In Vitro Testing of Crude Natural Plant Extracts from Costa Rica for Their Ability to Boost Innate Immune Cells against Staphylococcus aureus
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Plant Extracts
2.2. Bacteria
2.3. Isolation and Preparation of Monocytes and Neutrophils
2.4. Whole Blood, Monocyte and Neutrophil Killing Assay
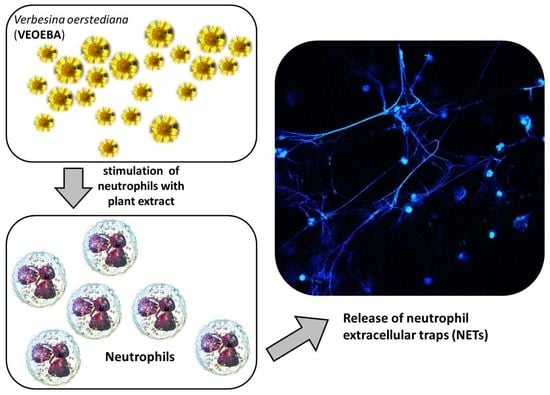
2.5. Neutrophil Extracellular Traps Induction Assay
2.6. NET Visualization and Quantification
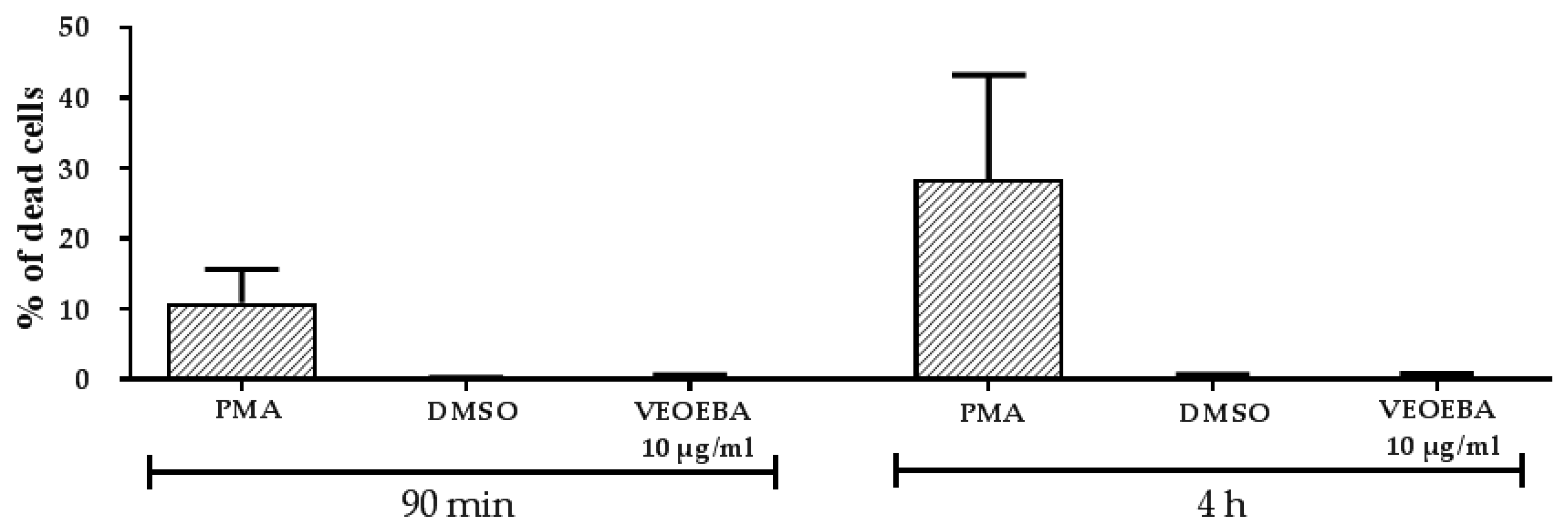
2.7. Cytotoxicity Test
2.8. Statistical Analysis
3. Results and Discussion
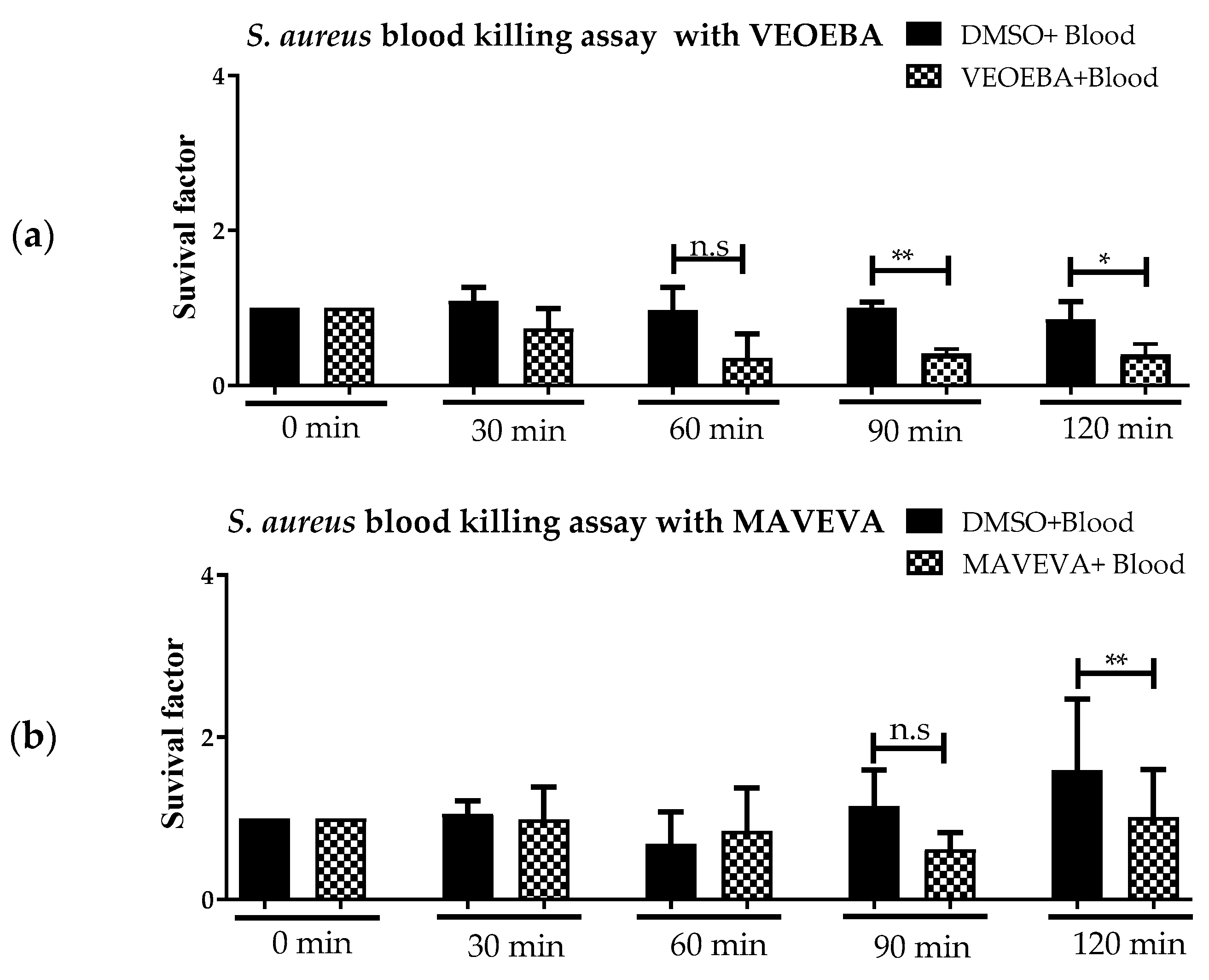
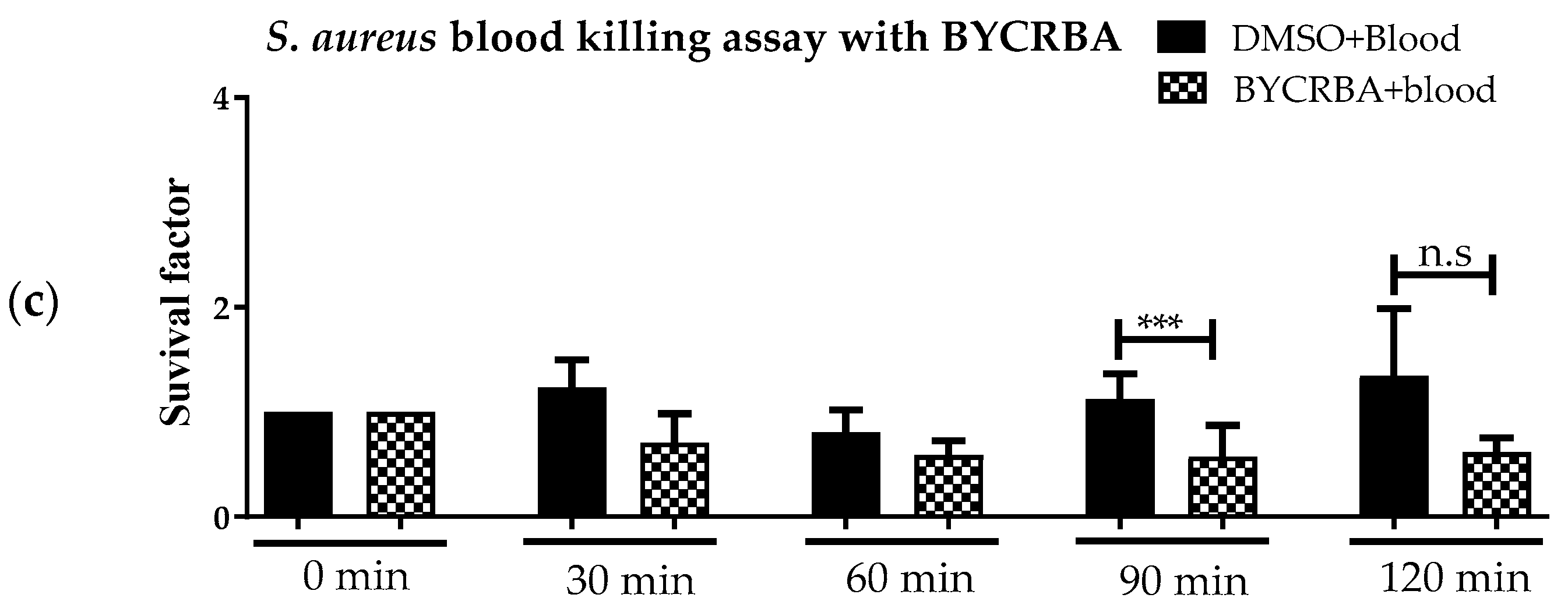
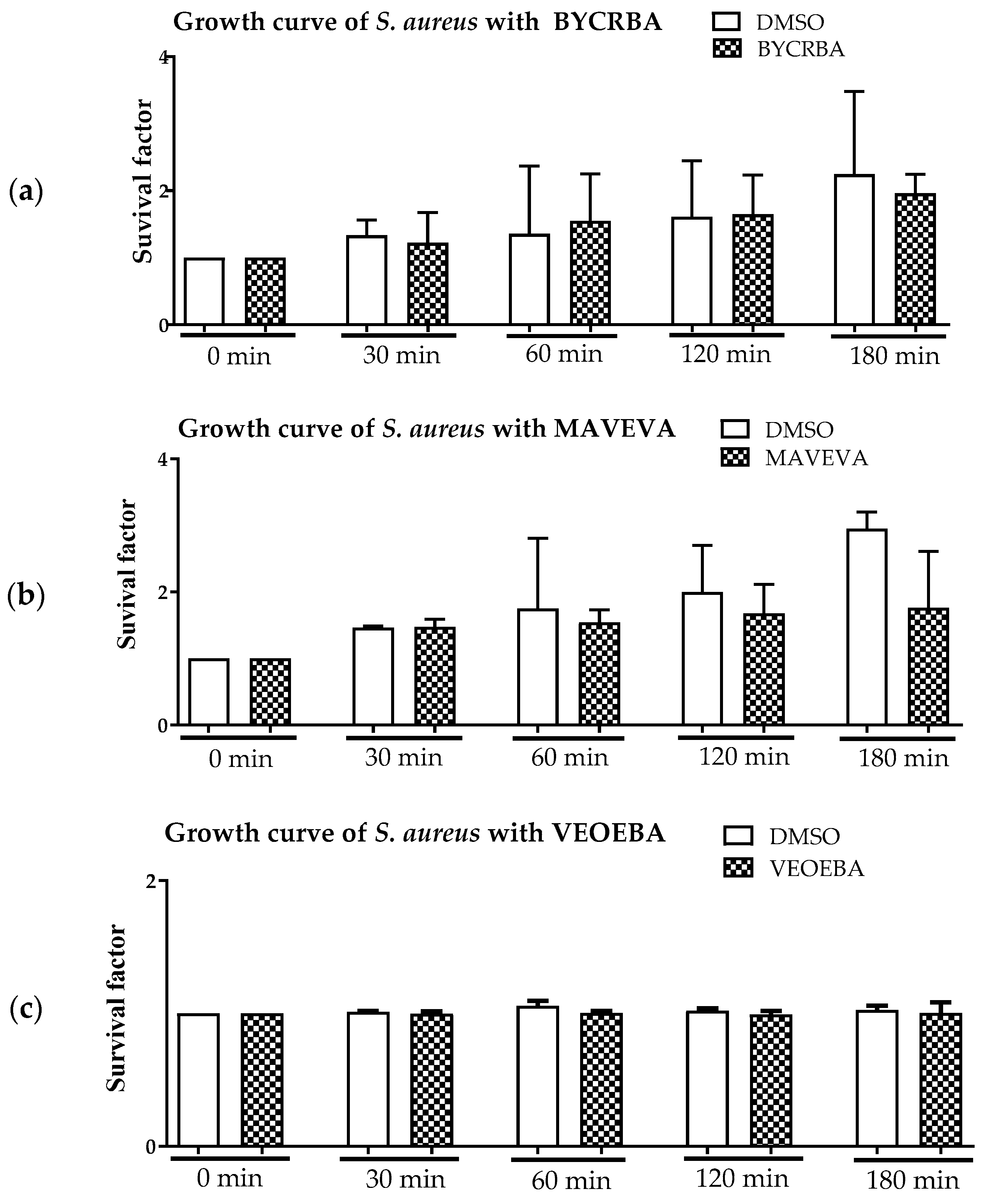
3.1. Effect of Plant Extracts on Antimicrobial Activity of Blood
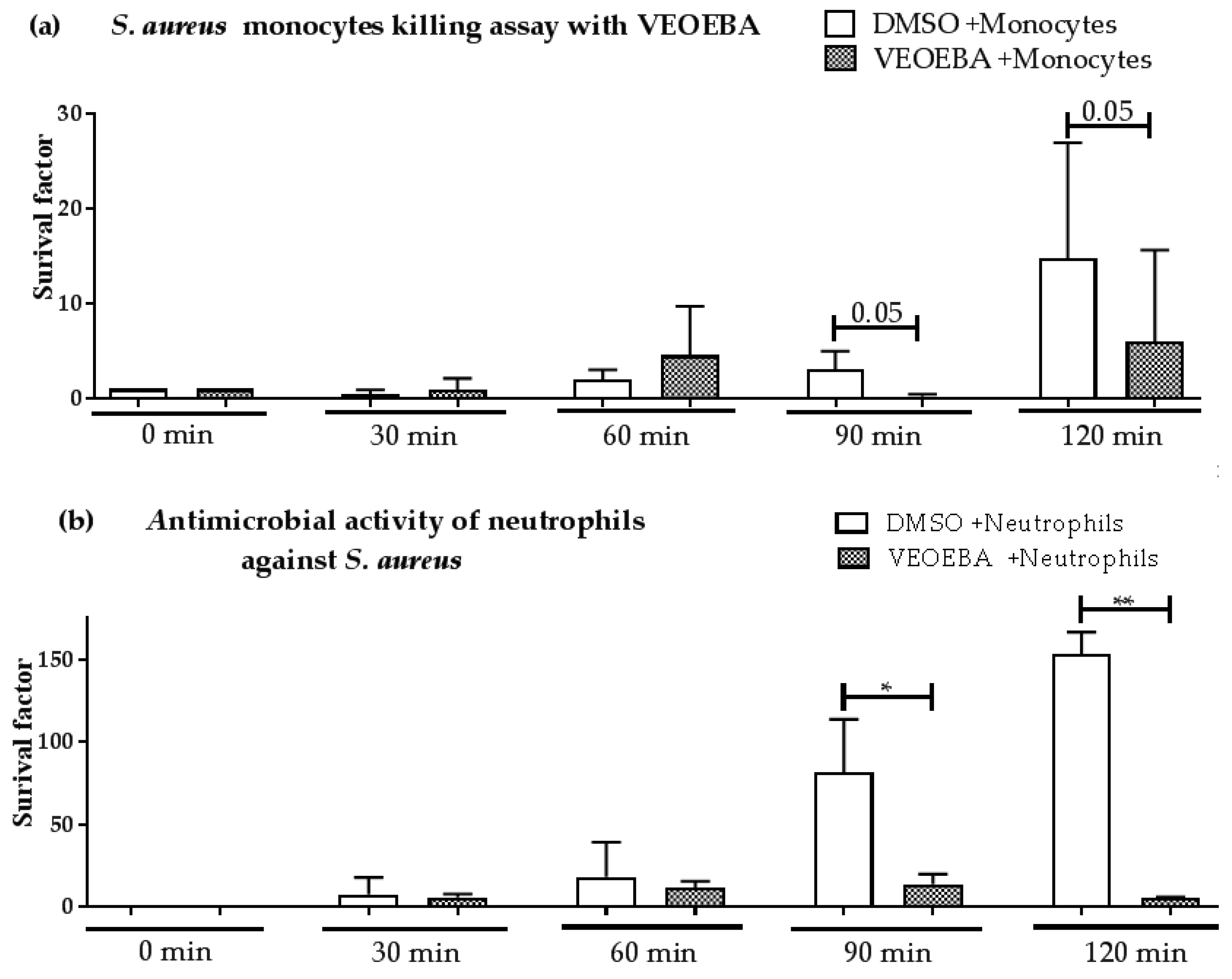
3.2. Effect of Plant Extracts on Antimicrobial Activity of Monocytes and Neutrophils
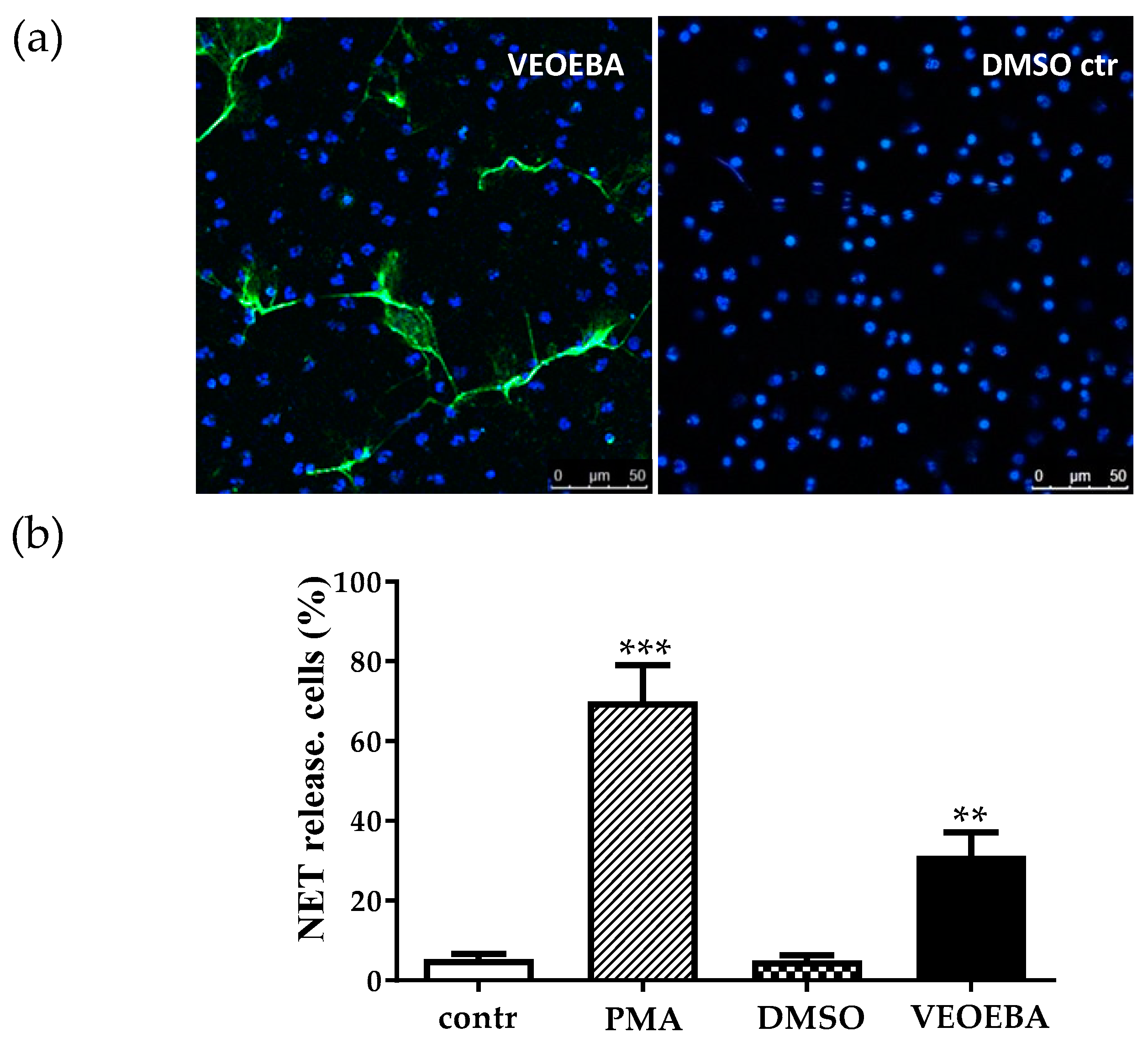
3.3. Effect of Plant Extracts on Formation of Neutrophil Extracellular Traps (NETs)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okumura, C.Y.; Nizet, V. Subterfuge and sabotage: Evasion of host innate defenses by invasive gram-positive bacterial pathogens. Annu. Rev. Microbiol. 2014, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Uhlemann, A.C.; Otto, M.; Lowy, F.D.; DeLeo, F.R. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. 2013, 21, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Rağbetli, C.; Parlak, M.; Bayram, Y.; Guducuoglu, H.; Ceylan, N. Evaluation of antimicrobial resistance in Staphylococcus aureus isolates by years. Interdiscip. Perspect. Infect. Dis. 2016, 9171395. [Google Scholar] [CrossRef]
- Kumari, J.; Shenoy, S.M.; Baliga, S.; Chakrapani, M.; Bhat, G.K. Healthcare-Associated Methicillin-Resistant Staphylococcus aureus. Clinical characteristics and antibiotic resistance profile with emphasis on macrolide-lincosamide-streptogramin B resistance. Sultan Qaboos Univ. Med. J. 2016, 16, e175–e181. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.W.; Newman, P.M.; Ocheltree, S.; Beaty, R.; Hassoun, A. Ceftaroline fosamil use in 2 pediatric patients with invasive methicillin-resistant Staphylococcus aureus infections. J. Pediatr. Pharmacol. Ther. 2015, 20, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Jerjomiceva, N.; Seri, H.; Yaseen, R.; de Buhr, N.; Setzer, W.N.; Naim, H.Y.; von Köckritz-Blickwede, M. Guarea kunthiana bark extract enhances the antimicrobial activities of human and bovine neutrophils. Nat. Prod. Commun. 2016, 11, 767–770. [Google Scholar] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Blood cells and endothelial barrier function. Tissue Barriers 2015, 3, e978720. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 3, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Hollands, A.; Corriden, R.; Gysler, G.; Dahesh, S.; Olson, J.; Ali, S.R.; Kunkel, M.T.; Lin, A.E.; Forli, S.; Newton, A.C.; et al. Natural product anacardic acid from cashew nut shells stimulates neutrophil extracellular trap production and bactericidal activity. J. Biol. Chem. 2016, 291, 13964–13973. [Google Scholar] [CrossRef] [PubMed]
- Duthie, E.S.; Lorenz, L.L. Staphylococcal coagulase: Mode of action and antigenicity. J. Gen. Microbiol. 1952, 6, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.A.; von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 2010, 9, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 10, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Ramirez, A.; Perez-Gutierrez, R.M.; Garcia-Baez, E.; Mota-Flores, J.M. Antimicrobial activities of diterpene labdane from seeds of Byrsonima crassifolia. Bol. Latinoam. Caribe Plantas Med. Aromát. 2014, 6, 31–37. [Google Scholar]
- Leonti, M.; Vibrans, H.; Sticher, O.; Heinrich, M. Ethnopharmacology of the Popoluca, Mexico: An evaluation. J. Pharm. Pharmacol. 2001, 12, 1653–1669. [Google Scholar] [CrossRef]
- Gutierrez, R.M.; Flores, J.M. Effect of chronic administration of hexane extract of Byrsonima crassifolia seed on B-cell and pancreatic oxidative parameters in streptozotocin-induced diabetic rat. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Martı́nez-Vázquez, M.; Gonzalez-Esquinca, A.R.; Luna, L.C.; Gutiérrez, M.M.; Garcı́a-Argáez, A.N. Antimicrobial activity of Byrsonima crassifolia (L.) H.B.K. J. Ethnopharmacol. 1999, 66, 79–82. [Google Scholar] [CrossRef]
- Helms, K.M.; Wilson, R.C.; Ogungbe, I.V.; Setzer, W.N.; Twigg, P.D. Vitexin inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K. Nat. Prod. Commun. 2011, 6, 1411–1416. [Google Scholar] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Das, R.; Sarkhel, S.; Mishra, R.; Mukherjee, S.; Bhattacharya, S.; Gomes, A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010, 48, 865–878. [Google Scholar] [PubMed]
| Name | Plant | Solvent | Origin |
|---|---|---|---|
| BRINBC | Bravaisia integerrima | chloroform | bark extract |
| BRINBM | Bravaisia integerrima | methanol | bark extract |
| BYCRBA | Byrsonima crassifolia | acetone | bark extract |
| CESOPA | Centropogon solanifolius | acetone | plant extract |
| DRALBA | Drypetes alba | acetone | bark extract |
| EUELBM | Euphorbia elata | methanol | bark extract |
| MATAYBA | Matayba oppositifolia | acetone | bark extract |
| MAVEVA | Mandevilla veraguasensis | acetone | vine extract |
| OCSIBA | Ocotea sinuata | acetone | bark extract |
| PAMABC | Paullinia cf. macrocarpa | chloroform | bark extract |
| PAMABM | Paullinia cf. macrocarpa | methanol | bark extract |
| PIAELA | Piper aequale | acetone | leaf extract |
| TALOBC | Tabernaemontana longipes | chloroform | bark extract |
| TALOBM | Tabernaemontana longipes | methanol | bark extract |
| TALOLC | Tabarnaemontana longipes | chloroform | leaf extract |
| TALOLM | Tabernaemontana longipes | methanol | leaf extract |
| TAMEBC | Tapirira mexicana | chloroform | bark extract |
| TRMABA | Trichilia martiana | acetone | bark extract |
| VEOEBA | Verbesina oerstediana | acetone | bark extract |
| VEOELA | Verbesina oerstediana | acetone | leaf extract |
| VETUBA | Verbesina turbacensis | acetone | bark extract |
| VIVELC | Viburnum venustum | chloroform | leaf extract |
| ZABRBA | Zanthoxylum sp. “brillante” | acetone | bark extract |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, R.; Branitzki-Heinemann, K.; Moubasher, H.; Setzer, W.N.; Naim, H.Y.; Von Köckritz-Blickwede, M. In Vitro Testing of Crude Natural Plant Extracts from Costa Rica for Their Ability to Boost Innate Immune Cells against Staphylococcus aureus. Biomedicines 2017, 5, 40. https://doi.org/10.3390/biomedicines5030040
Yaseen R, Branitzki-Heinemann K, Moubasher H, Setzer WN, Naim HY, Von Köckritz-Blickwede M. In Vitro Testing of Crude Natural Plant Extracts from Costa Rica for Their Ability to Boost Innate Immune Cells against Staphylococcus aureus. Biomedicines. 2017; 5(3):40. https://doi.org/10.3390/biomedicines5030040
Chicago/Turabian StyleYaseen, Ragheda, Katja Branitzki-Heinemann, Hani Moubasher, William N. Setzer, Hassan Y. Naim, and Maren Von Köckritz-Blickwede. 2017. "In Vitro Testing of Crude Natural Plant Extracts from Costa Rica for Their Ability to Boost Innate Immune Cells against Staphylococcus aureus" Biomedicines 5, no. 3: 40. https://doi.org/10.3390/biomedicines5030040