T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation
Abstract
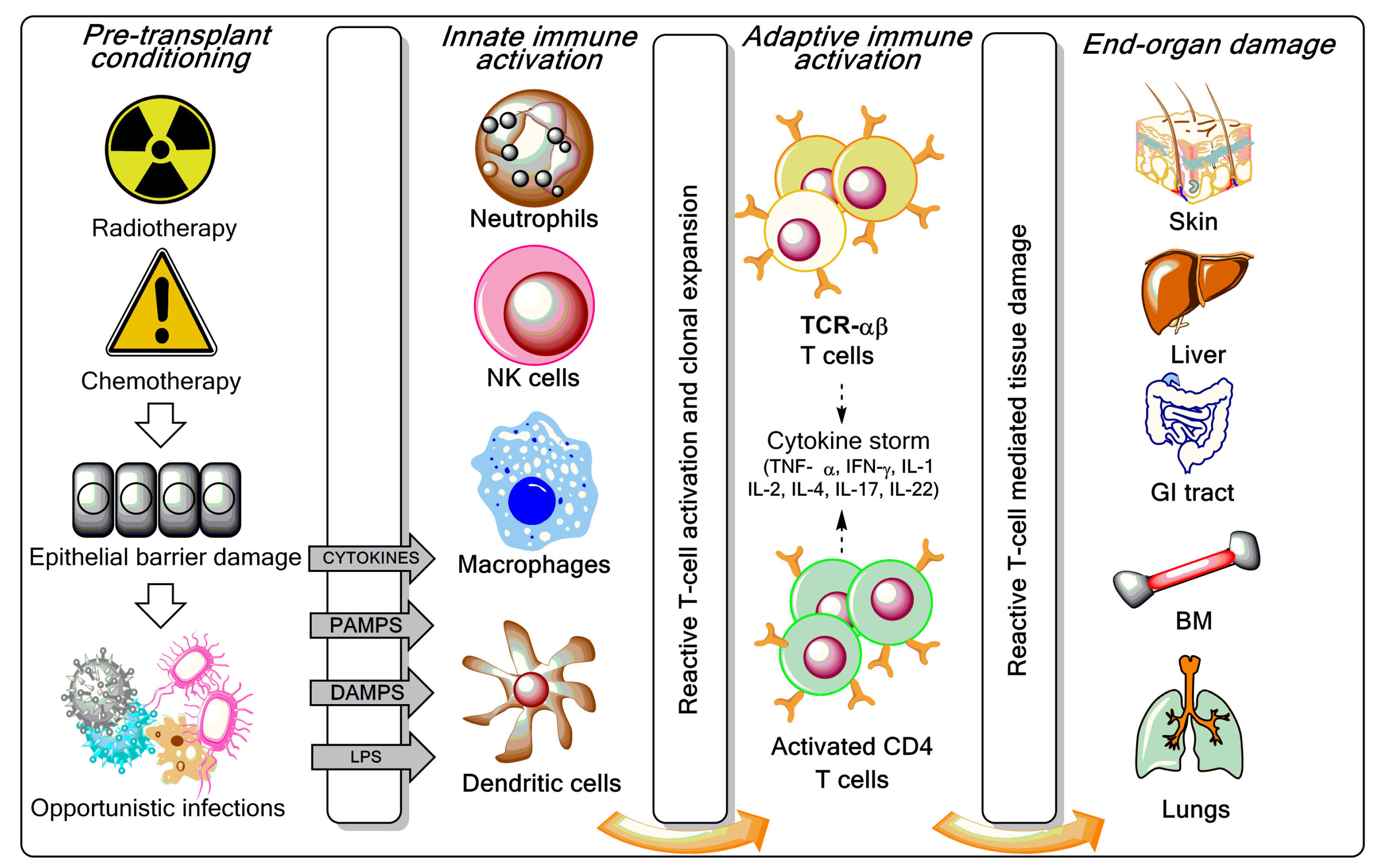
:1. Introduction
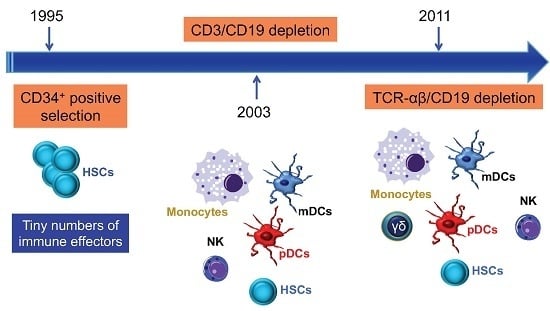
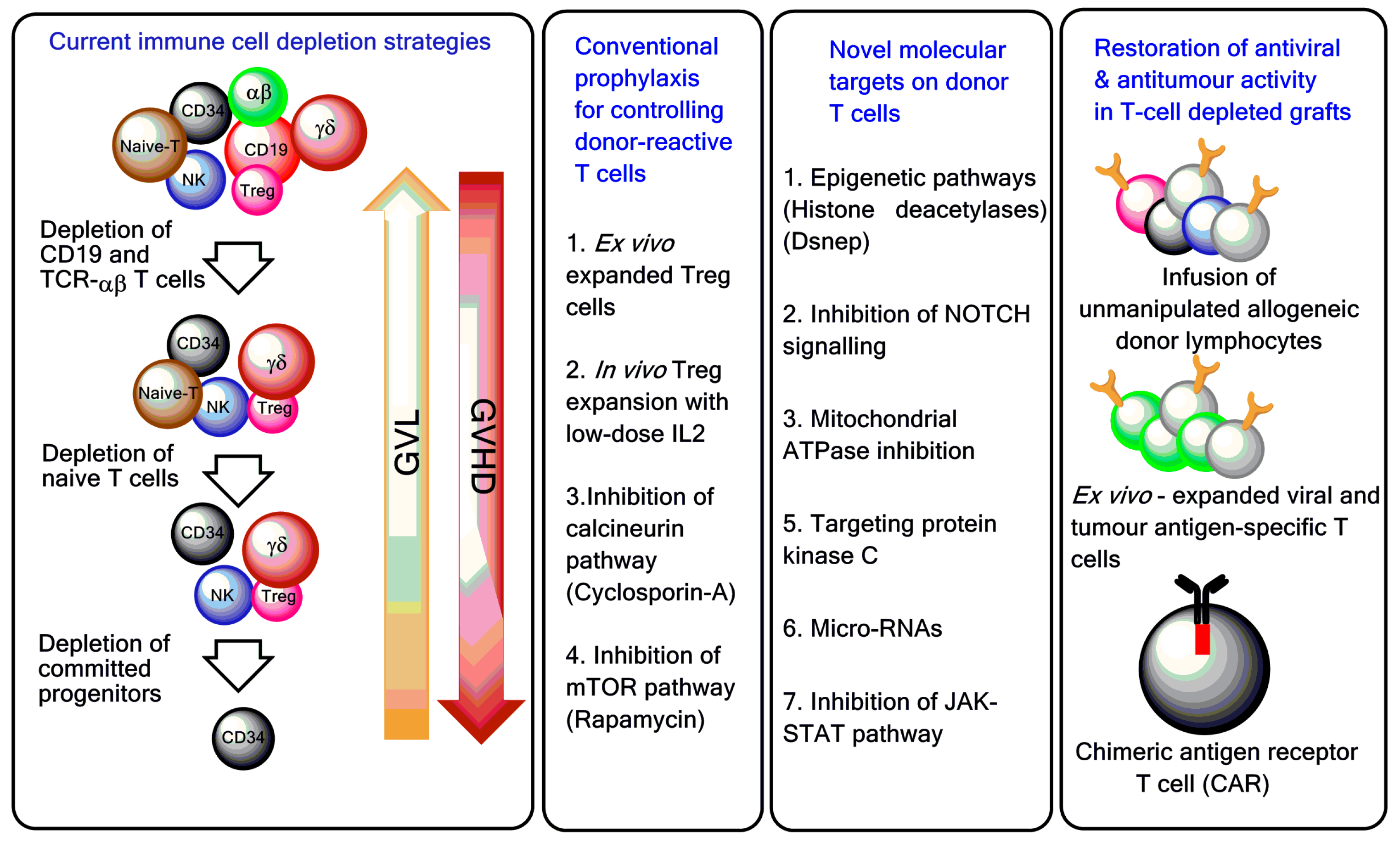
2. Depletion of T-Cell Subpopulations
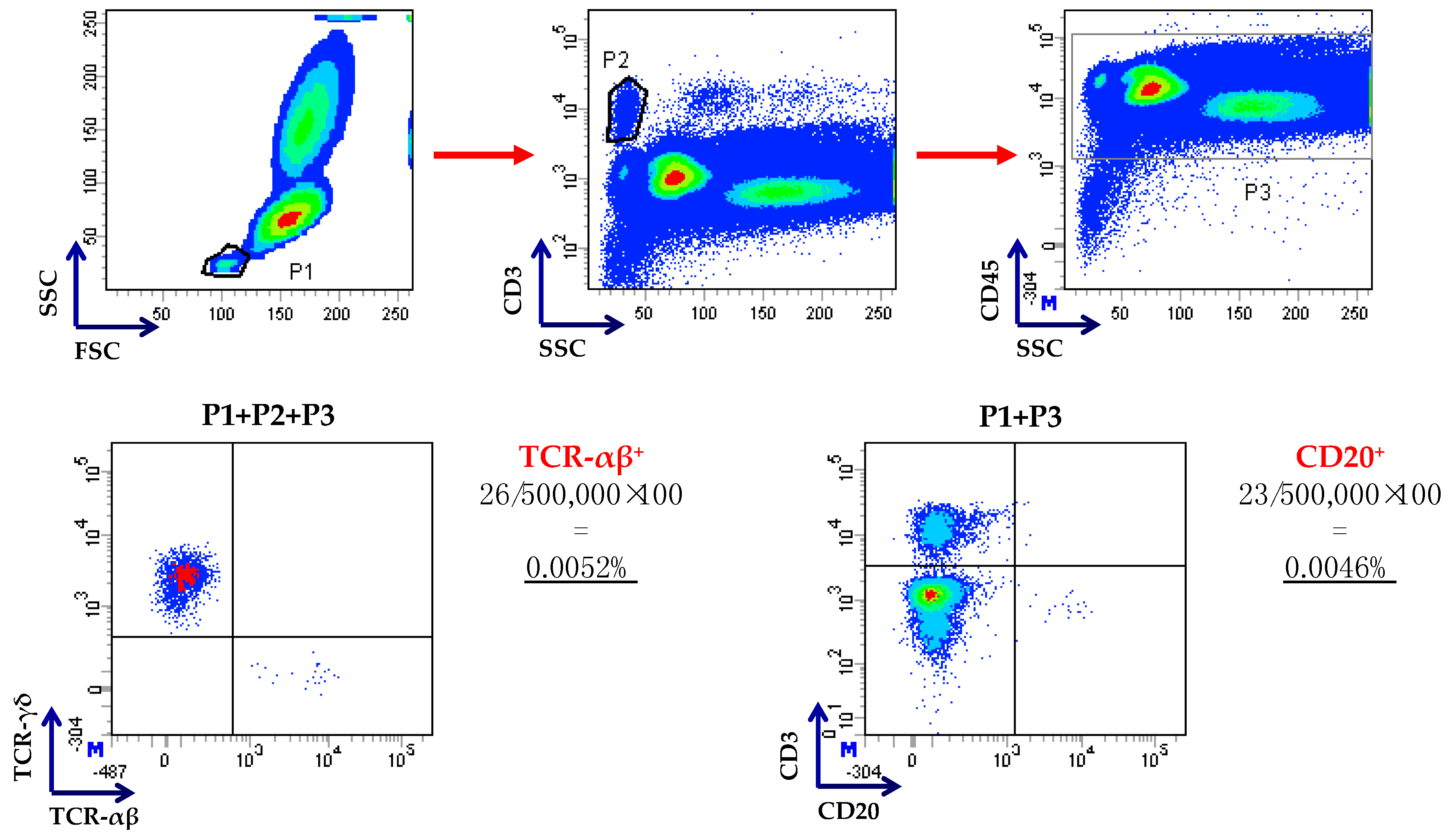
2.1. Depletion of T-Cell Receptor (TCR)-αβ T Cells and CD19+ B Cells
2.2. Depletion of Naïve T Cells
2.3. Depletion of Alloreactive T Cells
3. Treatment of Donors to Prevent Graft-Versus-Host Disease (GVHD)
3.1. Granulocyte Colony-Stimulating Factor (G-CSF)
3.2. Low and Ultra-Low Dose IL-2
4. T-Cell Depleted (TCD) Haploidentical Haematopoietic Stem Cell Transplantation (HSCT) as a Platform for Adoptive Immunotherapy
4.1. Suicide Gene-Transduced T Cells to Provide Graft-Versus-Leukaemia (GVL) Responses without GVHD
4.2. Virus-Specific T Cells
5. Novel Strategies for Controlling GVHD
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ballen, K.K.; Koreth, J.; Chen, Y.B.; Dey, B.R.; Spitzer, T.R. Selection of optimal alternative graft source: Mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood 2012, 119, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Kanakry, C.G.; Fuchs, E.J.; Luznik, L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat. Rev. Clin. Oncol. 2016, 13, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, W.D. Graft-versus-host disease. Nat. Rev. Immunol. 2007, 7, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Im, A.; Hakim, F.T.; Pavletic, S.Z. Novel targets in the treatment of chronic graft-versus-host disease. Leukemia 2017, 31, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Pearce, R.M.; Lee, J.; Kirkland, K.; Gilleece, M.; Veys, P.; Clark, R.E.; Kazmi, M.; Abinun, M.; Jackson, G.H.; et al. Haematopoietic stem cell transplantation (HSCT) in severe autoimmune diseases: Analysis of UK outcomes from the British Society of Blood and Marrow Transplantation (BSBMT) data registry 1997–2009. Br. J. Haematol. 2012, 157, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin. Oncol. 2012, 39, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Filippini, P.; Rutella, S. Recent advances on cellular therapies and immune modulators for graft-versus-host disease. Expert Rev. Clin. Immunol. 2014, 10, 1357–1374. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Ferrara, J.L.; Levine, J.E. Advances in predicting acute GVHD. Br. J. Haematol. 2013, 160, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.J.; Koehne, G.; Hasan, A.N.; Doubrovina, E.; Prockop, S. T-cell depleted allogeneic hematopoietic cell transplants as a platform for adoptive therapy with leukemia selective or virus-specific T-cells. Bone Marrow Transpl. 2015, 50, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Or-Geva, N.; Reisner, Y. The evolution of T-cell depletion in haploidentical stem-cell transplantation. Br. J. Haematol. 2016, 172, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Aversa, F.; Martelli, M.F.; Velardi, A. Haploidentical hematopoietic stem cell transplantation with a megadose T-cell-depleted graft: Harnessing natural and adaptive immunity. Semin. Oncol. 2012, 39, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Schumm, M.; Lang, P.; Bethge, W.; Faul, C.; Feuchtinger, T.; Pfeiffer, M.; Vogel, W.; Huppert, V.; Handgretinger, R. Depletion of T-cell receptor ab and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy 2013, 15, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Teltschik, H.M.; Feuchtinger, T.; Muller, I.; Pfeiffer, M.; Schumm, M.; Ebinger, M.; Schwarze, C.P.; Gruhn, B.; Schrauder, A.; et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br. J. Haematol. 2014, 165, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, A.; Merli, P.; Rutella, S.; Pagliara, D.; Bernardo, M.E.; Masetti, R.; Pende, D.; Falco, M.; Handgretinger, R.; Moretta, F.; et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B-cells in children with non-malignant disorders. Blood 2014, 124, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Feucht, J.; Opherk, K.; Lang, P.; Kayser, S.; Hartl, L.; Bethge, W.; Matthes-Martin, S.; Bader, P.; Albert, M.H.; Maecker-Kolhoff, B.; et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015, 125, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Maschan, M.; Shelikhova, L.; Ilushina, M.; Kurnikova, E.; Boyakova, E.; Balashov, D.; Persiantseva, M.; Skvortsova, Y.; Laberko, A.; Muzalevskii, Y.; et al. TCR-αβ and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transpl. 2016, 51, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Balashov, D.; Shcherbina, A.; Maschan, M.; Trakhtman, P.; Skvortsova, Y.; Shelikhova, L.; Laberko, A.; Livshits, A.; Novichkova, G.; Maschan, A.; et al. Single-center experience of unrelated and haploidentical stem cell transplantation with TCR-αβ and CD19 depletion in children with primary immunodeficiency syndromes. Biol. Blood Marrow Transpl. 2015, 21, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.; Feuchtinger, T.; Teltschik, H.M.; Schwinger, W.; Schlegel, P.; Pfeiffer, M.; Schumm, M.; Lang, A.M.; Lang, B.; Schwarze, C.P.; et al. Improved immune recovery after transplantation of TCR-αβ/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transpl. 2015, 50, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Filippini, P.; Bertaina, V.; Li Pira, G.; Altomare, L.; Ceccarelli, S.; Brescia, L.P.; Lucarelli, B.; Girolami, E.; Conflitti, G.; et al. Mobilization of healthy donors with plerixafor affects the cellular composition of T-cell receptor (TCR)-αβ/CD19-depleted haploidentical stem cell grafts. J. Transl. Med. 2014, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Kaynar, L.; Demir, K.; Turak, E.E.; Ozturk, C.P.; Zararsiz, G.; Gonen, Z.B.; Gokahmetoglu, S.; Sivgin, S.; Eser, B.; Koker, Y.; et al. TCR-αβ-depleted haploidentical transplantation results in adult acute leukemia patients. Hematology 2017, 22, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Merli, P.; Pagliara, D.; Li Pira, G.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood 2017. [Google Scholar] [CrossRef] [PubMed]
- Bremm, M.; Cappel, C.; Erben, S.; Jarisch, A.; Schumm, M.; Arendt, A.; Bonig, H.; Klingebiel, T.; Koehl, U.; Bader, P.; et al. Generation and flow cytometric quality control of clinical-scale TCR-αβ/CD19-depleted grafts. Cytom. B Clin. Cytom. 2017, 92, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.E.; McNiff, J.; Yan, J.; Doyle, H.; Mamula, M.; Shlomchik, M.J.; Shlomchik, W.D. Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Investig. 2003, 112, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, M.; Heimfeld, S.; Loeb, K.R.; Jones, L.A.; Chaney, C.; Seropian, S.; Gooley, T.A.; Sommermeyer, F.; Riddell, S.R.; Shlomchik, W.D. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J. Clin. Investig. 2015, 125, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, M.; Heimfeld, S.; Jones, L.A.; Turtle, C.; Krause, D.; Riddell, S.R.; Shlomchik, W. Engineering human peripheral blood stem cell grafts that are depleted of naive T cells and retain functional pathogen-specific memory T cells. Biol. Blood Marrow Transpl. 2014, 20, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; Nunes, R.; Rezvani, K.; Fellowes, V.S.; Venne, A.; Solomon, S.R.; Fan, Y.; Gostick, E.; Price, D.A.; Scotto, C.; et al. A clinical-scale selective allodepletion approach for the treatment of HLA-mismatched and matched donor-recipient pairs using expanded T lymphocytes as antigen-presenting cells and a TH9402-based photodepletion technique. Blood 2008, 111, 4392–4402. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; McIver, Z.A.; Shenoy, A.; Fellowes, V.; Khuu, H.; Stroncek, D.F.; Leitman, S.F.; Childs, R.; Battiwalla, M.; Koklanaris, E.; et al. Selectively T cell-depleted allografts from HLA-matched sibling donors followed by low-dose posttransplantation immunosuppression to improve transplantation outcome in patients with hematologic malignancies. Biol. Blood Marrow Transpl. 2011, 17, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- McIver, Z.A.; Melenhorst, J.J.; Grim, A.; Naguib, N.; Weber, G.; Fellowes, V.; Khuu, H.; Stroncek, D.S.; Leitman, S.F.; Battiwalla, M.; et al. Immune reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol. Blood Marrow Transpl. 2011, 17, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.C.; Lachance, S.; Roy, J.; Walker, I.; Maerten, J.; Delisle, J.S.; Foley, S.R.; Lewalle, P.; Olavarria, E.; Selleslag, D.; et al. Donor lymphocytes depleted of alloreactive T cells (ATIR101) improve event-free survival (GRFS) and overall survival in a T-cell depleted haploidentical HSCT: Phase 2 trial in patients with AML and ALL. Blood 2016, 128, 1226. [Google Scholar]
- Shook, D.R.; Triplett, B.M.; Eldridge, P.W.; Kang, G.; Srinivasan, A.; Leung, W. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatr. Blood Cancer 2015, 62, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Triplett, B.M.; Shook, D.R.; Eldridge, P.; Li, Y.; Kang, G.; Dallas, M.; Hartford, C.; Srinivasan, A.; Chan, W.K.; Suwannasaen, D.; et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transpl. 2015, 50, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.M.; O’Donnell, P.V.; Fuchs, E.J.; Luznik, L. Haploidentical bone marrow and stem cell transplantation: Experience with post-transplantation cyclophosphamide. Semin. Hematol. 2016, 53, 90–97. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.V.; Luznik, L.; Jones, R.J.; Vogelsang, G.B.; Leffell, M.S.; Phelps, M.; Rhubart, P.; Cowan, K.; Piantados, S.; Fuchs, E.J.; et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol. Blood Marrow Transpl. 2002, 8, 377–386. [Google Scholar] [CrossRef]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transpl. 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.R.; Kanakry, J.A.; Showel, M.M.; Tsai, H.L.; Bolanos-Meade, J.; Rosner, G.L.; Kanakry, C.G.; Perica, K.; Symons, H.J.; Brodsky, R.A.; et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 2015, 125, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Kanakry, C.G.; O’Donnell, P.V.; Furlong, T.; de Lima, M.J.; Wei, W.; Medeot, M.; Mielcarek, M.; Champlin, R.E.; Jones, R.J.; Thall, P.F.; et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J. Clin. Oncol. 2014, 32, 3497–3505. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.M.; Dominietto, A.; Ghiso, A.; di Grazia, C.; Lamparelli, T.; Gualandi, F.; Bregante, S.; van Lint, M.T.; Geroldi, S.; Luchetti, S.; et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol. Blood Marrow Transpl. 2013, 19, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Dominietto, A.; Ghiso, A.; di Grazia, C.; Lamparelli, T.; Gualandi, F.; Bregante, S.; van Lint, M.T.; Geroldi, S.; Luchetti, S.; et al. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: An update. Bone Marrow Transpl. 2015, 50, S37–S39. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Pierelli, L.; Bonanno, G.; Sica, S.; Ameglio, F.; Capoluongo, E.; Mariotti, A.; Scambia, G.; d’Onofrio, G.; Leone, G.; et al. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood 2002, 100, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Pierelli, L.; Mariotti, A.; Capoluongo, E.; Contemi, A.M.; Ameglio, F.; Curti, A.; de Ritis, D.G.; Voso, M.T.; et al. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-α. Eur. J. Immunol. 2004, 34, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Hill, G.R.; Pan, L.; Gerbitz, A.; Teshima, T.; Brinson, Y.; Ferrara, J.L. G-CSF modulates cytokine profile of dendritic cells and decreases acute graft-versus-host disease through effects on the donor rather than the recipient. Transplantation 2000, 69, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Geyer, S.; Bingman, A.; Auletta, J.J.; Jaglowski, S.; Elder, P.; Donnell, L.O.; Bradbury, H.; Kitzler, R.; Andritsos, L.; et al. Granulocyte colony-stimulating factor-mobilized allografts contain activated immune cell subsets associated with risk of acute and chronic graft-versus-host disease. Biol. Blood Marrow Transpl. 2016, 22, 658–668. [Google Scholar] [CrossRef] [PubMed]
- D’Aveni, M.; Rossignol, J.; Coman, T.; Sivakumaran, S.; Henderson, S.; Manzo, T.; Santos e Sousa, P.; Bruneau, J.; Fouquet, G.; Zavala, F.; et al. G-CSF mobilizes CD34+ regulatory monocytes that inhibit graft-versus-host disease. Sci. Transl. Med. 2015, 7, 281ra242. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, A.; Gimondi, S.; Bermema, A.; Longoni, P.; Rizzitano, S.; Corradini, P.; Carniti, C. Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol. Blood Marrow Transpl. 2014, 20, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Pessach, I.; Resnick, I.; Shimoni, A.; Nagler, A. G-CSF-primed BM for allogeneic SCT: Revisited. Bone Marrow Transpl. 2015, 50, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.X.; Jun, C.Y.; Yu, Z.X. A direct comparison of immunological characteristics of granulocyte colony-stimulating factor (G-CSF)-primed bone marrow grafts and G-CSF-mobilized peripheral blood grafts. Haematologica 2005, 90, 715–716. [Google Scholar] [PubMed]
- Huang, X.J.; Liu, D.H.; Liu, K.Y.; Xu, L.P.; Chen, H.; Han, W.; Chen, Y.H.; Zhang, X.H.; Lu, D.P. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol. Blood Marrow Transpl. 2009, 15, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Chang, Y.J.; Zhao, X.Y. Maintaining hyporesponsiveness and polarization potential of T cells after in vitro mixture of G-CSF mobilized peripheral blood grafts and G-CSF primed bone marrow grafts in different proportions. Transpl. Immunol. 2007, 17, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Bollard, C.M.; Carlsten, M.; Melenhorst, J.J.; Biancotto, A.; Wang, E.; Chen, J.; Kotliarov, Y.; Cheung, F.; Xie, Z.; et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol. Ther. 2014, 22, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhao, X.S.; Wang, Y.T.; Chen, Y.H.; Xu, L.P.; Zhang, X.H.; Han, W.; Chen, H.; Wang, Y.; Yan, C.H.; et al. Prophylactic use of low-dose interleukin-2 and the clinical outcomes of hematopoietic stem cell transplantation: A randomized study. Oncoimmunology 2016, 5, e1250992. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Matsuoka, K.; Kim, H.T.; McDonough, S.M.; Bindra, B.; Alyea, E.P., 3rd; Armand, P.; Cutler, C.; Ho, V.T.; Treister, N.S.; et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 2011, 365, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, M.; Li Pira, G.; Amodeo, A.; Cecchetti, C.; Giorda, E.; Ceccarelli, S.; Brescia, L.P.; Pirozzi, N.; Rutella, S.; Locatelli, F.; et al. Adoptive immunotherapy with antigen-specific T cells during extracorporeal membrane oxygenation (ECMO) for adenovirus-related respiratory failure in a child given haploidentical stem cell transplantation. Pediatr. Blood Cancer 2014, 61, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Tyler, E.M.; Jungbluth, A.A.; O’Reilly, R.J.; Koehne, G. WT1-specific T-cell responses in high-risk multiple myeloma patients undergoing allogeneic T cell-depleted hematopoietic stem cell transplantation and donor lymphocyte infusions. Blood 2013, 121, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, A.G.; Ragnarsson, G.B.; Nguyen, H.N.; Chaney, C.N.; Pufnock, J.S.; Schmitt, T.M.; Duerkopp, N.; Roberts, I.M.; Pogosov, G.L.; Ho, W.Y.; et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci. Transl. Med. 2013, 5, 174ra127. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, F.; Bonini, C.; Stanghellini, M.T.; Bondanza, A.; Traversari, C.; Salomoni, M.; Turchetto, L.; Colombi, S.; Bernardi, M.; Peccatori, J.; et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): A non-randomised phase I-II study. Lancet Oncol. 2009, 10, 489–500. [Google Scholar] [CrossRef]
- Zhou, X.; di Stasi, A.; Tey, S.K.; Krance, R.A.; Martinez, C.; Leung, K.S.; Durett, A.G.; Wu, M.F.; Liu, H.; Leen, A.M.; et al. Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood 2014, 123, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dotti, G.; Krance, R.A.; Martinez, C.A.; Naik, S.; Kamble, R.T.; Durett, A.G.; Dakhova, O.; Savoldo, B.; di Stasi, A.; et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015, 125, 4103–4113. [Google Scholar] [CrossRef] [PubMed]
- Tzannou, I.; Leen, A.M. Preventing stem cell transplantation-associated viral infections using T-cell therapy. Immunotherapy 2015, 7, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Bollard, C.M.; Mendizabal, A.M.; Shpall, E.J.; Szabolcs, P.; Antin, J.H.; Kapoor, N.; Pai, S.Y.; Rowley, S.D.; Kebriaei, P.; et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013, 121, 5113–5123. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Maeda, Y.; Hotary, K.; Liu, C.; Reznikov, L.L.; Dinarello, C.A.; Ferrara, J.L. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl. Acad. Sci. USA 2004, 101, 3921–3926. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Sun, Y.; Toubai, T.; Duran-Struuck, R.; Clouthier, S.G.; Weisiger, E.; Maeda, Y.; Tawara, I.; Krijanovski, O.; Gatza, E.; et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J. Clin. Investig. 2008, 118, 2562–2573. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Okiyama, N.; Villarroel, V.A.; Katz, S.I. Histone deacetylase 6 inhibition impairs effector CD8 T-cell functions during skin inflammation. J. Allergy Clin. Immunol. 2015, 135, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Kato, K.; Xie, F.; Varambally, S.; Mineishi, S.; Kuick, R.; Mochizuki, K.; Liu, Y.; Nieves, E.; et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood 2012, 119, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Xu, M.; Rong, R.; Wang, J.; Zhu, T. Inhibition of histone methyltransferase EZH2 ameliorates early acute renal allograft rejection in rats. BMC Immunol. 2016, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Sandy, A.R.; Chung, J.; Toubai, T.; Shan, G.T.; Tran, I.T.; Friedman, A.; Blackwell, T.S.; Reddy, P.; King, P.D.; Maillard, I. T cell-specific notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J. Immunol. 2013, 190, 5818–5828. [Google Scholar] [CrossRef] [PubMed]
- Gatza, E.; Wahl, D.R.; Opipari, A.W.; Sundberg, T.B.; Reddy, P.; Liu, C.; Glick, G.D.; Ferrara, J.L. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl. Med. 2011, 3, 67ra68. [Google Scholar] [CrossRef] [PubMed]
- Haarberg, K.M.; Li, J.; Heinrichs, J.; Wang, D.; Liu, C.; Bronk, C.C.; Kaosaard, K.; Owyang, A.M.; Holland, S.; Masuda, E.; et al. Pharmacologic inhibition of PKCα and PKCθ prevents GVHD while preserving GVL activity in mice. Blood 2013, 122, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cooper, M.L.; Alahmari, B.; Ritchey, J.; Collins, L.; Holt, M.; di Persio, J.F. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS ONE 2014, 9, e109799. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oravecz-Wilson, K.; Mathewson, N.; Wang, Y.; McEachin, R.; Liu, C.; Toubai, T.; Wu, J.; Rossi, C.; Braun, T.; et al. Mature T cell responses are controlled by microRNA-142. J. Clin. Investig. 2015, 125, 2825–2840. [Google Scholar] [CrossRef] [PubMed]
- Furlan, S.N.; Watkins, B.; Tkachev, V.; Flynn, R.; Cooley, S.; Ramakrishnan, S.; Singh, K.; Giver, C.; Hamby, K.; Stempora, L.; et al. Transcriptome analysis of GVHD reveals aurora kinase A as a targetable pathway for disease prevention. Sci. Transl. Med. 2015, 7, 315ra191. [Google Scholar] [CrossRef] [PubMed]
| Patients | Disease | Graft-versus-Host Disease (GVHD) Prophylaxis | Acute/Chronic GVHD | TRM | EFS(DFS)/OS | Reference |
|---|---|---|---|---|---|---|
| 28 | HR-AML | FK506, MTX | 39%/30% | 10% | 60%/67% (2 years) | [19] |
| 37 | PID | FK506, MTX; FK506, MMF; CYA, MTX | 22% | 3.3% (27% GF) | 96.7% (15 months) | [20] |
| 41 | AL | MMF | 10%/9% | N.A. | 21/41 patients alive after 1.6 years | [21] |
| 23 | Non-malignant | None | 13%/0% | 9.3% | 91% (2 years) | [16,22] |
| 34 | HR-AL | N.A. | 5.9%/6.1% | 14.7% | 42%/54% (1 year) | [23] |
| 80 | AL | None | 30%; no extensive chronic GVHD | 5% | 71%/72% (5 years) | [24] |
| Patients | Disease | Graft-Versus-Host Disease (GVHD) Prophylaxis | Acute/Chronic GVHD | TRM | EFS(DFS)/OS | Reference |
|---|---|---|---|---|---|---|
| 35 | High-risk leukaemia | Tacrolimus | 66%; 9% | 9% | 70%/78% (2 years) | [29] |
| 8 | Solid tumours | Sirolimus | No acute GVHD or GF | 1 patient died of sinusoidal obstruction syndrome | N.A. (median follow-up was 184 days) | [34] |
| 17 | Haematological malignancies | Sirolimus and MMF | 17.6% grades III–IV acute GVHD/6 patients with signs of oral or skin chronic GVHD | 11.7% | 76.5% of patients alive at a median of 223 days after haematopoietic stem cell transplantation (HSCT) | [35] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadakekolathu, J.; Rutella, S. T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation. Biomedicines 2017, 5, 33. https://doi.org/10.3390/biomedicines5020033
Vadakekolathu J, Rutella S. T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation. Biomedicines. 2017; 5(2):33. https://doi.org/10.3390/biomedicines5020033
Chicago/Turabian StyleVadakekolathu, Jayakumar, and Sergio Rutella. 2017. "T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation" Biomedicines 5, no. 2: 33. https://doi.org/10.3390/biomedicines5020033