; Futaki, S.; Takeuchi, T.; Nakase, I.; Jones, A.T. Intracellular traffic and fate of protein transduction domains HIV-1 TAT peptide and octaarginine. Implications for their utilization as drug delivery vectors. Bioconjug. Chem. 2006, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Olsnes, S.; Sandvig, K.; Petersen, O.W.; van Deurs, B. Immunotoxins—Entry into cells and mechanisms of action. Immunol. Today 1989, 10, 291–295. [Google Scholar] [PubMed]
- Pirker, R.; FitzGerald, D.J.; Hamilton, T.C.; Ozols, R.F.; Laird, W.; Frankel, A.E.; Willingham, M.C.; Pastan, I. Characterization of immunotoxins active against ovarian cancer cell lines. J. Clin. Investig. 1985, 76, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Ravel, S.; Colombatti, M.; Casellas, P. Internalization and intracellular fate of anti-CD5 monoclonal antibody and anti-CD5 ricin A-chain immunotoxin in human leukemic T cells. Blood 1992, 79, 1511–1517. [Google Scholar] [PubMed]
- Heisler, I.; Keller, J.; Tauber, R.; Sutherland, M.; Fuchs, H. A cleavable adapter to reduce nonspecific cytotoxicity of recombinant immunotoxins. Int. J. Cancer 2003, 103, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Swanson, J.A.; Lee, K.D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Baluna, R.; Vitetta, E.S. Vascular leak syndrome: A side effect of immunotherapy. Immunopharmacology 1997, 37, 117–132. [Google Scholar] [CrossRef]
- Kuus-Reichel, K.; Grauer, L.S.; Karavodin, L.M.; Knott, C.; Krusemeier, M.; Kay, N.E. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1994, 1, 365–372. [Google Scholar] [PubMed]
- Fuchs, H.; Bachran, C.; Flavell, D.J. Diving through membranes: Molecular cunning to enforce the endosomal escape of antibody-targeted anti-tumor toxins. Antibodies 2013, 2, 209–235. [Google Scholar] [CrossRef]
- Selbo, P.K.; Bostad, M.; Olsen, C.E.; Edwards, V.T.; Hogset, A.; Weyergang, A.; Berg, K. Photochemical internalisation, a minimally invasive strategy for light-controlled endosomal escape of cancer stem cell-targeting therapeutics. Photochem. Photobiol. Sci. 2015, 14, 1433–1450. [Google Scholar] [CrossRef] [PubMed]
- Wales, R.; Roberts, L.M.; Lord, J.M. Addition of an endoplasmic reticulum retrieval sequence to ricin A chain significantly increases its cytotoxicity to mammalian cells. J. Biol. Chem. 1993, 268, 23986–23990. [Google Scholar] [PubMed]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Bachran, S.; Sutherland, M.; Bachran, D.; Fuchs, H. Saponins in tumor therapy. Mini Rev. Med. Chem. 2008, 8, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Bachran, S.; Sutherland, M.; Bachran, D.; Fuchs, H. Preclinical Studies of Saponins for Tumor therapy. In Recent Advances in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 272–302. [Google Scholar]
- Fuchs, H.; Bachran, D.; Panjideh, H.; Schellmann, N.; Weng, A.; Melzig, M.F.; Sutherland, M.; Bachran, C. Saponins as tool for improved targeted tumor therapies. Curr. Drug Targets 2009, 10, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Weng, A.; Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Thakur, M. Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: a lethal cocktail with tumor specific efficacy. Curr. Pharm. Des. 2014, 20, 6584–6643. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J.N. Terpenoids and Steroids. In Pharmacognosy, Phytochemistry, Medicinal Plants, 1st ed.; Tec & Doc Lavoisier: Paris, France, 1995; pp. 537–573. [Google Scholar]
- Osbourn, A.; Goss, R.J.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorg. Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Tyler, V.E.; Brady, L.R.; Robbers, J.E. Pharmacognosy, 8th ed.; Lee and Febiger: Philadelphia, PA, USA, 1981; p. 67. [Google Scholar]
- Baumann, E.; Stoya, G.; Volkner, A.; Richter, W.; Lemke, C.; Linss, W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000, 102, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kemmerich, B.; Eberhardt, R.; Stammer, H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled clinical trial. Arzneimittel-Forschung 2006, 56, 652–660. [Google Scholar] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, F.; Zhang, L.; Han, B.; Zhu, M.; Zhang, X. Effects of escin on acute inflammation and the immune system in mice. Pharmacol. Rep. 2009, 61, 697–704. [Google Scholar] [CrossRef]
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C.J. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med. Sci. Monit. 2004, 10, RA187–RA192. [Google Scholar] [PubMed]
- Heisler, I.; Sutherland, M.; Bachran, C.; Hebestreit, P.; Schnitger, A.; Melzig, M.F.; Fuchs, H. Combined application of saponin and chimeric toxins drastically enhances the targeted cytotoxicity on tumor cells. J. Control. Release 2005, 106, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Hebestreit, P.; Melzig, M.F. Cytotoxic activity of the seeds from Agrostemma githago var. githago. Planta Med. 2003, 69, 921–925. [Google Scholar] [PubMed]
- Hebestreit, P.; Weng, A.; Bachran, C.; Fuchs, H.; Melzig, M.F. Enhancement of cytotoxicity of lectins by Saponinum album. Toxicon 2006, 47, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Thakur, M.; von Mallinckrodt, B.; Beceren-Braun, F.; Gilabert-Oriol, R.; Wiesner, B.; Eichhorst, J.; Bottger, S.; Melzig, M.F.; Fuchs, H. Saponins modulate the intracellular trafficking of protein toxins. J. Control. Release 2012, 164, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Hebestreit, P.; Gaidi, G.; Lacaille-Dubois, M.A. Structure-activity-relationship of saponins to enhance toxic effects of agrostin. Planta Med. 2005, 71, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Sutherland, M.; Heisler, I.; Hebestreit, P.; Melzig, M.F.; Fuchs, H. The saponin-mediated enhanced uptake of targeted saporin-based drugs is strongly dependent on the saponin structure. Exp. Biol. Med. 2006, 231, 412–420. [Google Scholar]
- Böttger, S.; Westhof, E.; Siems, K.; Melzig, M.F. Structure-activity relationships of saponins enhancing the cytotoxicity of ribosome-inactivating proteins type I (RIP-I). Toxicon 2013, 73, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Thakur, M.; Beceren-Braun, F.; Bachran, D.; Bachran, C.; Riese, S.B.; Jenett-Siems, K.; Gilabert-Oriol, R.; Melzig, M.F.; Fuchs, H. The toxin component of targeted anti-tumor toxins determines their efficacy increase by saponins. Mol. Oncol. 2012, 6, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Bachran, C.; Fuchs, H.; Weng, A.; Melzig, M.F.; Flavell, S.U.; Flavell, D.J. Triterpenoid saponin augmention of saporin-based immunotoxin cytotoxicity for human leukaemia and lymphoma cells is partially immunospecific and target molecule dependent. Immunopharmacol. Immunotoxicol. 2015, 37, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Jenett-Siems, K.; Schmieder, P.; Bachran, D.; Bachran, C.; Gorick, C.; Thakur, M.; Fuchs, H.; Melzig, M.F. A convenient method for saponin isolation in tumour therapy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S. Untersuchungen zur synergistischen Zytotoxizität zwischen Saponinen und Ribosomen inaktivierenden Proteinen Typ I. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2013. [Google Scholar]
- Gilabert-Oriol, R.; Thakur, M.; Haussmann, K.; Niesler, N.; Bhargava, C.; Gorick, C.; Fuchs, H.; Weng, A. Saponins from Saponaria officinalis L. augment the efficacy of a rituximab-immunotoxin. Planta Med. 2016, 82, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Manunta, M.D.; Thakur, M.; Gilabert-Oriol, R.; Tagalakis, A.D.; Eddaoudi, A.; Munye, M.M.; Vink, C.A.; Wiesner, B.; Eichhorst, J.; et al. Improved intracellular delivery of peptide- and lipid-nanoplexes by natural glycosides. J. Control. Release 2015, 206, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Weng, A.; von Mallinckrodt, B.; Stoshel, A.; Nissi, L.; Melzig, M.F.; Fuchs, H.; Thakur, M. Electrophoretic mobility as a tool to separate immune adjuvant saponins from Quillaja saponaria Molina. Int. J. Pharm. 2015, 487, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Weng, A.; Bachran, D.; Riese, S.B.; Bottger, S.; Melzig, M.F.; Fuchs, H. Electrophoretic isolation of saponin fractions from Saponinum album and their evaluation in synergistically enhancing the receptor-specific cytotoxicity of targeted toxins. Electrophoresis 2011, 32, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Weng, A.; Pieper, A.; Mergel, K.; von Mallinckrodt, B.; Gilabert-Oriol, R.; Gorick, C.; Wiesner, B.; Eichhorst, J.; Melzig, M.F.; et al. Macromolecular interactions of triterpenoids and targeted toxins: Role of saponins charge. Int. J. Biol. Macromol. 2013, 61, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Bachran, D.; Gorick, C.; Bachran, C.; Fuchs, H.; Melzig, M.F. A simple method for isolation of Gypsophila saponins for the combined application of targeted toxins and saponins in tumor therapy. Planta Med. 2009, 75, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Thakur, M.; Schindler, A.; Fuchs, H.; Melzig, M.F. Liquid-chromatographic profiling of Saponinum album (Merck). Die Pharm. 2011, 66, 744–746. [Google Scholar]
- Thakur, M.; Jerz, G.; Tuwalska, D.; Gilabert-Oriol, R.; Wybraniec, S.; Winterhalter, P.; Fuchs, H.; Weng, A. High-speed countercurrent chromatographic recovery and off-line electrospray ionization mass spectrometry profiling of bisdesmodic saponins from Saponaria officinalis possessing synergistic toxicity enhancing properties on targeted antitumor toxins. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 955–956, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, C.; Dürkop, H.; Zhao, X.; Weng, A.; Melzig, M.F.; Fuchs, H. Targeted dianthin is a powerful toxin to treat pancreatic carcinoma when applied in combination with the glycosylated triterpene SO1861. Mol. Oncol. 2017. in revision. [Google Scholar]
- Morrissey, J.P.; Osbourn, A.E. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [PubMed]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.K.; Nagao, T.; Okabe, H.; Shinoda, T. Resistance in the plant, Barbarea vulgaris, and counter-adaptations in flea beetles mediated by saponins. J. Chem. Ecol. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Aladesanmi, O. Medication adherence and physician communication skills. Arch. Intern. Med. 2007, 167, 859–860. [Google Scholar] [PubMed]
- Fuchs, H.; Weng, A.; Gilabert-Oriol, R. Augmenting the efficacy of immunotoxins and other targeted protein toxins by endosomal escape enhancers. Toxins 2016, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Cheng, D.; Iles, G.H. Structure of membrane holes in osmotic and saponin hemolysis. J. Cell Biol. 1973, 56, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Dourmashkin, R.R.; Dougherty, R.M.; Harris, R.J. Electron microscopic observations on Rous sarcoma virus and cell membranes. Nature 1962, 194, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Horne, R.W.; Glauert, A.M.; Dingle, J.T.; Lucy, J.A. Action of saponin on biological cell membranes. Nature 1962, 196, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.; de Vrije, T.; Fabrie, C.H.; Demel, R.A.; Jongen, W.M.; de Kruijff, B. Dual specificity of sterol-mediated glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1992, 1110, 127–136. [Google Scholar] [CrossRef]
- Keukens, E.A.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1995, 1240, 216–228. [Google Scholar] [CrossRef]
- Lin, F.; Wang, R. Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. J. Mol. Model. 2010, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Melzig, M.F. The influence of saponins on cell membrane cholesterol. Bioorg. Med. Chem. 2013, 21, 7118–7124. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, E.; Suprynowicz, F.A.; Sudarshan, S.R.; Schlegel, R. Membrane orientation of the human papillomavirus type 16 E5 oncoprotein. J. Virol. 2010, 84, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Shatkovsky, P.; Milo-Goldzweig, I. On the mechanism of saponin hemolysis—I. Hydrolysis of the glycosidic bond. Biochem. Pharmacol. 1974, 23, 973–981. [Google Scholar] [CrossRef]
- Gilabert-Oriol, R.; Mergel, K.; Thakur, M.; von Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Weng, A. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorg. Med. Chem. 2013, 21, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Legault, J.; Girard-Lalancette, K.; Mshvildadze, V.; Pichette, A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins. Bioorg. Med. Chem. 2009, 17, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Legault, J.; Piochon, M.; Lavoie, S.; Tremblay, S.; Pichette, A. Synthesis, cytotoxicity, and haemolytic activity of chacotrioside lupane-type neosaponins and their germanicane-type rearrangement products. Bioorg. Med. Chem. Lett. 2009, 19, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Melzig, M.F.; Bader, G.; Loose, R. Investigations of the mechanism of membrane activity of selected triterpenoid saponins. Planta Med. 2001, 67, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yudt, M.R.; Cidlowski, J.A. The glucocorticoid receptor: Coding a diversity of proteins and responses through a single gene. Mol. Endocrinol. 2002, 16, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Wina, E.; Muetzel, S.; Becker, K. The impact of saponins or saponin-containing plant materials on ruminant production—A review. J. Agric. Food Chem. 2005, 53, 8093–8105. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Transient holes in the erythrocyte membrane during hypotonic hemolysis and stable holes in the membrane after lysis by saponin and lysolecithin. J. Cell Biol. 1967, 32, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dungan, S.R. Micellar properties of Quillaja saponin. 1. Effects of temperature, salt, and pH on solution properties. J. Agric. Food Chem. 1997, 45, 1587–1595. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Lacaille-Dubois, M.A.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Rajput, Z.I.; Hu, S.H.; Xiao, C.W.; Arijo, A.G. Adjuvant effects of saponins on animal immune responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Cabral de Oliveira, A.C.; Perez, A.C.; Merino, G.; Prieto, J.G.; Alvarez, A.I. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 369–377. [Google Scholar] [CrossRef]
- Haridas, V.; Arntzen, C.J.; Gutterman, J.U. Avicins, a family of triterpenoid saponins from Acacia victoriae (Bentham), inhibit activation of nuclear factor-κB by inhibiting both its nuclear localization and ability to bind DNA. Proc. Natl. Acad. Sci. USA 2001, 98, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, P.; Dalsgaard, K.; Madsen, M. Prolonged survival of AKR mice treated with the saponin adjuvant Quil A. Acta Pathol. Microbiol. Scand. A 1976, 84, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Li, Q.; Murakamio, T.; Takahashi, M. Anti-growth effects with components of Sho-saiko-to (TJ-9) on cultured human hepatoma cells. Eur. J. Cancer Prev. 1993, 2, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Horimoto, T.; Hirayama, R.; Mukai, T.; Nakashima, M.; Sasaki, H.; Nishida, K. Effect of the absorption enhancer saponin on the intrarenal distribution of 5-fluorouracil following its kidney surface application in rats. Biol. Pharm. Bull. 2003, 26, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Gaidi, G.; Correia, M.; Chauffert, B.; Beltramo, J.L.; Wagner, H.; Lacaille-Dubois, M.A. Saponins-mediated potentiation of cisplatin accumulation and cytotoxicity in human colon cancer cells. Planta Med. 2002, 68, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Elbandy, M.; Miyamoto, T.; Chauffert, B.; Delaude, C.; Lacaille-Dubois, M.A. Novel acylated triterpene glycosides from Muraltia heisteria. J. Nat. Prod. 2002, 65, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Bachran, C.; Fuchs, H.; Melzig, M.F. Soapwort saponins trigger clathrin-mediated endocytosis of saporin, a type I ribosome-inactivating protein. Chem. Biol. Interact. 2008, 176, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Gorick, C.; Melzig, M.F. Enhancement of toxicity of saporin-based toxins by Gypsophila saponins--kinetic of the saponin. Exp. Biol. Med. 2009, 234, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Bachran, D.; Schneider, S.; Bachran, C.; Weng, A.; Melzig, M.F.; Fuchs, H. The endocytic uptake pathways of targeted toxins are influenced by synergistically acting Gypsophila saponins. Mol. Pharm. 2011, 8, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Thakur, M.; von Mallinckrodt, B.; Bhargava, C.; Wiesner, B.; Eichhorst, J.; Melzig, M.F.; Fuchs, H.; Weng, A. Reporter assay for endo/lysosomal escape of toxin-based therapeutics. Toxins 2014, 6, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Huang, L. Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Hum. Gene Ther. 2001, 12, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Tokatlian, T.; Segura, T. siRNA applications in nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Mellman, I.S.; Muller, W.A.; Cohn, Z.A. Endocytosis and the recycling of plasma membrane. J. Cell Biol. 1983, 96, 1–27. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.S.; Rosenblum, M.G.; Philips, M.R.; Scheinberg, D.A. Immunotoxin resistance in multidrug resistant cells. Cancer Res. 2003, 63, 72–79. [Google Scholar] [PubMed]
- Li, M.; Tao, Y.; Shu, Y.; LaRochelle, J.R.; Steinauer, A.; Thompson, D.; Schepartz, A.; Chen, Z.Y.; Liu, D.R. Discovery and characterization of a peptide that enhances endosomal escape of delivered proteins in vitro and in vivo. J. Am. Chem. Soc. 2015, 137, 14084–14093. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Helenius, A. Virus entry: Open sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J.; van der Goot, F.G. Mechanisms of pathogen entry through the endosomal compartments. Nat. Rev. Mol. Cell Biol. 2006, 7, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Moskaug, J.O.; Sletten, K.; Sandvig, K.; Olsnes, S. Translocation of diphtheria toxin A-fragment to the cytosol. Role of the site of interfragment cleavage. J. Biol. Chem. 1989, 264, 15709–15713. [Google Scholar] [PubMed]
- Shete, H.K.; Prabhu, R.H.; Patravale, V.B. Endosomal escape: A bottleneck in intracellular delivery. J. Nanosci. Nanotechnol. 2014, 14, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.C.; Skehel, J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 1987, 56, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Takeda, K.; Ezawa, R.; Ishii, J.; Ogino, C.; Kondo, A. A display of pH-sensitive fusogenic GALA peptide facilitates endosomal escape from a Bio-nanocapsule via an endocytic uptake pathway. J. Nanobiotechnol. 2014, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Seglen, P.O.; Grinde, B.; Solheim, A.E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur. J. Biochem. 1979, 95, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Niesen, J.; Hehmann-Titt, G.; Woitok, M.; Fendel, R.; Barth, S.; Fischer, R.; Stein, C. A novel fully-human cytolytic fusion protein based on granzyme B shows in vitro cytotoxicity and ex vivo binding to solid tumors overexpressing the epidermal growth factor receptor. Cancer Lett. 2016, 374, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.G.; Firman, P.; Schneider, H. Sodium ion-proton exchange reactions of the carboxylic acid ionophore monensin. J. Am. Chem. Soc. 1985, 107, 4297–4300. [Google Scholar] [CrossRef]
- Grinde, B. Effect of carboxylic ionophores on lysosomal protein degradation in rat hepatocytes. Exp. Cell Res. 1983, 149, 27–35. [Google Scholar] [CrossRef]
- Akiyama, S.; Gottesman, M.M.; Hanover, J.A.; Fitzgerald, D.J.; Willingham, M.C.; Pastan, I. Verapamil enhances the toxicity of conjugates of epidermal growth factor with Pseudomonas exotoxin and antitransferrin receptor with Pseudomonas exotoxin. J. Cell. Physiol. 1984, 120, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R.; FitzGerald, D.J.; Willingham, M.C.; Pastan, I. Enhancement of the activity of immunotoxins made with either ricin A chain or Pseudomonas exotoxin in human ovarian and epidermoid carcinoma cell lines. Cancer Res. 1988, 48, 3919–3923. [Google Scholar] [PubMed]
- Shin, M.C.; Zhao, J.; Zhang, J.; Huang, Y.; He, H.; Wang, M.; Min, K.A.; Yang, V.C. Recombinant TAT-gelonin fusion toxin: Synthesis and characterization of heparin/protamine-regulated cell transduction. J. Biomed. Mater. Res. A 2015, 103, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.C.; Zhang, J.; Min, K.A.; Lee, K.; Moon, C.; Balthasar, J.P.; Yang, V.C. Combination of antibody targeting and PTD-mediated intracellular toxin delivery for colorectal cancer therapy. J. Control. Release 2014, 194, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Bachran, D.; Schneider, S.; Bachran, C.; Urban, R.; Weng, A.; Melzig, M.F.; Hoffmann, C.; Kaufmann, A.M.; Fuchs, H. Epidermal growth factor receptor expression affects the efficacy of the combined application of saponin and a targeted toxin on human cervical carcinoma cells. Int. J. Cancer 2010, 127, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R.; Thakur, M.; von Mallinckrodt, B.; Hug, T.; Wiesner, B.; Eichhorst, J.; Melzig, M.F.; Fuchs, H.; Weng, A. Modified trastuzumab and cetuximab mediate efficient toxin delivery while retaining antibody-dependent cell-mediated cytotoxicity in target cells. Mol. Pharm. 2013, 10, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Jenett-Siems, K.; Gorick, C.; Melzig, M.F. Enhancement of cytotoxicity of ribosome-inactivating-protein type I by saponinum album is not based on stimulation of phagocytosis. J. Pharm. Pharmacol. 2008, 60, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.; Melzig, M.F.; Bachran, C.; Fuchs, H. Enhancement of saporin toxicity against U937 cells by Gypsophila saponins. J. Immunotoxicol. 2008, 5, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Serresi, M.; Bizzarri, R.; Cardarelli, F.; Beltram, F. Real-time measurement of endosomal acidification by a novel genetically encoded biosensor. Anal. Bioanal. Chem. 2009, 393, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Gilabert-Oriol, R. Development of a Platform Technology for Enhanced Endo/Lysosomal Escape of Targeted Toxins by Structurally Specific Oleanane Saponins. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2014. [Google Scholar]
- Shapira, A.; Benhar, I. Toxin-based therapeutic approaches. Toxins 2010, 2, 2519–2583. [Google Scholar] [CrossRef] [PubMed]
- Antignani, A.; Fitzgerald, D. Immunotoxins: The role of the toxin. Toxins 2013, 5, 1486–1502. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kachel, K.; Kim, H.; Malenbaum, S.E.; Collier, R.J.; London, E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 1999, 284, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.K.; Jinno, Y.; FitzGerald, D.; Pastan, I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc. Natl. Acad. Sci. USA 1990, 87, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Boston, R.S. Ribosome-inactivating proteins: A plant perspective. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 785–816. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F.; Battelli, M.G. Ribosome-inactivating proteins: Progress and problems. Cell. Mol. Life Sci. 2006, 63, 1850–1866. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Weyergang, A.; Prasmickaite, L.; Bonsted, A.; Hogset, A.; Strand, M.T.; Wagner, E.; Selbo, P.K. Photochemical internalization (PCI): A technology for drug delivery. Methods Mol. Biol. 2010, 635, 133–145. [Google Scholar] [PubMed]
- Weyergang, A.; Selbo, P.K.; Berstad, M.E.; Bostad, M.; Berg, K. Photochemical internalization of tumor-targeted protein toxins. Lasers Surg. Med. 2011, 43, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Bachran, C.; Li, T.; Heisler, I.; Durkop, H.; Sutherland, M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J. Control. Release 2007, 117, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Pirie, C.M.; Liu, D.V.; Wittrup, K.D. Targeted cytolysins synergistically potentiate cytoplasmic delivery of gelonin immunotoxin. Mol. Cancer Ther. 2013, 12, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Provoda, C.J.; Stier, E.M.; Lee, K.D. Tumor cell killing enabled by listeriolysin O-liposome-mediated delivery of the protein toxin gelonin. J. Biol. Chem. 2003, 278, 35102–35108. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Bomsel, M.; Devilliers, G.; vanderSpek, J.; Murphy, J.R.; Lukianov, E.V.; Olsnes, S.; Boquet, P. Membrane translocation of diphtheria toxin fragment A exploits early to late endosome trafficking machinery. Mol. Microbiol. 1997, 23, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Lahiji, A.; Patel, S.; Franklin, M.; Jimenez, X.; Hicklin, D.J.; Kang, X. Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Anticancer Res. 2007, 27, 3355–3366. [Google Scholar] [PubMed]
- Vu, T.; Claret, F.X. Trastuzumab: Updated mechanisms of action and resistance in breast cancer. Front. Oncol. 2012, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Seita, T.; Kuwabara, T.; Sugiyama, Y. Kinetic analysis of receptor-mediated endocytosis (RME) of proteins and peptides: Use of RME as a drug delivery system. J. Control. Release 1996, 39, 191–200. [Google Scholar] [CrossRef]
- Seidel, U.J.; Schlegel, P.; Lang, P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front. Immunol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, R.; Lund, J. Interaction sites on human IgG-Fc for FcγR: Current models. Immunol. Lett. 2002, 82, 57–65. [Google Scholar] [CrossRef]
- Gilabert-Oriol, R.; Weng, A.; Trautner, A.; Weise, C.; Schmid, D.; Bhargava, C.; Niesler, N.; Wookey, P.J.; Fuchs, H.; Thakur, M. Combinatorial approach to increase efficacy of Cetuximab, Panitumumab and Trastuzumab by dianthin conjugation and co-application of SO1861. Biochem. Pharmacol. 2015, 97, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Heisler, I.; Bachran, D.; Dassler, K.; Ervens, J.; Melzig, M.F.; Fuchs, H. Chimeric toxins inhibit growth of primary oral squamous cell carcinoma cells. Cancer Biol. Ther. 2008, 7, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Schneider, S.; Riese, S.B.; Bachran, D.; Urban, R.; Schellmann, N.; Zahn, C.; Sutherland, M.; Fuchs, H. A lysine-free mutant of epidermal growth factor as targeting moiety of a targeted toxin. Life Sci. 2011, 88, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Weng, A.; Bachran, D.; Riese, S.B.; Schellmann, N.; Melzig, M.F.; Fuchs, H. The distribution of saponins in vivo affects their synergy with chimeric toxins against tumours expressing human epidermal growth factor receptors in mice. Br. J. Pharmacol. 2010, 159, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Deng, W.; Sun, H.; Li, D. Platycodin D2 is a potential less hemolytic saponin adjuvant eliciting Th1 and Th2 immune responses. Int. Immunopharmacol. 2008, 8, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Maes, L. Intravenous and subcutaneous toxicity and absorption kinetics in mice and dogs of the antileishmanial triterpene saponin PX-6518. Molecules 2013, 18, 4803–4815. [Google Scholar] [CrossRef] [PubMed]
- Bachran, C.; Durkop, H.; Sutherland, M.; Bachran, D.; Muller, C.; Weng, A.; Melzig, M.F.; Fuchs, H. Inhibition of tumor growth by targeted toxins in mice is dramatically improved by saponinum album in a synergistic way. J. Immunother. 2009, 32, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Mergel, K.; Weng, A.; von Mallinckrodt, B.; Gilabert-Oriol, R.; Durkop, H.; Melzig, M.F.; Fuchs, H. Targeted tumor therapy by epidermal growth factor appended toxin and purified saponin: An evaluation of toxicity and therapeutic potential in syngeneic tumor bearing mice. Mol. Oncol. 2013, 7, 475–483. [Google Scholar] [CrossRef] [PubMed]
- von Mallinckrodt, B.; Thakur, M.; Weng, A.; Gilabert-Oriol, R.; Durkop, H.; Brenner, W.; Lukas, M.; Beindorff, N.; Melzig, M.F.; Fuchs, H. Dianthin-EGF is an effective tumor targeted toxin in combination with saponins in a xenograft model for colon carcinoma. Future Oncol. 2014, 10, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, Q.; Chen, W.; Wu, M.; Pan, G.; Yang, K.; Li, X.; Man, S.; Teng, Y.; Yu, P.; Gao, W. Chemosensitizing effect of Paris Saponin I on Camptothecin and 10-hydroxycamptothecin in lung cancer cells via p38 MAPK, ERK, and Akt signaling pathways. Eur. J. Med. Chem. 2017, 125, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Du, L.; Jiang, H.; Zhu, X.; Li, J.; Xu, J. Paris Saponin I Sensitizes Gastric Cancer Cell Lines to Cisplatin via Cell Cycle Arrest and Apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 3798–3803. [Google Scholar] [CrossRef]
- Gaumann, A.K.; Kiefer, F.; Alfer, J.; Lang, S.A.; Geissler, E.K.; Breier, G. Receptor tyrosine kinase inhibitors: Are they real tumor killers? Int. J. Cancer 2016, 138, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Alewine, C.; Hassan, R.; Pastan, I. Advances in anticancer immunotoxin therapy. Oncologist 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Bachran, C. Targeted tumor therapies at a glance. Curr. Drug Targets 2009, 10, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yamaizumi, M.; Mekada, E.; Uchida, T.; Okada, Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 1978, 15, 245–250. [Google Scholar] [CrossRef]
- Kurita, K.L.; Linington, R.G. Connecting phenotype and chemotype: High-content discovery strategies for natural products research. J. Nat. Prod. 2015, 78, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Laval, S.; Yu, B. Chemical synthesis of saponins. Adv. Carbohydr. Chem. Biochem. 2014, 71, 137–226. [Google Scholar] [PubMed]
- Fernandez-Tejada, A.; Walkowicz, W.E.; Tan, D.S.; Gin, D.Y. Semisynthesis of Analogues of the Saponin Immunoadjuvant QS-21. Methods Mol. Biol. 2017, 1494, 45–71. [Google Scholar] [PubMed]
- Huang, T.K.; McDonald, K.A. Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol. Adv. 2012, 30, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Lalaleo, L.; Khojasteh, A.; Fattahi, M.; Bonfill, M.; Cusido, R.M.; Palazon, J. Plant Anti-cancer Agents and their Biotechnological Production in Plant Cell Biofactories. Curr. Med. Chem. 2016, 23, 4418–4441. [Google Scholar] [CrossRef] [PubMed]

| Toxin | Ligand | Saponin | GI50 withoutSaponin [nM] | GI50 with Saponin [nM] | Enhancement Factor | Cell Line | Reference |
|---|---|---|---|---|---|---|---|
| Dianthin | EGF | SA1641 | 0.45 | <0.0000001 | >4,000,000 | HER14 | [40] |
| Cetuximab | SO1861 | >10 | 0.0053 | >1886 | HCT116 | [133] | |
| Panitumumab | >10 | 0.0015 | >6666 | HCT116 | |||
| Trastuzumab | >10 | 0.023 | >434 | BT-474 | |||
| Saporin | EGF | Saponinum album | 80 | 0.0011 | 76,000 | PHCC1 | [110] |
| 24.5 | 0.0027 | 9000 | PHCC2 | ||||
| >300 | 0.00012 | >2,500,000 | SiHa | ||||
| 53 | 0.0007 | 75,700 | HeLa | ||||
| 5 | 0.00013 | 38,000 | CaSki | ||||
| 2.5 | 0.0009 | 2800 | HER14 | ||||
| 206 | 0.012 | 17,100 | MDA-MB-435S | ||||
| Adapter-EGF | Quillaja saponin | 2.4 | 0.0017 | 1434 | HER14 | [38] | |
| 27.2 | 0.013 | 2113 | NIH-3T3 | ||||
| Saponinum album | 2.4 | 0.00018 0.00067 | 13,647 3560 | HER14 | [33,38] | ||
| 27.2 135 | 0.014 0.13 | 1977 1050 | NIH-3T3 | ||||
| 1040 | 0.0027 | 385,000 | MCF-7 | [33] | |||
| EGF | SA1641 | 57 | <0.0000001 | >4,000,000 | HER14 | [40] | |
| Rituximab | SO1861 | 7 | 0.01 | 700 | Ramos | [44] | |
| Anti-CD22 | 0.5 | 0.003 | 170 | ||||
| Anti-CD25 | 1 | 0.04 | 25 | ||||
| HB2 | Saponinum album | 139 | 0.31 | 448 | Daudi | [41] | |
| 1000 * | 0.88 * | 1130 | Ramos | ||||
| 0.5 * | 0.003 * | 146 | HSB-2 | ||||
| BU12 | 965 | 0.00003 | 31,500,000 | Daudi | |||
| 1.5 * | 0.000867 * | 1730 | Ramos | ||||
| 250 * | 0.08 * | 3140 | HSB-2 | ||||
| 4KB128 | 0.0139 | 0.0000226 | 615 | Daudi | |||
| 0.15 * | 0.0000331 * | 4520 | Ramos | ||||
| 1000 * | 25 * | 39 | HSB-2 | ||||
| OKT10 | 0.0532 | 0.000222 | 242 | Daudi | |||
| 1 * | 0.000346 * | 2890 | Ramos | ||||
| 0.2 * | 0.0007246 * | 276 | HSB-2 | ||||
| DF1513 | 0.0143 | 0.0000413 | 346 | Daudi | |||
| 0.8 * | 0.000396 * | 2020 | Ramos | ||||
| 0.03 * | 0.0001724 * | 174 | HSB-2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, H.; Niesler, N.; Trautner, A.; Sama, S.; Jerz, G.; Panjideh, H.; Weng, A. Glycosylated Triterpenoids as Endosomal Escape Enhancers in Targeted Tumor Therapies. Biomedicines 2017, 5, 14. https://doi.org/10.3390/biomedicines5020014
Fuchs H, Niesler N, Trautner A, Sama S, Jerz G, Panjideh H, Weng A. Glycosylated Triterpenoids as Endosomal Escape Enhancers in Targeted Tumor Therapies. Biomedicines. 2017; 5(2):14. https://doi.org/10.3390/biomedicines5020014
Chicago/Turabian StyleFuchs, Hendrik, Nicole Niesler, Alexandra Trautner, Simko Sama, Gerold Jerz, Hossein Panjideh, and Alexander Weng. 2017. "Glycosylated Triterpenoids as Endosomal Escape Enhancers in Targeted Tumor Therapies" Biomedicines 5, no. 2: 14. https://doi.org/10.3390/biomedicines5020014