Photodynamic Therapy for Eye Cancer
Abstract
:1. Introduction
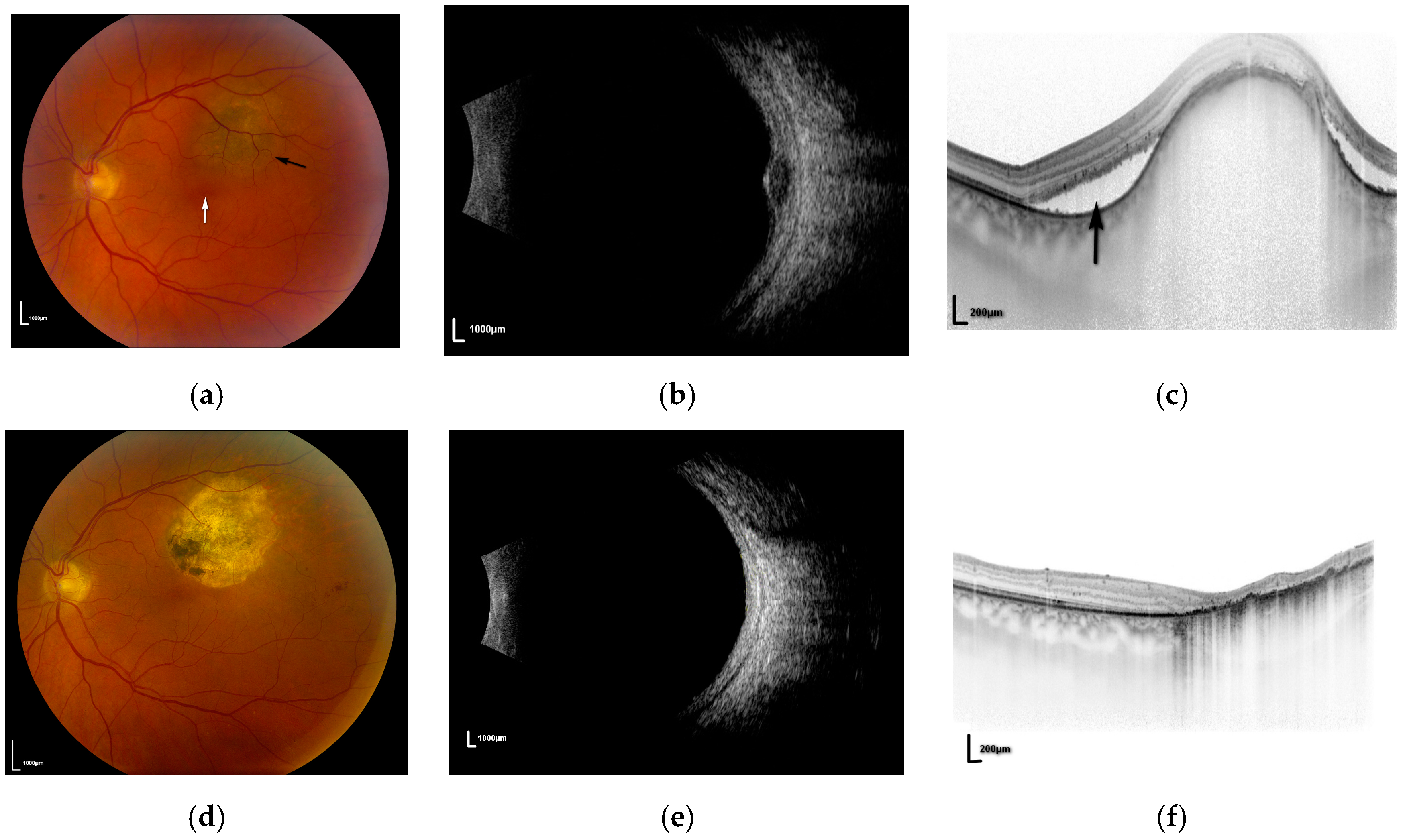
2. Choroidal Melanoma
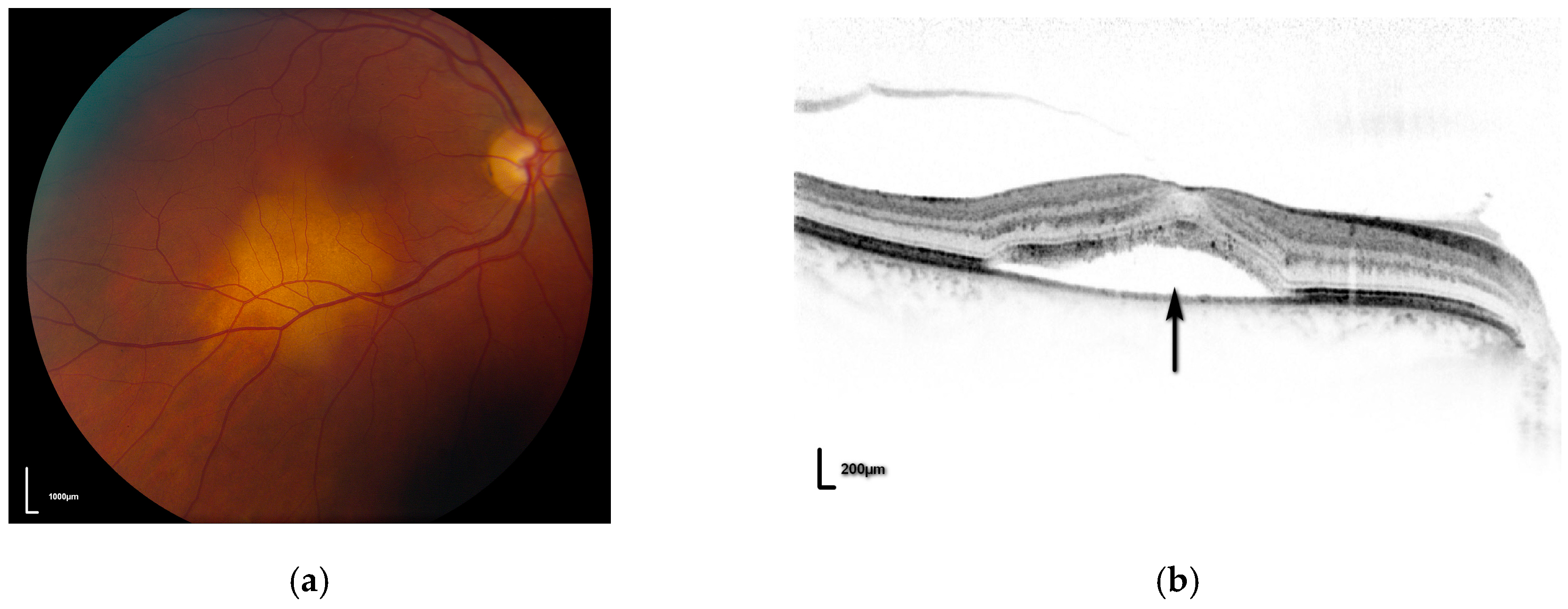
3. Choroidal Metastases
4. Conclusions
Conflicts of Interest
References
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Mork, T. Incidence of malignant neoplasms of the eye in Norway: Incidence, treatment and prognosis. Acta Ophthalmol. 1961, 39, 824–831. [Google Scholar] [CrossRef]
- Mahendraraj, K.; Lau, C.S.M.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7516 patients from the surveillance, epidemiology, and end results database (1973–2012). Clin. Ophthalmol. 2016, 10, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Patel, K.A.; Edmondson, N.D.; Talbot, F.; Parsons, M.A.; Rennie, I.G.; Sisley, K. Prediction of prognosis in patients with uveal melanoma using fluorescence in situ hybridisation. Br. J. Ophthalmol. 2001, 85, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, P.K.; Werschnik, C.; Schuster, E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 129–137. [Google Scholar] [CrossRef]
- Singh, A.; Kivela, T. The Collaborative Ocular Melanoma Study. Ophthalmol. Clin. N. Am. 2005, 18, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Marie Lane, A. Uveal melanoma: Proton beam irradiation. Ophthalmol. Clin. N. Am. 2005, 18, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dinca, E.B.; Yianni, J.; Rowe, J.; Radatz, R.W.R.; Preotiuc-Pietro, D.; Rundle, P.; Rennie, I.; Kemeny, A.A. Survival and complications following γ knife radiosurgery or enucleation for ocular melanoma: A 20-year experience. Acta Neurochi. 2012, 154, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Cairns, J.D. Laser photocoagulation treatment of choroidal melanoma. Aust. N. Z. J. Ophthalmol. 1993, 21, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Rundle, P.A.; Berry-Brincat, A.; Parsons, M.A.; Rennie, I.G. Extrascleral extension of choroidal malignant melanoma following transpupillary thermotherapy. Eye 2004, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Barbazetto, I.A.; Lee, T.C.; Rollins, I.S.; Chang, S.; Abramson, D.H. Treatment of choroidal melanoma using photodynamic therapy. Am. J. Ophthalmol. 2003, 135, 898–899. [Google Scholar] [CrossRef]
- Soucek, P.; Cihelkova, I. Photodynamic therapy with verteporfin in subfoveal amelanotic choroidal melanoma (A controlled case). Neuro Endocrinol. Lett. 2006, 27, 145–148. [Google Scholar] [PubMed]
- Rundle, P. Treatment of posterior uveal melanoma with multi-dose photodynamic therapy. Br. J. Ophthalmol. 2014, 98, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.G.; Pejnovic, T.M. Treatment of amelanotic choroidal melanoma with photodynamic therapy. Retina 2012, 32, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Fabian, I.D.; Stacey, A.W.; Papastefanou, V.; Al Harby, L.; Arora, A.K.; Sagoo, M.S.; Cohen, V.M.L. Primary photodynamic therapy with verteporfin for small pigmented posterior pole choroidal melanoma. Eye 2017, 31, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Group Toa-rmdwptTS. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials—TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch. Ophthalmol. 1999, 117, 1329–1345. [Google Scholar]
- Schnurrbusch, U.E.K.; Jochmann, C.; Einbock, W.; Wolf, S. Complications after photodynamic therapy. Arch. Ophthalmol. 2005, 123, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Sikuade, M.J.; Salvi, S.; Rundle, P.A.; Errington, D.G.; Kacperek, A.; Rennie, I.G. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye 2015, 29, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Böhringer, T.; Chamot, L.; Goitein, G. Eye retention after proton beam radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 867–880. [Google Scholar] [CrossRef]
- Wackernagel, W.; Holl, E.; Tarmann, L.; Mayer, C.; Avian, A.; Schneider, M.; Kapp, K.S.; Langmann, G. Local tumour control and eye preservation after gamma-knife radiosurgery of choroidal melanomas. Br. J. Ophthalmol. 2014, 98, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Bloch, R.S.; Gartner, S. The incidence of ocular metastatic carcinoma. Arch. Ophthalmol. 1971, 85, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Gross, N.E.; Schwartz, G.P.; Lally, S.E. Survey of 520 Eyes with Uveal Metastases. Ophthalmology 1997, 104, 1265–1276. [Google Scholar] [CrossRef]
- Wiegel, T.; Bottke, D.; Kreusel, K.M.; Schmidt, S.; Bornfeld, N.; Foerster, M.H.; Hinkelbein, W. External beam radiotherapy of choroidal metastases—Final results of a prospective study of the German Cancer Society (ARO 95–08). Radiother. Oncol. 2002, 64, 13–18. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; De Potter, P.; Quaranta, M.; Freire, J.; Brady, L.W.; Barrett, J. Plaque radiotherapy for the management of uveal metastasis. Arch. Ophthalmol. 1997, 115, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Manquez, M.E.; Brown, M.M.; Shields, C.L.; Shields, J.A. Management of choroidal metastases from breast carcinomas using aromatase inhibitors. Curr. Opin. Ophthalmol. 2006, 17, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ghodasra, D.H.; Demirci, H. Photodynamic Therapy for Choroidal Metastasis. Am. J. Ophthalmol. 2016, 161, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Fenicia, V.; Abdolrahimzadeh, S.; Mannino, G.; Verrilli, S.; Balestrieri, M.; Recupero, S.M. Intravitreal bevacizumab in the successful management of choroidal metastases secondary to lung and breast cancer unresponsive to systemic therapy: A case series. Eye 2014, 28, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Maudgil, A.; Sears, K.S.; Rundle, P.A.; Rennie, I.G.; Salvi, S.M. Failure of intravitreal bevacizumab in the treatment of choroidal metastasis. Eye 2015, 29, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L.; Al-Dahmash, S.A.; Mashayekhi, A.; Shields, J.A. Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology 2012, 119, 1218–1222. [Google Scholar] [CrossRef] [PubMed]

| Reference | Number Treated | Pigmented (%) | Thickness Range/mm | Fluence J/cm2 | Follow-Up/Months |
|---|---|---|---|---|---|
| [15] | 18 | 9 (50%) | 0.5–4.4 | 100 | 10–42 |
| [17] | 15 | 15 (100%) | 0.9–2.7 | 100 | 8–18 |
| [16] | 9 | 0 | 1.3–5.7 | 50 (50%) 100 (50%) | 34–90 |
| Reference | Regressed (%) | Recurrence/Failure (%) | Vision Improved (%) | Vision Stable (%) | Visual Impairment (%) |
|---|---|---|---|---|---|
| [15] | 16 (88) | 2 (12) | 6 (33) | 10 (56) | 2 (11) |
| [17] | 12 (80) | 3 (20) | Not stated | Not stated | 0 |
| [16] | 8 (89) | 1 (11) | 3 (33) | 6 (67) | 0 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rundle, P. Photodynamic Therapy for Eye Cancer. Biomedicines 2017, 5, 69. https://doi.org/10.3390/biomedicines5040069
Rundle P. Photodynamic Therapy for Eye Cancer. Biomedicines. 2017; 5(4):69. https://doi.org/10.3390/biomedicines5040069
Chicago/Turabian StyleRundle, Paul. 2017. "Photodynamic Therapy for Eye Cancer" Biomedicines 5, no. 4: 69. https://doi.org/10.3390/biomedicines5040069




