Estrogen Repression of MicroRNAs Is Associated with High Guanine Content in the Terminal Loop Sequences of Their Precursors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Estrogen-Treated Zebrafish Dataset
2.2. Breast Cancer Dataset
2.3. Bioinformatic Tools
2.4. Nucleotide Composition Analysis
2.5. Statistical Data Analysis
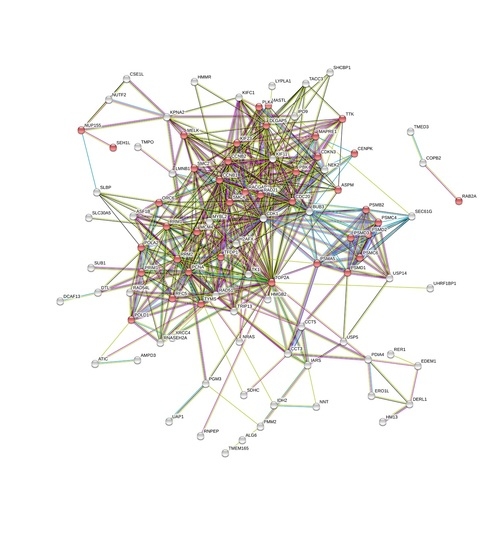
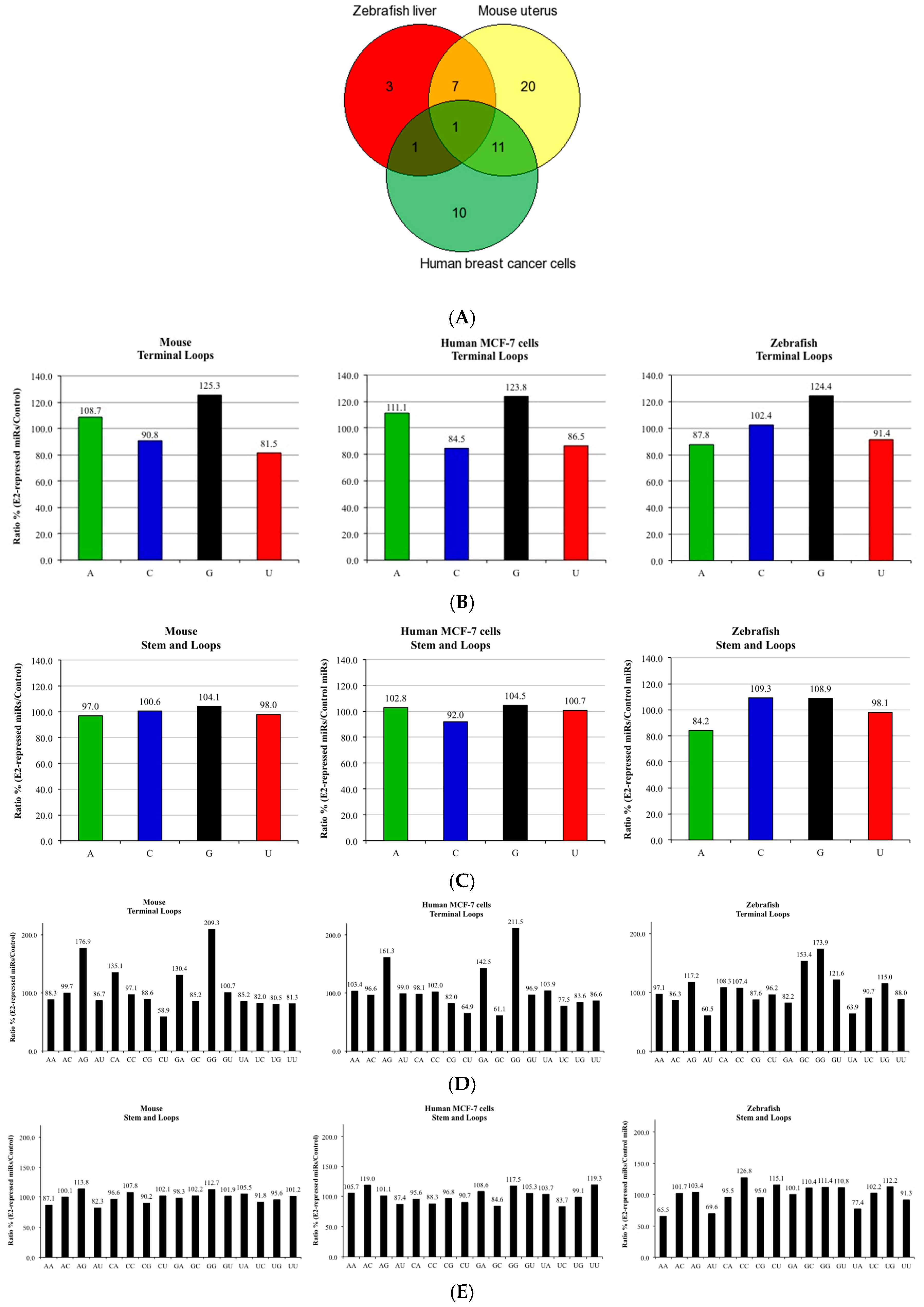
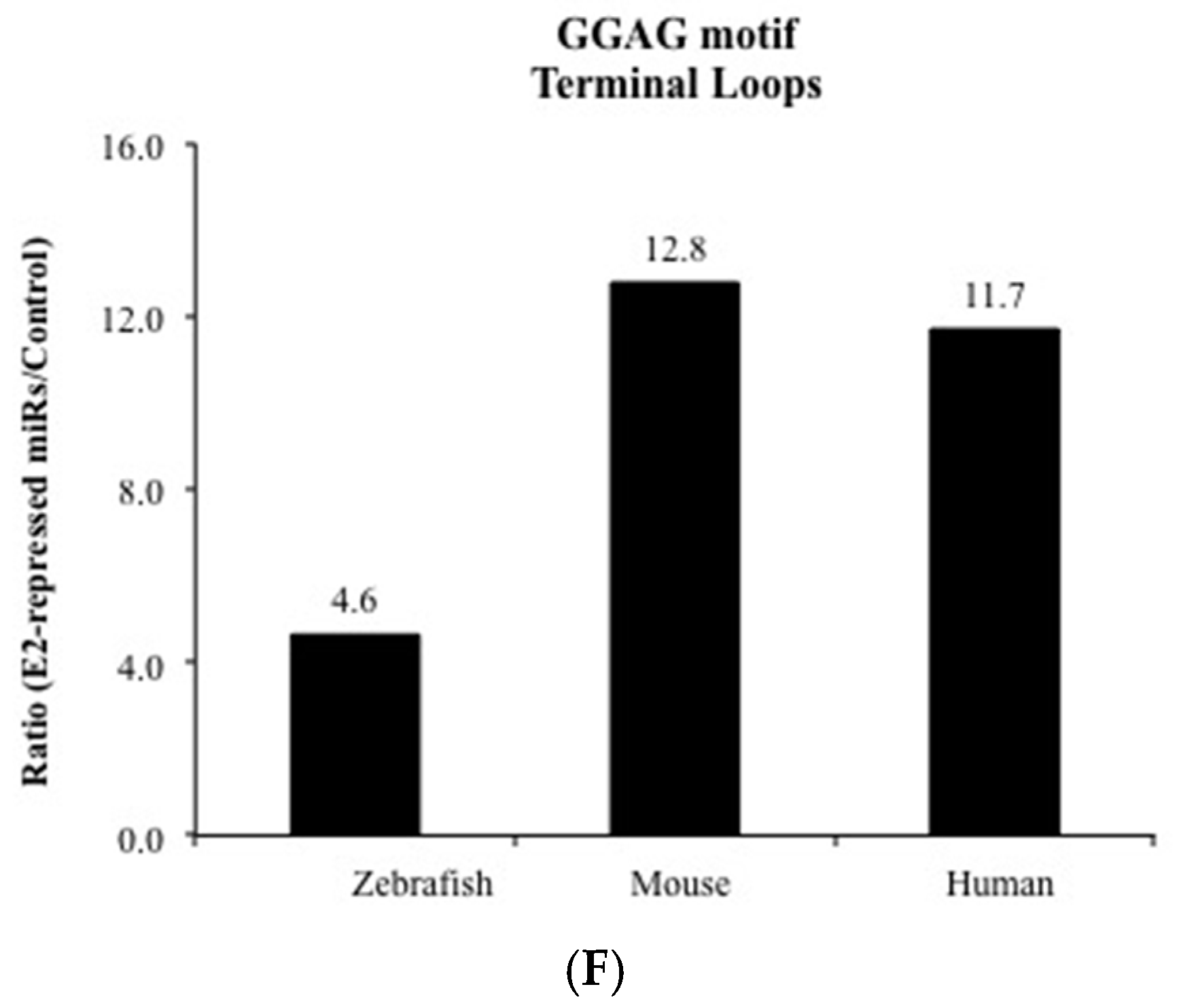
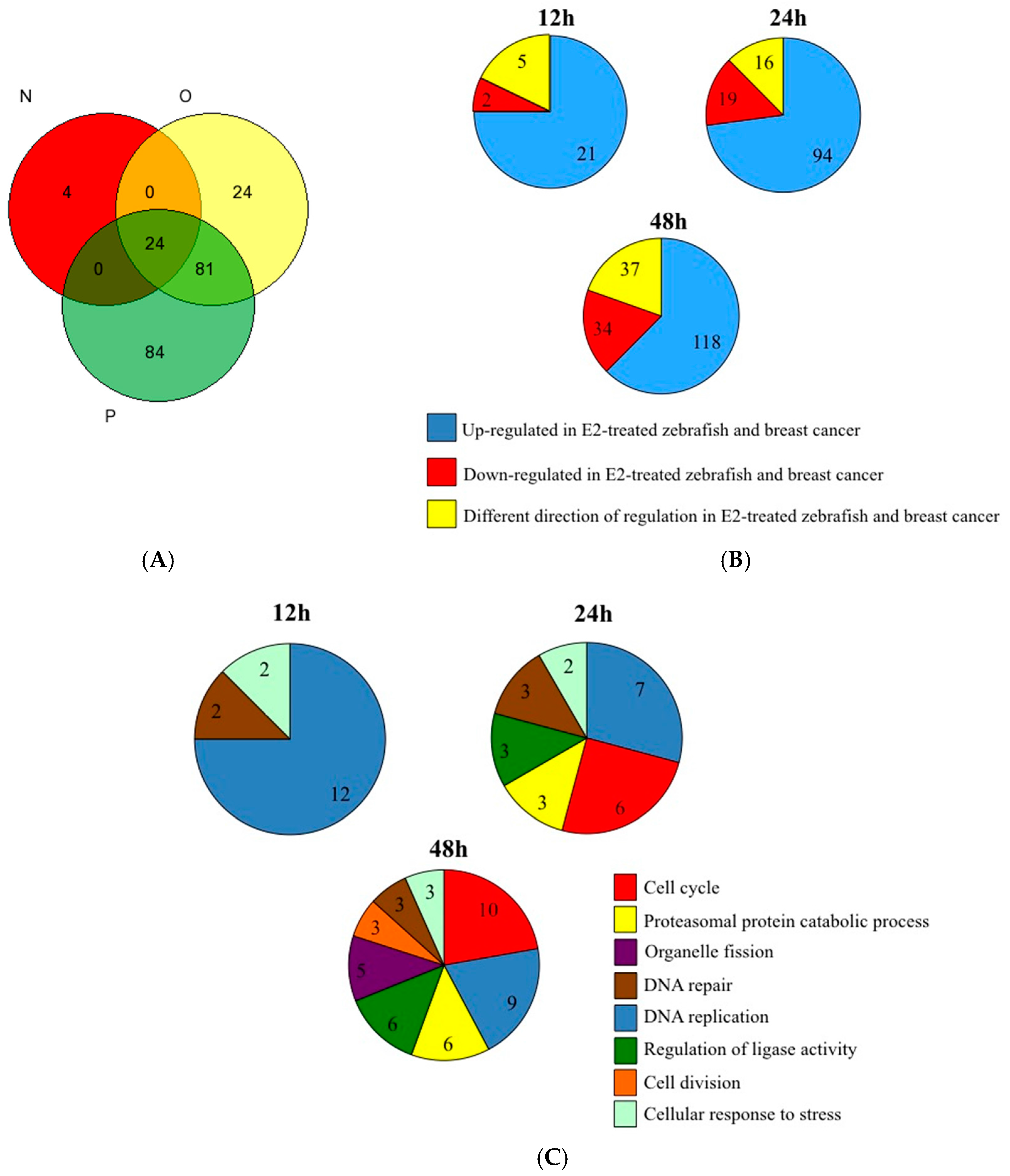
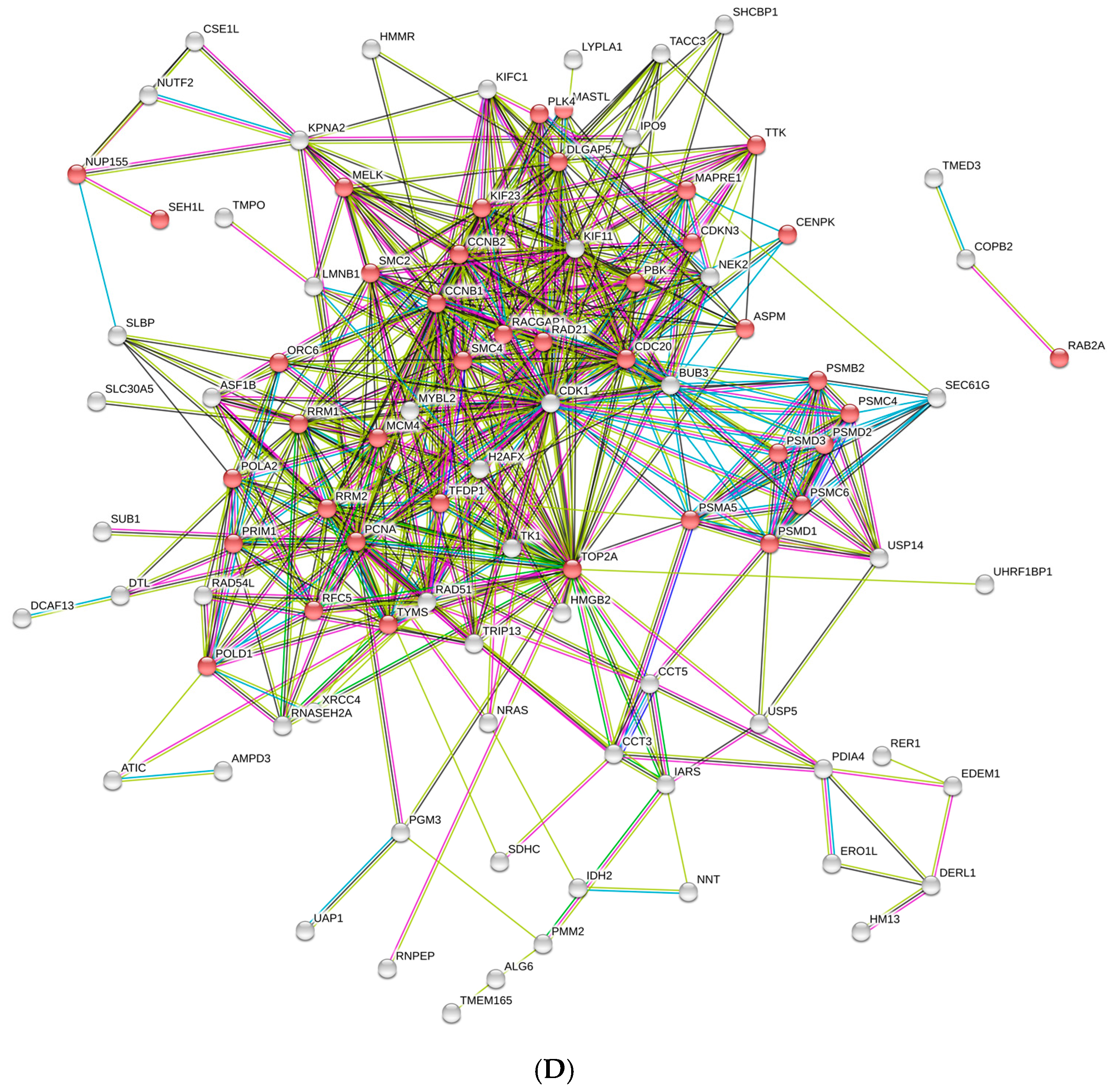
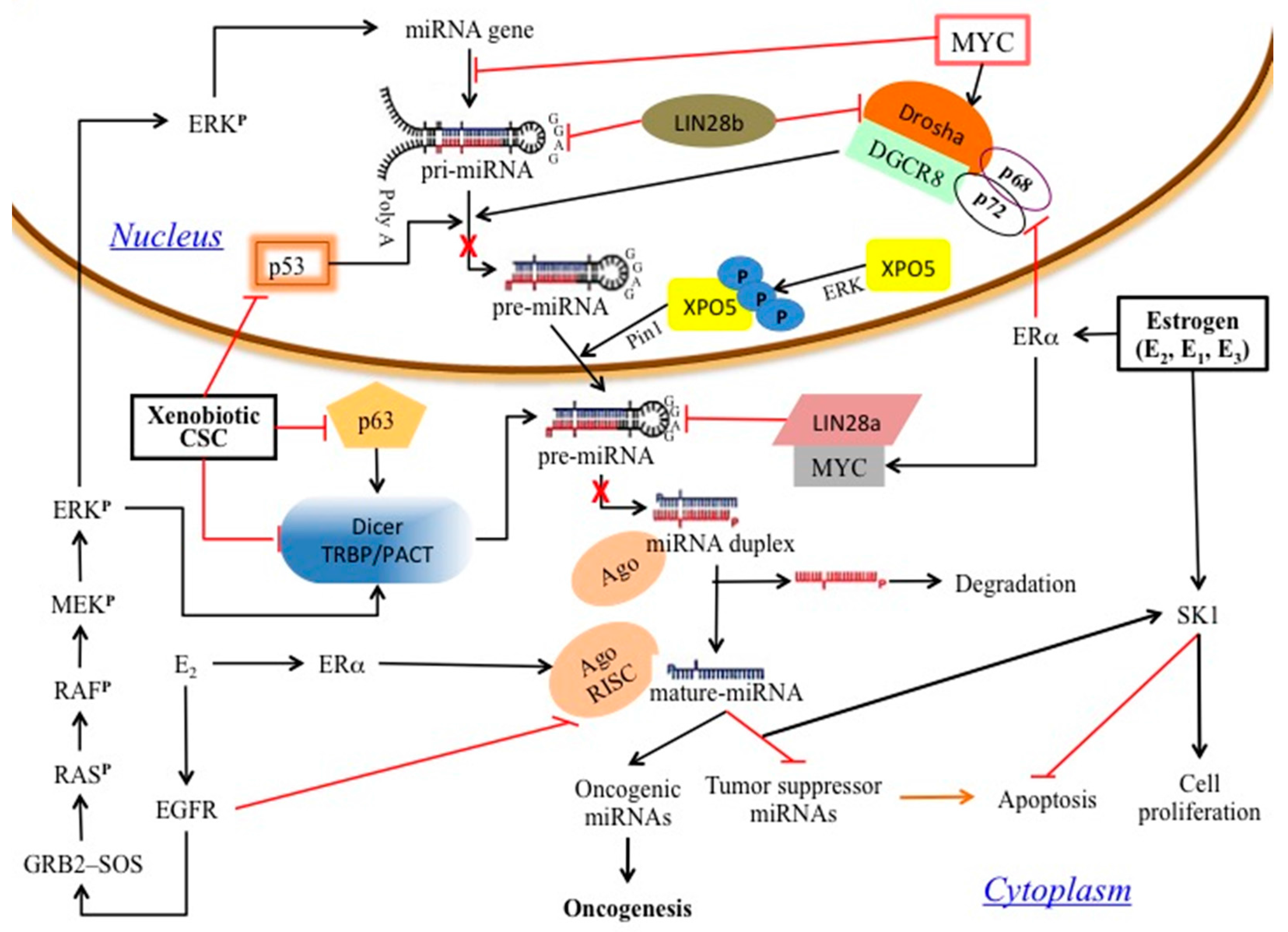
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Simpson, E.R.; Misso, M.; Hewitt, K.N.; Hill, R.A.; Boon, W.C.; Jones, M.E.; Kovacic, A.; Zhou, J.; Clyne, C.D. Estrogen—The good, the bad, and the unexpected. Endocr. Rev. 2005, 26, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Persson, I. Estrogen in the causation of breast, endometrial and ovarian cancer-evidence and hypotheses from epidemiological findings. J. Steroid Biochem. Mol. Biol. 2000, 74, 357–364. [Google Scholar] [CrossRef]
- Gruber, C.; Tschugguel, W.; Schneeberger, C.; Huber, J. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 116, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Burgos-Aceves, M.A.; Smith, Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed. Pharmacother. 2016, 78, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Maillot, G.; Lacroix-Triki, M.; Pierredon, S.; Gratadou, L.; Schmidt, S.; Bénès, V.; Roché, H.; Dalenc, F.; Auboeuf, D.; Millevoi, S.; et al. Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res. 2009, 69, 8332–8340. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fujiyama, S.; Ito, S.; Ueda, T.; Murata, T.; Naitou, M.; Takeyama, K.; Minami, Y.; O’Malley, B.W.; Kato, S.; et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol. Cell 2009, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Smith, Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014, 11, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012, 23, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Yu, D.; Lee, Y.S.; Wentzel, E.A.; Arking, D.E.; West, K.M.; Dang, C.V.; Thomas-Tikhonenko, A.; Mendell, J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 2008, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Cui, R.; Zhou, J.; Teng, K.; Hsiao, Y.H.; Nakanishi, K.; Fassan, M.; Luo, Z.; Shi, G.; Tili, E.; et al. ERK activation globally downregulates miRNAs through phosphorylating exportin-5. Cancer Cell 2016, 30, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.; Rogan, E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: Keynote lecture. Ann. N. Y. Acad. Sci. 2006, 1089, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.L.; Rogan, E.G. Depurinating estrogen–DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010, 6, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, E.L.; Rogan, E.G. Depurinating estrogen-DNA adducts, generators of cancer initiation: Their minimization leads to cancer prevention. Clin. Transl. Med. 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Wambua, D.M.; Brownstone, A.L.; Barnes, C.A.; Chiu, N.H.L. Isolation and Detection of Carcinogenic Nucleic Acid Adducts. In Carcinogen; InTech: Rijeka, Croatia, 2012; pp. 111–130. [Google Scholar]
- Izzotti, A.; Pulliero, A. The effects of environmental chemical carcinogens on the microRNA machinery. Int. J. Hyg. Environ. Health 2014, 217, 601–627. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, M.; Izzotti, A.; Pulliero, A.; Arrigo, P. Mutagens interfere with microRNA maturation by inhibiting DICER. An in silico biology analysis. Mutat. Res. 2011, 717, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Calin, G.A.; Steele, V.E.; Cartiglia, C.; Longobardi, M.; Croce, C.M.; De Flora, S. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev. Res. 2010, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Burgos-Aceves, M.A.; Smith, Y. A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer. Transl. Lung Cancer Res. 2016, 5, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, X.; Mace, B.E.; Vanderveer, L.A.; Workman, L.R.; Slifker, M.J.; Sullivan, P.M.; Veenstra, T.D.; Clapper, M.L. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis 2013, 34, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Belous, A.R.; Hachey, D.L.; Dawling, S.; Roodi, N.; Parl, F.F. Cytochrome P450 1B1-mediated estrogen metabolism results in estrogen-deoxyribonucleoside adduct formation. Cancer Res. 2007, 67, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Harrell, J.C.; Prat, A.; Parker, J.S.; Fan, C.; He, X.; Carey, L.; Anders, C.; Ewend, M.; Perou, C.M. Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res. Treat. 2012, 132, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Libri, V.; Miesen, P.; van Rij, R.P.; Buck, A.H. Regulation of microRNA biogenesis and turnover by animals and their viruses. Cell. Mol. Life Sci. 2013, 70, 3525–3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, I.; Joo, C.; Kim, Y.K.; Ha, M.; Yoon, M.J.; Cho, J.; Yeom, K.H.; Han, J.; Kim, V.N. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009, 138, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Trabucchi, M.; Briata, P.; Garcia-Mayoral, M.; Haase, A.D.; Filipowicz, W.; Ramos, A.; Gherzi, R.; Rosenfeld, M.G. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009, 459, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, G.; García-Mayoral, M.F.; Hollingworth, D.; Kelly, G.; Martin, S.R.; Briata, P.; Gherzi, R.; Ramos, A. Noncanonical G recognition mediates KSRP regulation of let-7 biogenesis. Nat. Struct. Mol. Biol. 2012, 19, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, L.; Wang, Y.; Wang, X.; Liu, N.; You, Y. Regulation of let-7 and its target oncogenes. Oncol. Lett. 2012, 3, 955–960. [Google Scholar] [PubMed]
- He, X.Y.; Chen, J.X.; Zhang, Z.; Li, C.L.; Peng, Q.L.; Peng, H.M. The let-7a microRNA protects from growth of lung carcinoma by suppression of k-Ras and c-Myc in nude mice. J. Cancer Res. Clin. Oncol. 2010, 136, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.Y.; Wang, R.; Chen, L.B. MicroRNA-145: A potent tumour suppressor that regulates multiple cellular pathways. J. Cell. Mol. Med. 2014, 18, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Kent, O.A.; Chivukula, R.R.; Mullendore, M.; Wentzel, E.A.; Feldmann, G.; Lee, K.H.; Liu, S.; Leach, S.D.; Maitra, A.; Mendell, J.T. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010, 24, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, R.; Nicoloso, M.S.; Lupini, L.; Lu, Y.; Fogarty, J.; Rossi, S.; Zagatti, B.; Fabbri, M.; Veronese, A.; Liu, X.; et al. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010, 17, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of microRNA processing by p53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, X.; Yan, Y.; Guo, F.; Li, J.; Hu, Y.; Zhou, H.; Xun, Q. Estrogen regulates the tumour suppressor miRNA-30c and its target gene, MTA-1, in endometrial cancer. PLoS ONE 2014, 9, e90810. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Smith, A.; Lang, J.C.; Islam, M.; Dutt, D.; Teknos, T.N.; Pan, Q. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cε. Oncogene 2012, 31, 4045–4053. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Lotterman, C.D.; Bao, C.; Hruban, R.H.; Karim, B.; Mendell, J.T.; Huso, D.; Lowenstein, C.J. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 6334–6339. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ding, K.; Li, R.; Zhang, W.; Li, G.; Kong, X.; Qian, P.; Lobie, P.E.; Zhu, T. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Res. 2014, 16, R40. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Wu, Y.L.; Vega, V.B.; Miller, L.D.; Spitsbergen, J.; Tong, Y.; Zhan, H.; Govindarajan, K.R.; Lee, S.; Mathavan, S.; et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat. Biotechnol. 2006, 24, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Lam, S.H.; Lee, S.G.; Lin, C.Y.; Thomsen, J.S.; Fu, P.Y.; Murthy, K.R.K.; Li, H.; Govindarajan, K.R.; Nick, L.C.H.; Bourque, G.; et al. Molecular conservation of estrogen-response associated with cell cycle regulation, hormonal carcinogenesis and cancer in zebrafish and human cancer cell lines. BMC Med. Genom. 2011, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A.; Goksoyr, A. Eggshell and egg yolk proteins in fish: Hepatic proteins for the next generation: Oogenetic, population, and evolutionary implications of endocrine disruption. Comp. Hepatol. 2003, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. Estrogen regulation of gene expression in the teleost fish immune system. Fish. Shellfish Immunol. 2016, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Amat, A.; Burgeot, T.; Castegnaro, M.; Pfohl-Leszkowicz, A. DNA adducts in fish following an oil spill exposure. Environ. Chem. Lett. 2006, 4, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Schlehe, B.; Hemminki, K.; Sutter, C.; Bugert, P.; Wappenschmidt, B.; Volkmann, J.; Varon, R.; Weber, B.H.F.; Niederacher, D.; et al. A genetic variant in the pre-miR-27a oncogene is associated with a reduced familial breast cancer risk. Breast Cancer Res. Treat. 2010, 121, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gu, H.; Zeng, Y.; Xia, Y.; Wang, Y.; Jing, Y.; Yang, L.; Wang, B. Hsa-miR-27a genetic variant contributes to gastric cancer susceptibility through affecting miR-27a and target gene expression. Cancer Sci. 2010, 101, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, P.; Ma, L.; Zhong, D.; Chu, H.; Yan, F.; Lv, Q.; Qin, C.; Wang, W.; Wang, M.; et al. A genetic variant in pre-miR-27a is associated with a reduced renal cell cancer risk in a Chinese population. PLoS ONE 2012, 7, e46566. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.; Cordiner, R.A.; Young, R.S.; Hug, N.; Macias, S.; Cáceres, J.F. Genetic variation and RNA structure regulate microRNA biogenesis. Nat. Commun. 2017, 8, 15114. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging role of micro-RNA in the effect of the endocrine disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.; Burgos-Aceves, M.A.; Kahan, T.; Smith, Y. Estrogen Repression of MicroRNAs Is Associated with High Guanine Content in the Terminal Loop Sequences of Their Precursors. Biomedicines 2017, 5, 47. https://doi.org/10.3390/biomedicines5030047
Cohen A, Burgos-Aceves MA, Kahan T, Smith Y. Estrogen Repression of MicroRNAs Is Associated with High Guanine Content in the Terminal Loop Sequences of Their Precursors. Biomedicines. 2017; 5(3):47. https://doi.org/10.3390/biomedicines5030047
Chicago/Turabian StyleCohen, Amit, Mario Alberto Burgos-Aceves, Tamar Kahan, and Yoav Smith. 2017. "Estrogen Repression of MicroRNAs Is Associated with High Guanine Content in the Terminal Loop Sequences of Their Precursors" Biomedicines 5, no. 3: 47. https://doi.org/10.3390/biomedicines5030047