Targeting of Tumor Neovasculature with GrB/VEGF121, a Novel Cytotoxic Fusion Protein
Abstract
:1. Introduction
1.1. Angiogenesis and Vascular Targeting Agents in the Clinic
1.2. VEGF Receptor Targeting to Inhibit Angiogenesis
1.3. Development of Fusion Proteins for Targeted Therapy
2. Granzyme B as a Cytolytic Agent
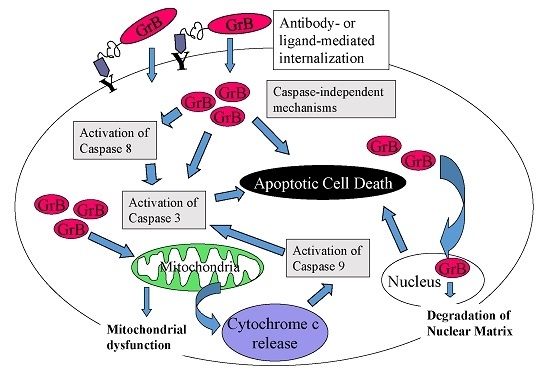
2.1. Granzyme B Mechanism of Action
2.2. Advantages of GrB-Based Constructs over Other Immunotoxins or ADCs
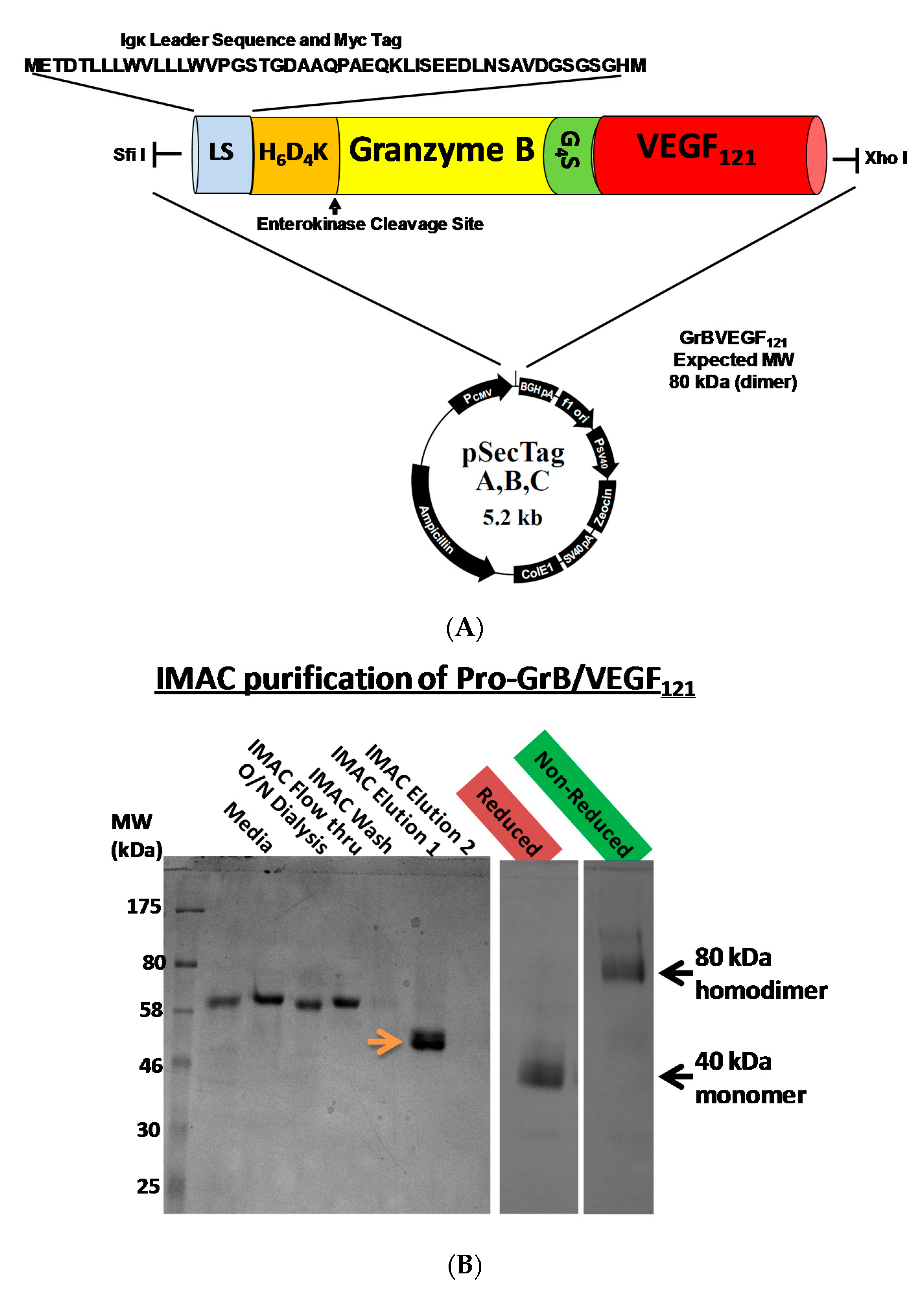
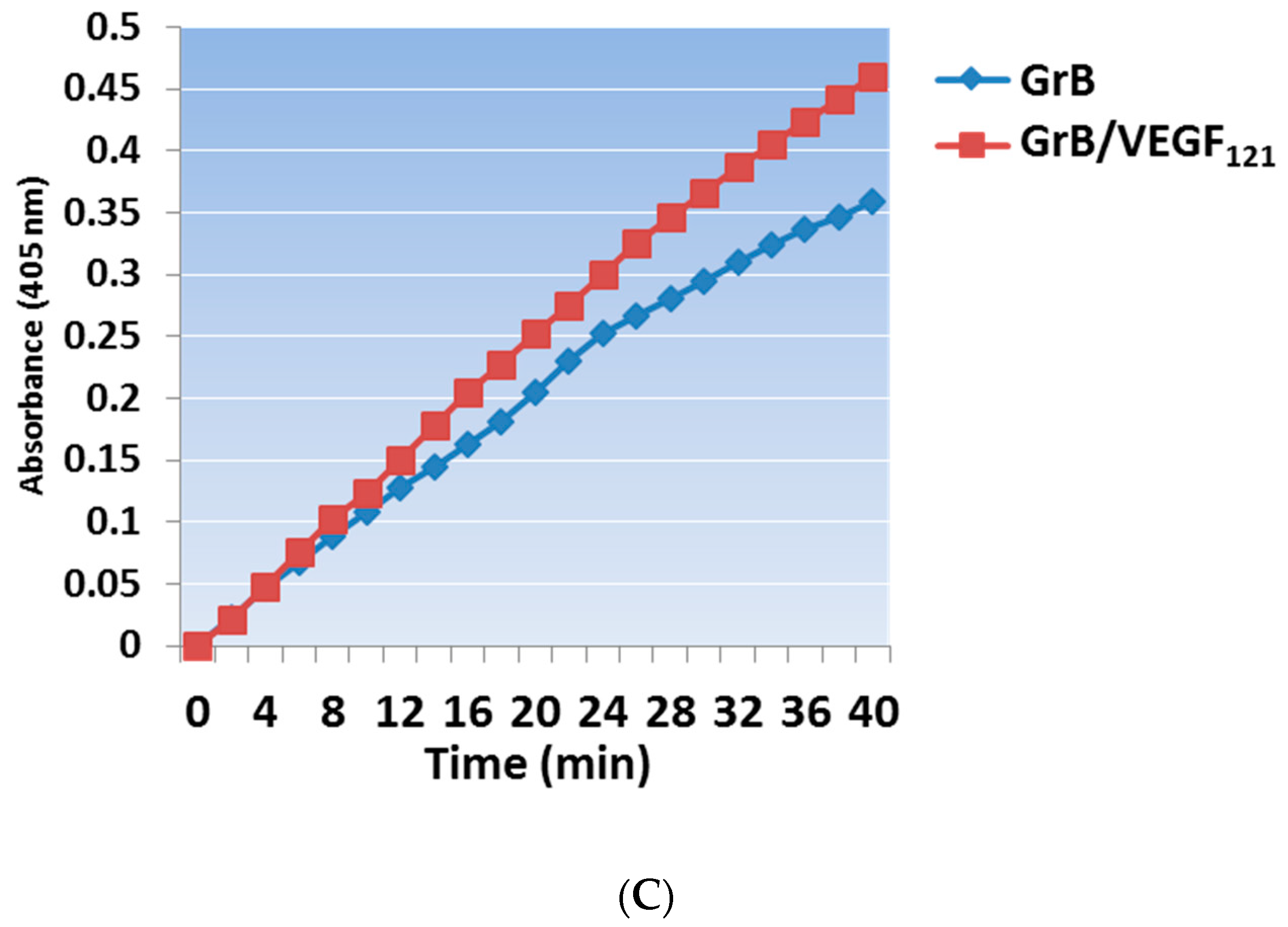
3. Construction, Expression and Purification of GrB/VEGF121
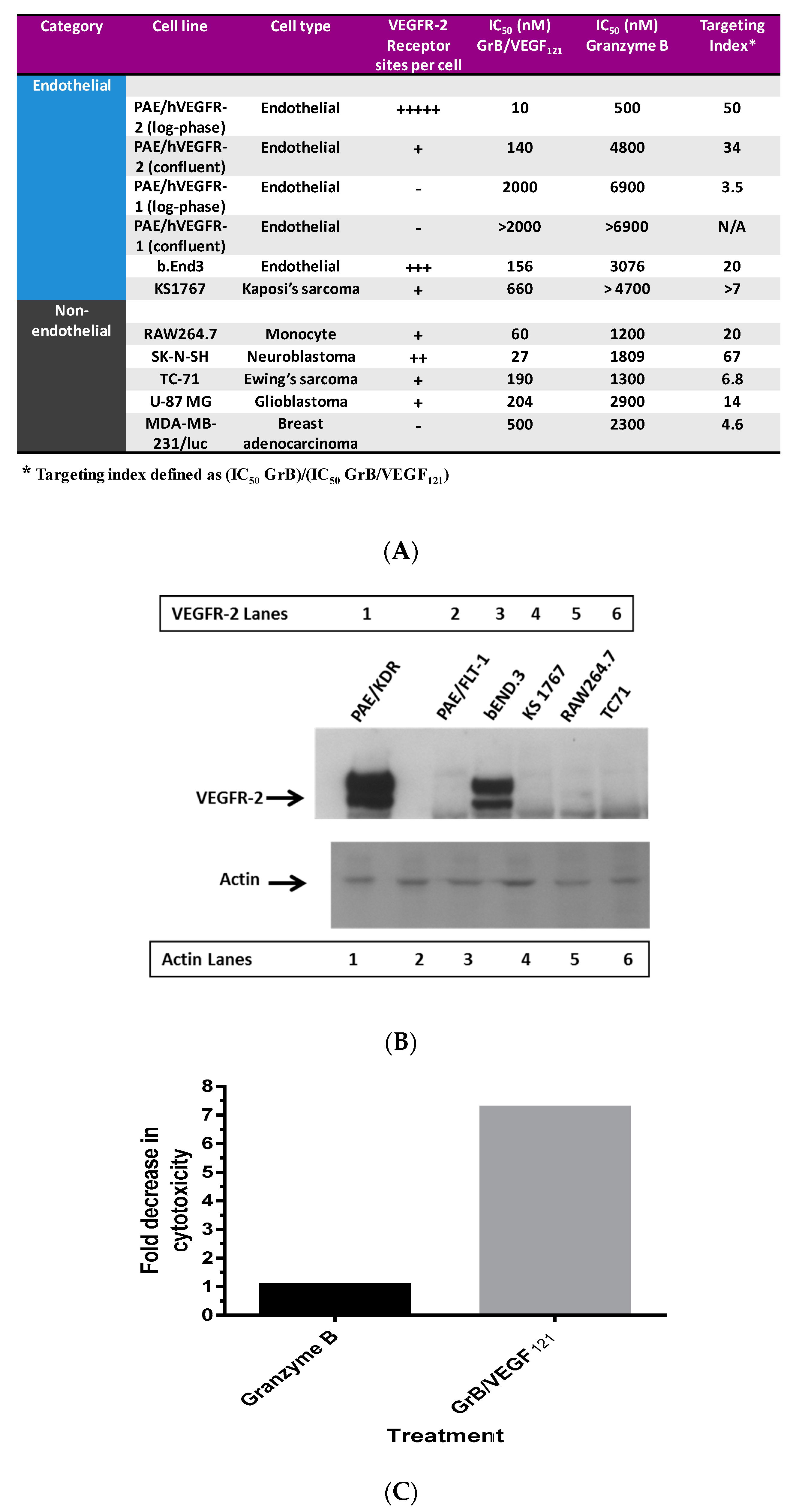
3.1. In Vitro Internalization and Cytotoxic Activity
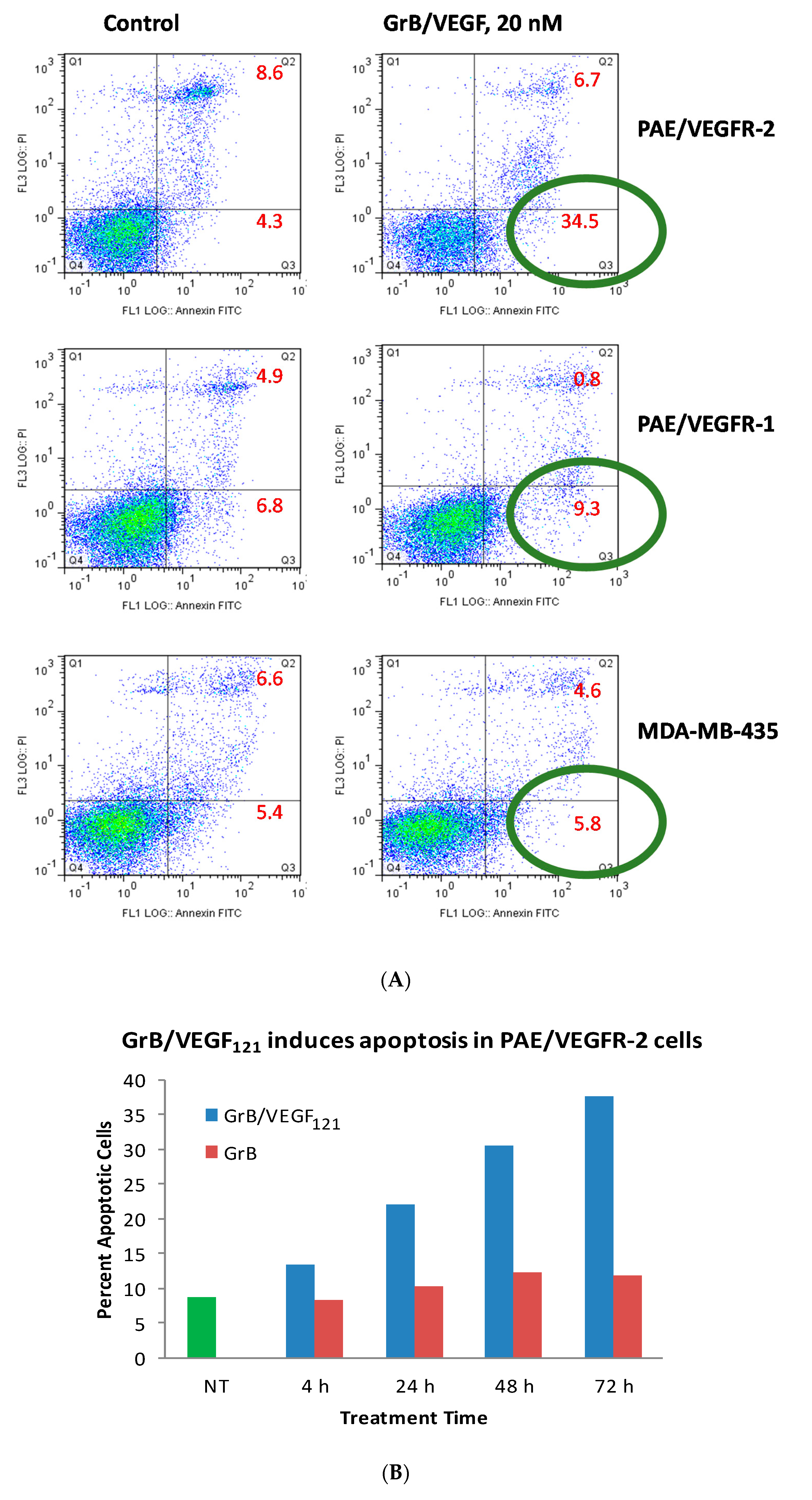
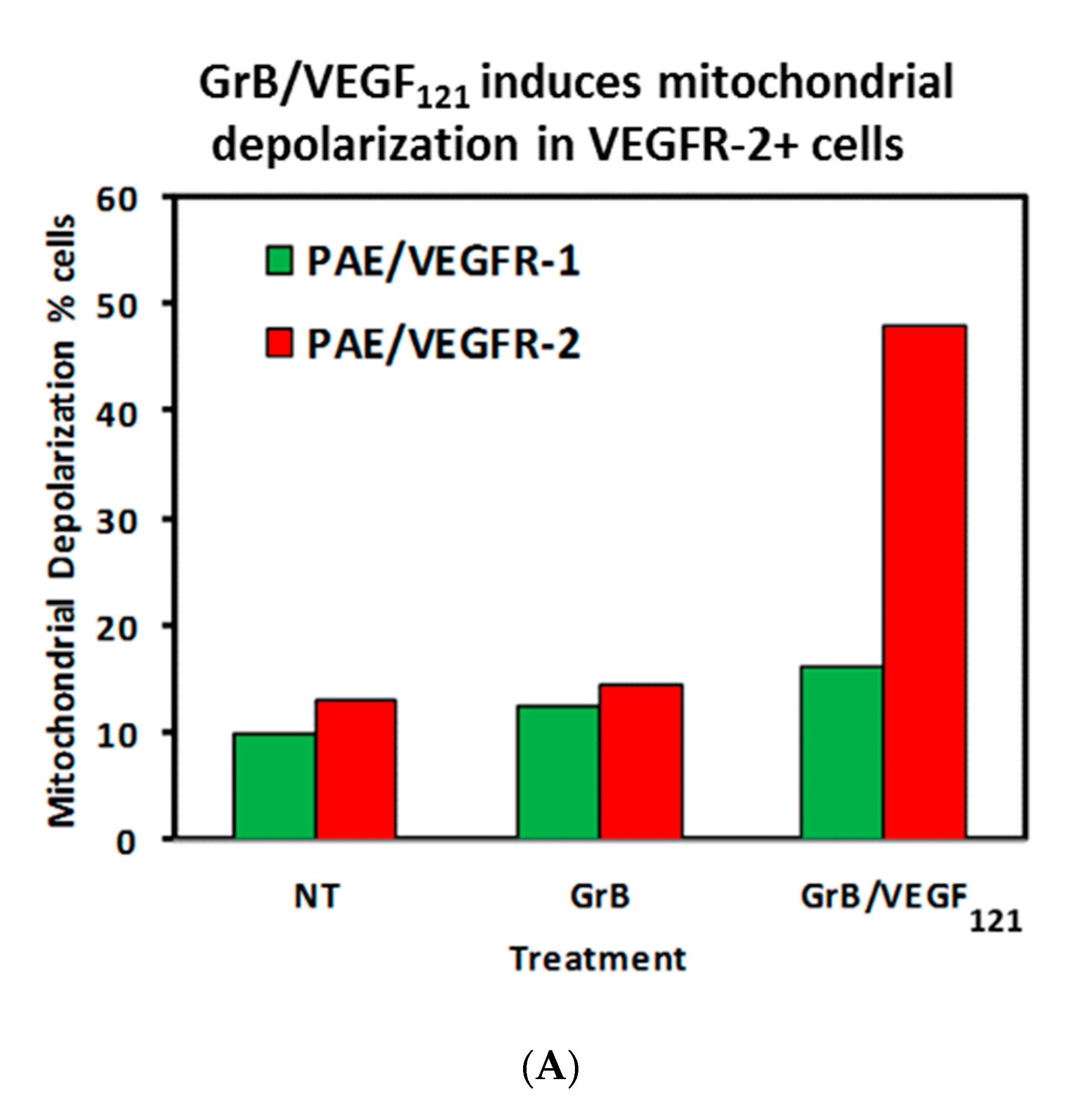
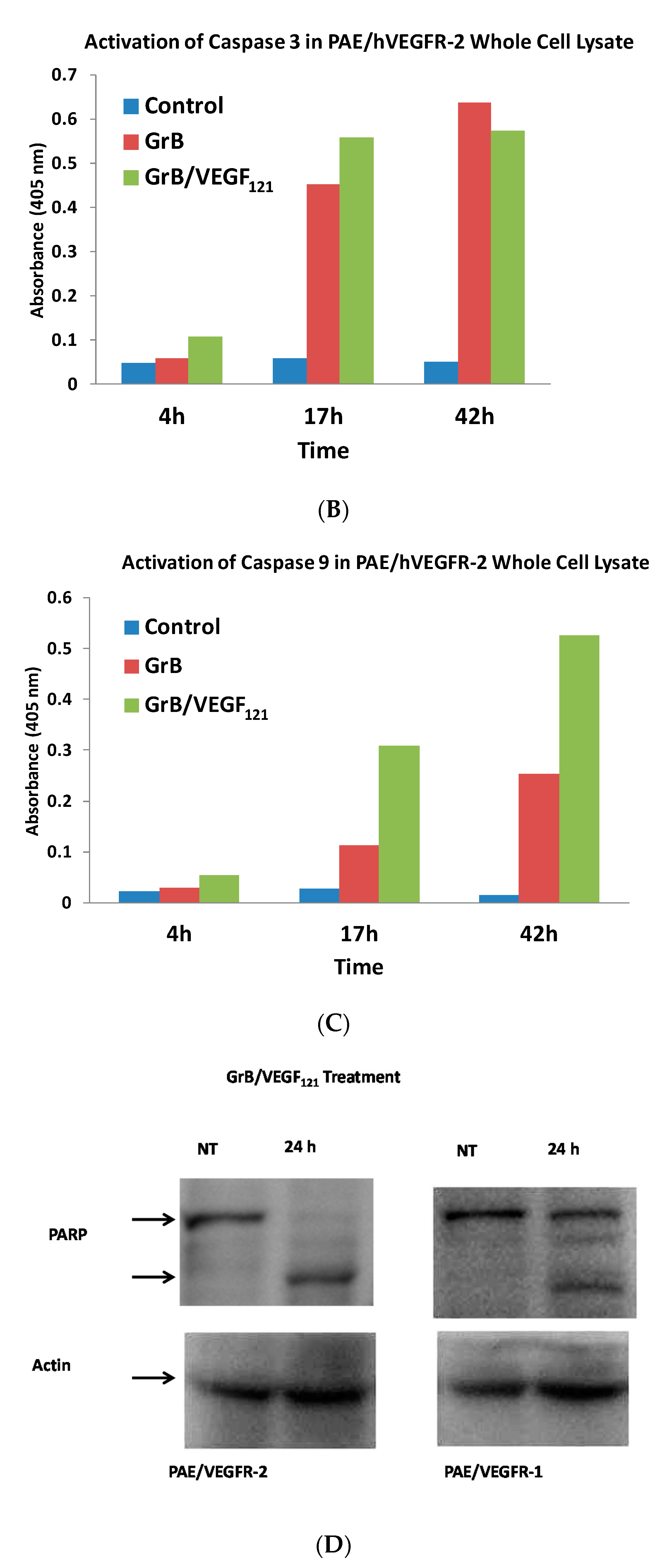
3.2. Pro-Apoptotic Activity of GrB/VEGF121
4. In Vivo Studies with GrB/VEGF121
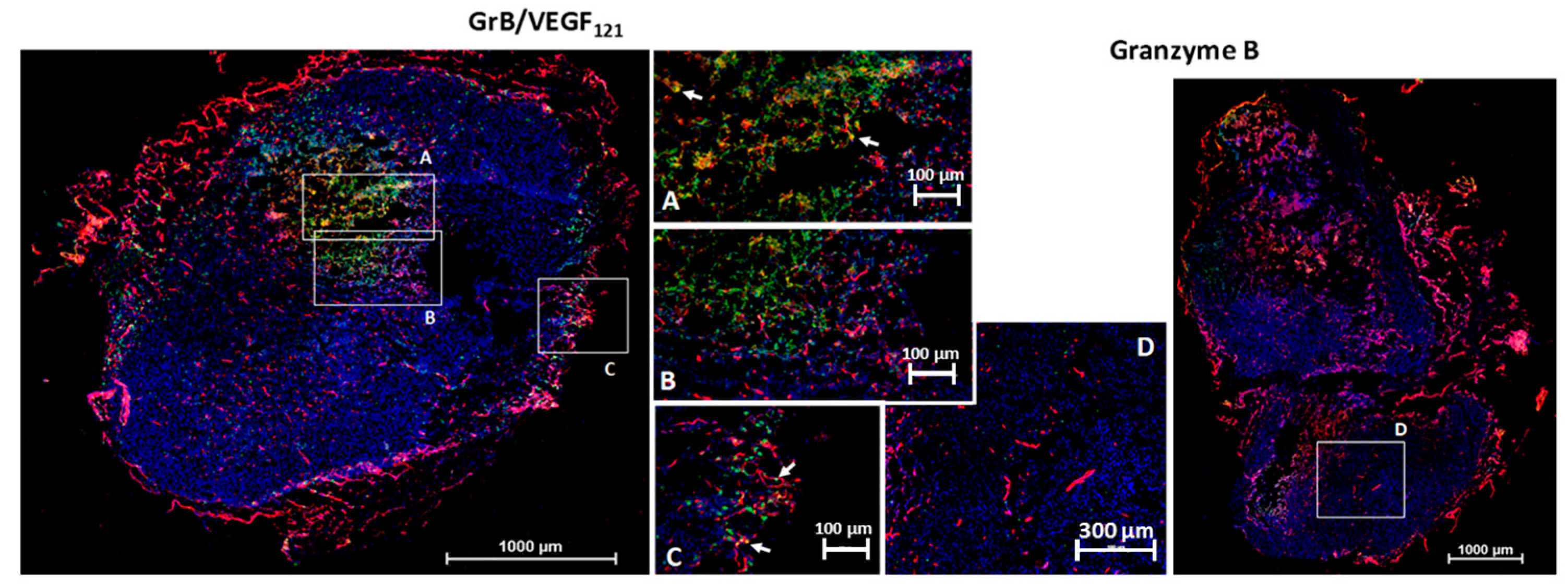
4.1. Localization of GrB/VEGF121 into Tumor Tissue
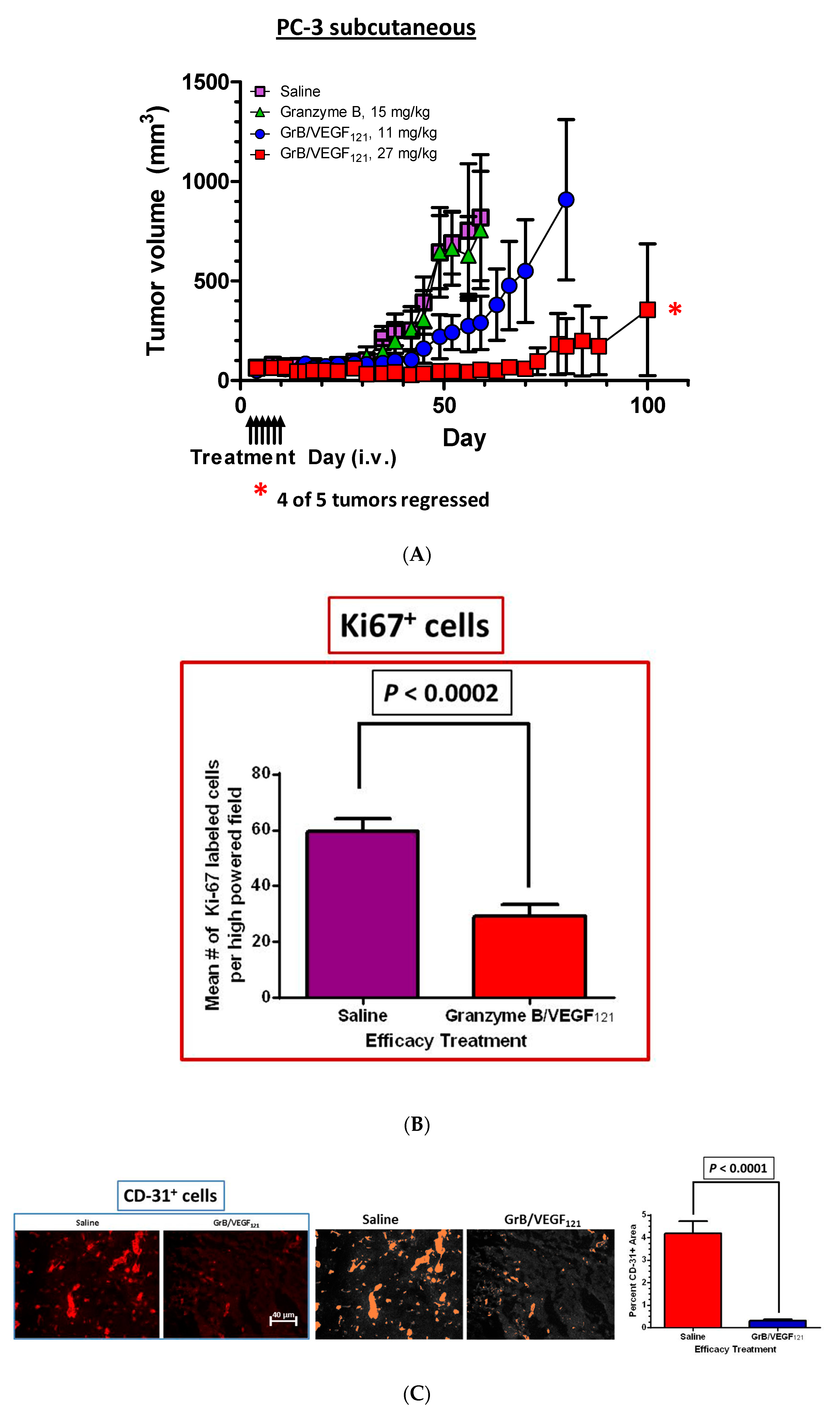
4.2. In Vivo Efficacy of GrB/VEGF121
5. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Aoun, E.; Taher, A. The clinical implications of angiogenesis in the treatment of cancer. J. Med. Liban. 2002, 50, 32–38. [Google Scholar] [PubMed]
- Bogenrieder, T.; Herlyn, M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene 2003, 22, 6524–6536. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Diagnostic and therapeutic applications of angiogenesis research. C. R. Acad. Sci. III 1993, 316, 909–918. [Google Scholar] [PubMed]
- Verheul, H.M.; Voest, E.E.; Schlingemann, R.O. Are tumours angiogenesis-dependent? J. Pathol. 2004, 202, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S.; Meyer-Morse, N.; Bergsland, E.; Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Investig. 2003, 111, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Klohs, W.D.; Fry, D.W.; Kraker, A.J. Inhibitors of tyrosine kinase. Curr. Opin. Oncol. 1997, 9, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.W.; Martiny-Baron, G.; Schlaeppi, J.M.; Wood, J.M. Therapies directed at vascular endothelial growth factor. Expert. Opin. Investig. Drugs 2002, 11, 1715–1736. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S. Inhibitors of the vascular endothelial growth factor receptor. Hematol. Oncol. Clin. N. Am. 2002, 16, 1173–1187. [Google Scholar] [CrossRef]
- Ruegg, C.; Dormond, O.; Foletti, A. Suppression of tumor angiogenesis through the inhibition of integrin function and signaling in endothelial cells: Which side to target? Endothelium 2002, 9, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Veronese, M.L.; Stevenson, J.P.; Sun, W.; Redlinger, M.; Algazy, K.; Giantonio, B.; Hahn, S.; Vaughn, D.; Thorn, C.; Whitehead, A.S.; et al. Phase I trial of UFT/leucovorin and irinotecan in patients with advanced cancer. Eur. J. Cancer 2004, 40, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Zondor, S.D.; Medina, P.J. Bevacizumab: An angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann. Pharmacother. 2004, 38, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. Vegf-trap: A vegf blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Serur, A.; Huang, J.; Manley, C.A.; McCrudden, K.W.; Frischer, J.S.; Soffer, S.Z.; Ring, L.; New, T.; Zabski, S.; et al. Potent vegf blockade causes regression of coopted vessels in a model of neuroblastoma. Proc. Natl. Acad. Sci. USA 2002, 99, 11399–11404. [Google Scholar] [CrossRef] [PubMed]
- Wulff, C.; Wilson, H.; Rudge, J.S.; Wiegand, S.J.; Lunn, S.F.; Fraser, H.M. Luteal angiogenesis: Prevention and intervention by treatment with vascular endothelial growth factor trap(A40). J. Clin. Endocrinol. Metab. 2001, 86, 3377–3386. [Google Scholar] [PubMed]
- Azzouzi, A.R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
- Ciric, E.; Sersa, G. Radiotherapy in combination with vascular-targeted therapies. Radiol. Oncol. 2010, 44, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Molecular mechanisms of tumor angiogenesis and tumor progression. J. Neurooncol. 2000, 50, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Shinkaruk, S.; Bayle, M.; Lain, G.; Deleris, G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr. Med. Chem. Anticancer Agents 2003, 3, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer 2000, 1, S80–S84. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, G.; Hagemeister, F. Denileukin diftitox: A novel immunotoxin. Expert Opin. Biol. Ther. 2009, 9, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Masood, R.; Zheng, T.; Cai, J.; Smith, D.L.; Gill, P.S. Vascular endothelial growth factor chimeric toxin is highly active against endothelial cells. Cancer Res. 1999, 59, 183–188. [Google Scholar] [PubMed]
- Backer, M.V.; Budker, V.G.; Backer, J.M. Shiga-like toxin-VEGF fusion proteins are selectively cytotoxic to endothelial cells overexpressing VEGFR-2. J. Control Release 2001, 74, 349–355. [Google Scholar] [CrossRef]
- Hotz, H.G.; Gill, P.S.; Masood, R.; Hotz, B.; Buhr, H.J.; Foitzik, T.; Hines, O.J.; Reber, H.A. Specific targeting of tumor vasculature by diphtheria toxin-vascular endothelial growth factor fusion protein reduces angiogenesis and growth of pancreatic cancer. J. Gastrointest. Surg. 2002, 6, 159–166. [Google Scholar] [CrossRef]
- Masood, R.; Kundra, A.; Zhu, S.; Xia, G.; Scalia, P.; Smith, D.L.; Gill, P.S. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int. J. Cancer 2003, 104, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Wild, R.; Nojima, D. Targeting tumor vasculature using VEGF-toxin conjugates. Methods Mol. Biol. 2001, 166, 219–234. [Google Scholar] [PubMed]
- Mohamedali, K.A.; Kedar, D.; Sweeney, P.; Kamat, A.; Davis, D.W.; Eve, B.Y.; Huang, S.; Thorpe, P.E.; Dinney, C.P.; Rosenblum, M.G. The vascular-targeting fusion toxin VEGF121/rGel inhibits the growth of orthotopic human bladder carcinoma tumors. Neoplasia 2005, 7, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A.; Li, Z.G.; Starbuck, M.W.; Wan, X.; Yang, J.; Kim, S.; Zhang, W.; Rosenblum, M.G.; Navone, N.M. Inhibition of prostate cancer osteoblastic progression with VEGF121/rGel, a single agent targeting osteoblasts, osteoclasts, and tumor neovasculature. Clin. Cancer Res. 2011, 17, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A.; Niu, G.; Luster, T.A.; Thorpe, P.E.; Gao, H.; Chen, X.; Rosenblum, M.G. Pharmacodynamics, tissue distribution, toxicity studies and antitumor efficacy of the vascular targeting fusion toxin VEGF121/rGel. Biochem. Pharmacol. 2012, 84, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A.; Poblenz, A.T.; Sikes, C.R.; Navone, N.M.; Thorpe, P.E.; Darnay, B.G.; Rosenblum, M.G. Inhibition of prostate tumor growth and bone remodeling by the vascular targeting agent VEGF121/rGel. Cancer Res. 2006, 66, 10919–10928. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A.; Ran, S.; Gomez-Manzano, C.; Ramdas, L.; Xu, J.; Kim, S.; Cheung, L.H.; Hittelman, W.N.; Zhang, W.; Waltenberger, J.; et al. Cytotoxicity of VEGF121/rGel on vascular endothelial cells resulting in inhibition of angiogenesis is mediated via VEGFR-2. BMC Cancer 2011, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Mohamedali, K.A.; Luster, T.A.; Thorpe, P.E.; Rosenblum, M.G. The vascular-ablative agent VEGF121/rGel inhibits pulmonary metastases of MDA-MB-231 breast tumors. Neoplasia 2005, 7, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Veenendaal, L.M.; Jin, H.; Ran, S.; Cheung, L.; Navone, N.; Marks, J.W.; Waltenberger, J.; Thorpe, P.; Rosenblum, M.G. In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7866–7871. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Beers, R.; Xiang, L.; Lee, B.; Weldon, J.E.; Kreitman, R.J.; Pastan, I. Recombinant immunotoxin against B-cell malignancies with no immunogenicity in mice by removal of B-cell epitopes. Proc. Natl. Acad. Sci. USA 2011, 108, 5742–5747. [Google Scholar] [CrossRef] [PubMed]
- Onda, M.; Beers, R.; Xiang, L.; Nagata, S.; Wang, Q.C.; Pastan, I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc. Natl. Acad. Sci. USA 2008, 105, 11311–11316. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Lieberman, J. Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu. Rev. Immunol. 2008, 26, 389–420. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Brunet, M.; Martin, S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010, 17, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.J.; Rajotte, R.V.; Korbutt, G.S.; Bleackley, R.C. Granzyme B: A natural born killer. Immunol. Rev. 2003, 193, 31–38. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, C.; D’Alessio, G. From immunotoxins to immunornases. Curr. Pharm. Biotechnol. 2008, 9, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E. Reducing the immune response to immunotoxin. Clin. Cancer Res. 2004, 10, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Posey, J.A.; Khazaeli, M.B.; Bookman, M.A.; Nowrouzi, A.; Grizzle, W.E.; Thornton, J.; Carey, D.E.; Lorenz, J.M.; Sing, A.P.; Siegall, C.B.; et al. A phase i trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin. Cancer Res. 2002, 8, 3092–3099. [Google Scholar] [PubMed]
- Smallshaw, J.E.; Ghetie, V.; Rizo, J.; Fulmer, J.R.; Trahan, L.L.; Ghetie, M.A.; Vitetta, E.S. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat. Biotechnol. 2003, 21, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Gutierrez, E.; Muglia, J.; McDonald, C.J.; Guzzo, C.; Gottlieb, A.; Pappert, A.; Garland, W.T.; Bagel, J.; Bacha, P. A multicenter dose-escalation trial with denileukin diftitox (ONTAK, DAB(389)IL-2) in patients with severe psoriasis. J. Am. Acad. Dermatol. 2001, 45, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.S. Immunogenicity of biological therapeutics: A hierarchy of concerns. Dev. Biol. 2003, 112, 15–21. [Google Scholar]
- Hall, P.D.; Virella, G.; Willoughby, T.; Atchley, D.H.; Kreitman, R.J.; Frankel, A.E. Antibody response to DT-GM, a novel fusion toxin consisting of a truncated diphtheria toxin (DT) linked to human granulocyte-macrophage colony stimulating factor (GM), during a phase I trial of patients with relapsed or refractory acute myeloid leukemia. Clin. Immunol. 2001, 100, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hertler, A.A.; Spitler, L.E.; Frankel, A.E. Humoral immune response to a ricin a chain immunotoxin in patients with metastatic melanoma. Cancer Drug Deliv. 1987, 4, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Verma, R.S. Humanized immunotoxins: A new generation of immunotoxins for targeted cancer therapy. Cancer Sci. 2009, 100, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Todhunter, D.A.; Panoskaltsis-Mortari, A.; Buchsbaum, D.J.; Toma, S.; Vallera, D.A. A deimmunized bispecific ligand-directed toxin that shows an impressive anti-pancreatic cancer effect in a systemic nude mouse orthotopic model. Pancreas 2012, 41, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Okeley, N.M.; Senter, P.D. Antibody-drug conjugates: Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 2010, 14, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Casi, G.; Neri, D. Antibody-drug conjugates: Basic concepts, examples and future perspectives. J. Control. Release 2012, 161, 422–428. [Google Scholar] [CrossRef] [PubMed]
- King, H.D.; Dubowchik, G.M.; Mastalerz, H.; Willner, D.; Hofstead, S.J.; Firestone, R.A.; Lasch, S.J.; Trail, P.A. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: Inhibition of aggregation by methoxytriethyleneglycol chains. J. Med. Chem. 2002, 45, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mohamedali, K.A.; Marks, J.W.; Cheung, L.H.; Hittelman, W.N.; Rosenblum, M.G. Construction and characterization of novel, completely human serine protease therapeutics targeting her2/neu. Mol. Cancer Ther. 2013, 12, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A.; Cao, Y.; Cheung, L.H.; Hittelman, W.N.; Rosenblum, M.G. The functionalized human serine protease granzyme B/VEGF121 targets tumor vasculature and ablates tumor growth. Mol. Cancer Ther. 2013, 12, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mohamedali, K.A.; Gonzalez-Angulo, A.M.; Cao, Y.; Migliorini, M.; Cheung, L.H.; LoBello, J.; Lei, X.; Qi, Y.; Hittelman, W.N.; et al. Development of human serine protease-based therapeutics targeting Fn14 and identification of Fn14 as a new target overexpressed in TNBC. Mol. Cancer Ther. 2014, 13, 2688–2705. [Google Scholar] [CrossRef] [PubMed]
- Hilgeroth, A.; Hemmer, M.; Coburger, C. The impact of the induction of multidrug resistance transporters in therapies by used drugs: Recent studies. Mini Rev. Med. Chem. 2012, 12, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Cohen, S.; Perrot, J.Y.; Faussat, A.M.; Zuany-Amorim, C.; Marjanovic, Z.; Morjani, H.; Fava, F.; Corre, E.; Legrand, O.; et al. P-gp activity is a critical resistance factor against AVE9633 and DM4 cytotoxicity in leukaemia cell lines, but not a major mechanism of chemoresistance in cells from acute myeloid leukaemia patients. BMC Cancer 2009, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Amoury, M.; Kolberg, K.; Pham, A.T.; Hristodorov, D.; Mladenov, R.; Di Fiore, S.; Helfrich, W.; Kiessling, F.; Fischer, R.; Pardo, A.; et al. Granzyme B-based cytolytic fusion protein targeting EpCAM specifically kills triple negative breast cancer cells in vitro and inhibits tumor growth in a subcutaneous mouse tumor model. Cancer Lett. 2016, 372, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, I.; Lin, X.; Yuan, X.; Manorek, G.; Shang, X.; Cheung, L.H.; Rosenblum, M.G.; Howell, S.B. Targeting granzyme B to tumor cells using a yoked human chorionic gonadotropin. Cancer Chemother. Pharmacol. 2011, 68, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Kurschus, F.C.; Jenne, D.E. Delivery and therapeutic potential of human granzyme B. Immunol. Rev. 2010, 235, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Niesen, J.; Hehmann-Titt, G.; Woitok, M.; Fendel, R.; Barth, S.; Fischer, R.; Stein, C. A novel fully-human cytolytic fusion protein based on granzyme B shows in vitro cytotoxicity and ex vivo binding to solid tumors overexpressing the epidermal growth factor receptor. Cancer Lett. 2016, 374, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, P.; Jabulowsky, R.A.; Bahr-Mahmud, H.; Wels, W.S. EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PLoS ONE 2013, 8, e61267. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.G.; Barth, S. Development of novel, highly cytotoxic fusion constructs containing granzyme B: Unique mechanisms and functions. Curr. Pharm. Des. 2009, 15, 2676–2692. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, S.; Hansen, H.P.; Hehmann-Titt, G.; Huhn, M.; Fischer, R.; Barth, S.; Thepen, T. Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical hodgkin lymphoma cells in a murine model. Blood Cancer J. 2013, 3, e106. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, B.; Thepen, T.; Stocker, M.; Rosinke, R.; Jost, E.; Fischer, R.; Tur, M.K.; Barth, S. Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol. Cancer Ther. 2008, 7, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Waltenberger, J. A novel function of VEGF receptor-2 (KDR): Rapid release of nitric oxide in response to VEGF-A stimulation in endothelial cells. Biochem. Biophys. Res. Commun. 1999, 265, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Mohamedali, K.A. (University of Texas M.D. Anderson Cancer Center, Houston, TX, USA). Unpublished work. 2013. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamedali, K.A.; Rosenblum, M.G. Targeting of Tumor Neovasculature with GrB/VEGF121, a Novel Cytotoxic Fusion Protein. Biomedicines 2017, 5, 42. https://doi.org/10.3390/biomedicines5030042
Mohamedali KA, Rosenblum MG. Targeting of Tumor Neovasculature with GrB/VEGF121, a Novel Cytotoxic Fusion Protein. Biomedicines. 2017; 5(3):42. https://doi.org/10.3390/biomedicines5030042
Chicago/Turabian StyleMohamedali, Khalid A., and Michael G. Rosenblum. 2017. "Targeting of Tumor Neovasculature with GrB/VEGF121, a Novel Cytotoxic Fusion Protein" Biomedicines 5, no. 3: 42. https://doi.org/10.3390/biomedicines5030042