Immune Landscape of Breast Cancers
Abstract
:1. Breast Cancer
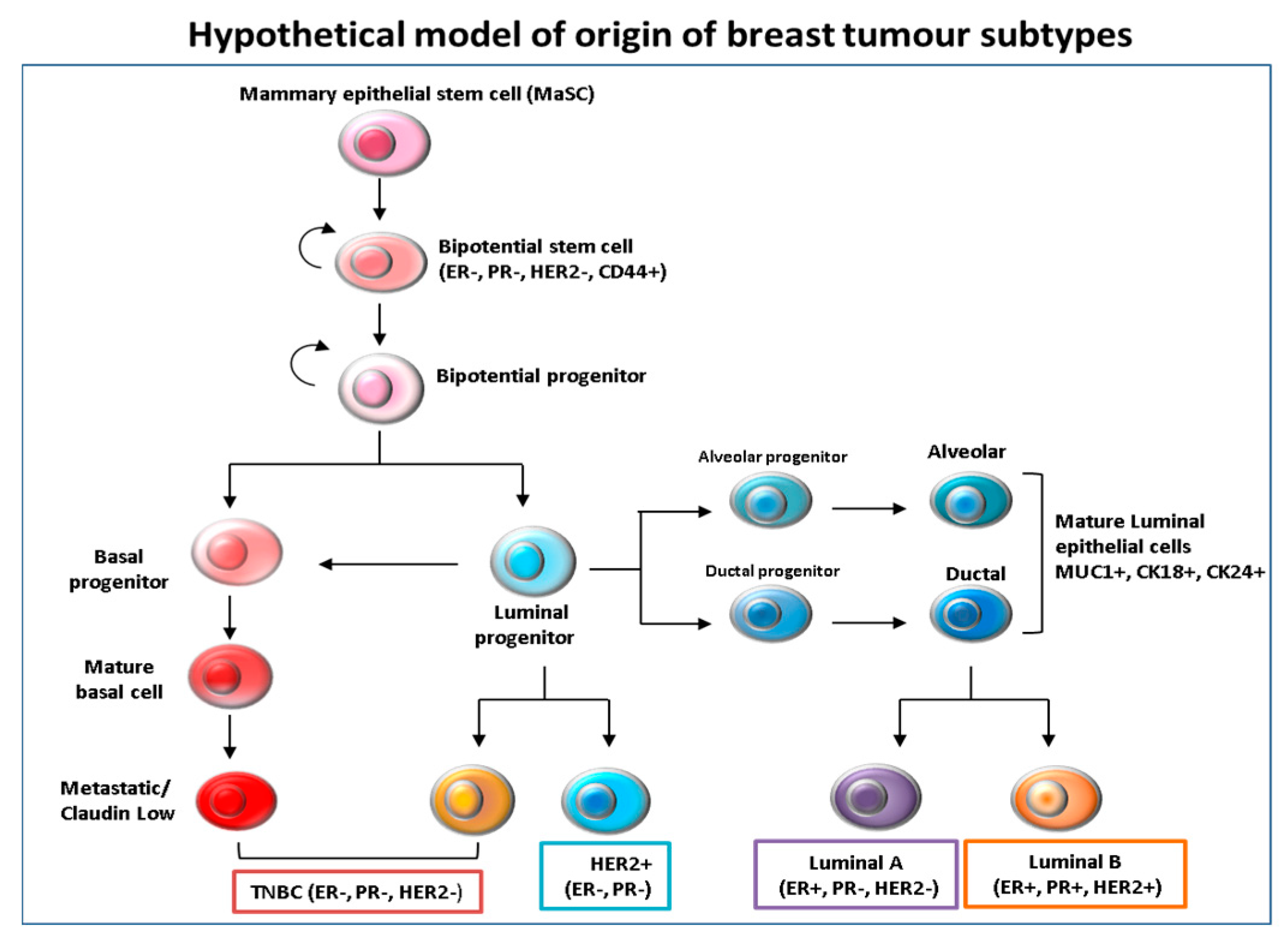
2. Tumor Heterogeneity
3. Crosstalk between Immune Cells and Breast Cancer
4. Prognostic Values of Tumor Infiltrating Lymphocytes (TILS) in TNBC
5. Prognostic Values of TILS in HER2 Expressing Breast Cancers
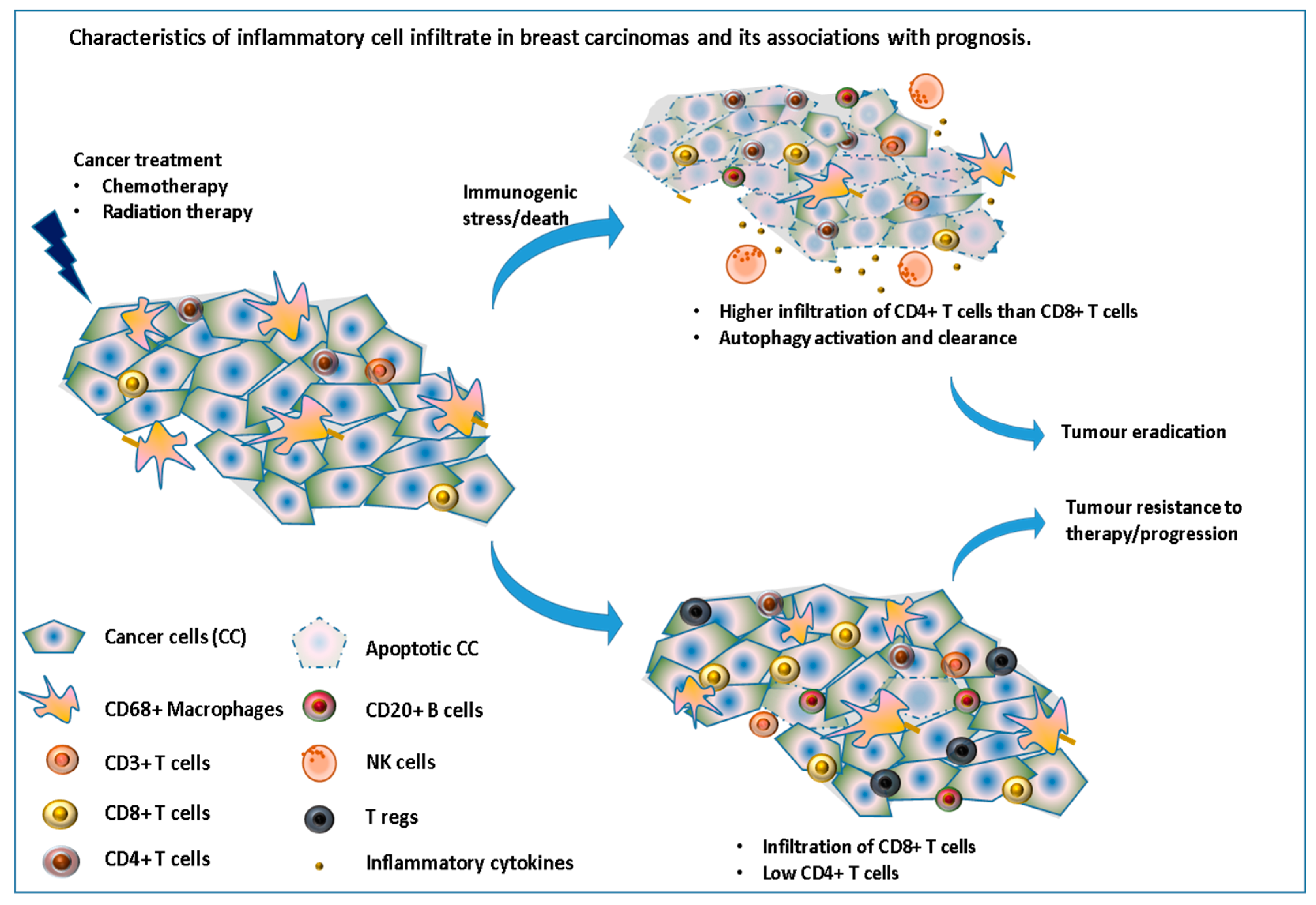
6. The Role of the Immune System in the Response to Chemo/Radiation Therapy
7. Future of Immunotherapy (IT) in Breast Cancer (BC)
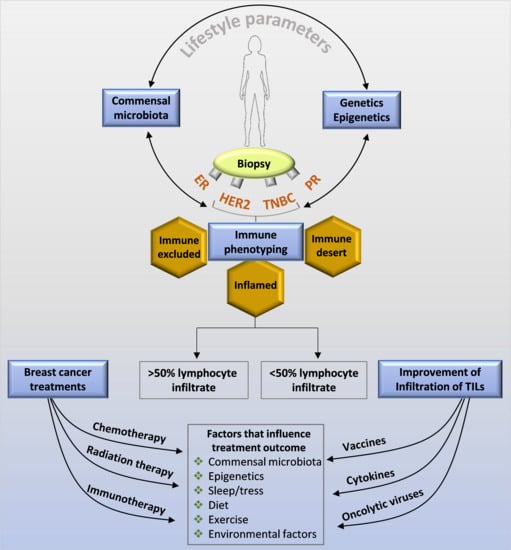
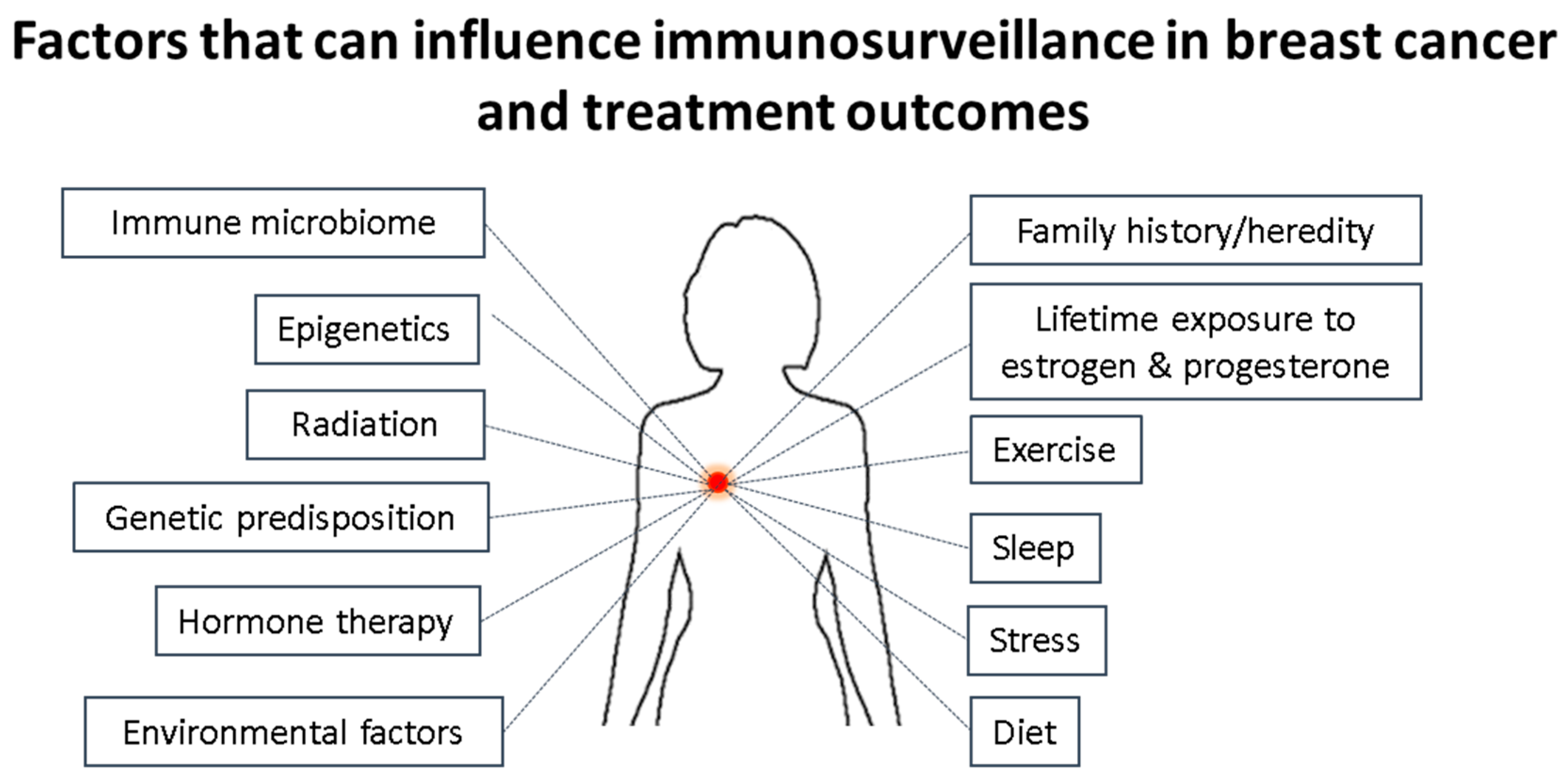
8. Role of Microbiome in Breast Carcinogenesis
9. Conclusions
Author Contributions
Conflicts of Interest
References
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumour subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Vaillant, F.; Wu, D.; Forrest, N.C.; Pal, B.; Hart, A.H.; Asselin-Labat, M.L.; Gyorki, D.E.; Ward, T.; Partanen, A.; et al. Aberrant luminal progenitors as the candidate target population for basal tumour development in BRCA1 mutation carriers. Nat. Med. 2009, 15, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumour heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Morale, M.C.; Sambataro, D.; Farinella, Z.; Scapagnini, U.; Marchetti, B. The immune system response during development and progression of carcinogen-induced rat mammary tumours: Prevention of tumour growth and restoration of immune system responsiveness by thymopentin. Breast Cancer Res. Treat. 1993, 27, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumour Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Scott, J.; Maecker, H.T.; Park, J.W.; Esserman, L.J. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res. Treat. 2005, 91, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Kanelopoulos, P.; Boulamatsis, D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer. 2002, 87, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.Z.; Terunuma, H.; Takada, M.; Tanaka, Y.; Abe, H.; Sata, T.; Toi, M.; Yamamoto, N. Role of natural killer cells in hormone-independent rapid tumour formation and spontaneous metastasis of breast cancer cells in vivo. Breast Cancer Res. Treat. 2007, 104, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Syed Khaja, A.S.; Toor, S.M.; El Salhat, H.; Faour, I.; Ul Haq, N.; Ali, B.R.; Elkord, E. Preferential accumulation of regulatory T cells with highly immunosuppressive characteristics in breast tumour microenvironment. Oncotarget 2017, 8, 33159–33171. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Kim, I.S.; Tian, L.; Gao, X.; Wang, H.; Li, J.; Holdman, X.B.; Herschkowitz, J.I.; Pond, A.; Xie, G.; et al. Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nat. Cell Biol. 2016, 18, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Dushyanthen, S.; Beavis, P.A.; Savas, P.; Teo, Z.L.; Zhou, C.; Mansour, M.; Darcy, P.K.; Loi, S. Relevance of tumour-infiltrating lymphocytes in breast cancer. BMC Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Loi, S. Tumour-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2013, 2, e24720. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumour-infiltrating lymphocytes in triple-negative breast cancers from two phase-III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The Prognostic Value of Tumour-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumour-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Chlon, L.; Pharoah, P.D.P.; Markowetz, F. Caldas Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study. PLoS Med. 2016, 13, e1002194. [Google Scholar] [CrossRef] [PubMed]
- Wells, A. EGFR receptor. Int. J. Biochem. Cell Biol. 1999, 31, 637–643. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), S9–S15. [Google Scholar] [CrossRef]
- Moasser, M.M.; Basso, A.; Averbuch, S.D.; Rosen, N. The tyrosine kinase inhibitor ZD1839 (‘Iressa’) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001, 61, 7184–7188. [Google Scholar] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.M.; Pinnaduwage, D.; Tchatchou, S.; Bull, S.B.; Andrulis, I.L. Validation of Intratumoral T-bet+ Lymphoid Cells as Predictors of Disease-Free Survival in Breast Cancer. Cancer Immunol. Res. 2016, 4, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Ali, R.; Wendt, M.K. The paradoxical functions of EGFR during breast cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16042. [Google Scholar] [CrossRef] [PubMed]
- Bouchalova, M.; Cizkova, K.; Cwiertka, R.; Trojanec, M. Hajduch Triple negative breast cancer—Current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and C-MYC gene assessment. Biomed. Pap. Med. Fac Univ. Palacky Olomouc 2009, 153, 13–18. [Google Scholar] [CrossRef]
- Bear, H.D.; Anderson, S.; Smith, R.E.; Geyer, C.E.; Mamounas, E.P.; Fisher, B.; Brown, A.M.; Robidoux, A.; Margolese, R.; Kahlenberg, M.S.; et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2006, 24, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Nakashoji, A.; Matsui, A.; Nagayama, A.; Iwata, Y.; Sasahara, M.; Murata, Y. Clinical predictors of pathological complete response to neoadjuvant chemotherapy in triple-negative breast cancer. Oncol. Lett. 2017, 14, 4135–4141. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Bloy, N.; Garcia, P.; Laumont, C.M.; Pitt, J.M.; Sistigu, A.; Stoll, G.; Yamazaki, T.; Bonneil, E.; Buqué, A.; Humeau, J.; et al. Immunogenic stress and death of cancer cells: Contribution of antigenicity vs. adjuvanticity to immunosurveillance. Immunol. Rev. 2017, 280, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kareva, I. A Combination of Immune Checkpoint Inhibition with Metronomic Chemotherapy as a Way of Targeting Therapy-Resistant Cancer Cells. Int. J. Mol. Sci. 2017, 18, 2134. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, E.; Gil, G.L.; Benito, A.C.; González-Billalabeitia, E.; Conesa, M.A.; García García, T.; García-Garre, E.; Vicente, V.; de la Peña, A. Tumour-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.; Garber, J.E.; Hacker, M.R. Prevalence and predictors of androgen receptor and programmed death-ligand 1 in BRCA1-associated and sporadic triple-negative breast cancer. NPJ Breast Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Kitano, A.; Ono, M.; Yoshida, M.; Noguchi, E.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yunokawa, M.; Yonemori, K.; Shimizu, C.; et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017, 2, e000150. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, N.; Dhakal, A.; Opyrchal, M. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer. Theranostics Can Res. 2017, 1, 1–4. [Google Scholar]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.; Firek, A.F.; Langridge, W.H.R. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.; Cho, M.S.; Lim, W.; Moon, B.; Sung, S.H. Strong Correlation of Indoleamine 2,3-Dioxygenase 1 Expression with Basal-Like Phenotype and Increased Lymphocytic Infiltration in Triple-Negative Breast Cancer. J. Cancer 2017, 8, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cancer Immunity Peptide Database. Available online: http://cancerimmunity.org/peptide/ (accessed on 5 January 2018).
- Abdel-Fatah, T.M.A.; McArdle, S.E.; Agarwal, D.; Moseley, P.M.; Green, A.R.; Ball, G.R.; Pockley, A.G.; Ellis, I.O.; Rees, R.C.; Chan, S.Y.T. HAGE in triple negative breast cancer (TNBC) is a novel prognostic, predictive and actionable biomarker: A transcriptomic and protein expression analysis. Clin. Cancer Res. 2015, 22, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Bardowell, S.; Parker, J.; Fan, C.; Crandell, J.; Perou, C.; Swift-Scanlan, T. Differential methylation relative to breast cancer subtype and matched normal tissue. Breast Cancer Res. Treat. 2013, 142, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Toland, A.E. Aberrant Epigenetic Regulation in Breast Cancer. In Patho-Epigenetics of Disease; Minarovits, J., Niller, H., Eds.; Springer: New York, NY, USA, 2012; pp. 91–122. ISBN 978-1-4614-3345-3. [Google Scholar]
- Takahashi, K. Influence of bacteria on epigenetic gene control. Cell. Mol. Life Sci. 2014, 71, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.J.; Schairer, C.; Gail, M.H.; Boyd-Morin, J.; Xu, X.; Sue, L.Y.; Buys, S.S.; Isaacs, C.; Keefer, L.K.; Veenstra, T.D.; et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2012, 104, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.; Ley, R.; Hamady, M.; Fraser-Liggett, C.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Poutahidis, T.; Ge, Z.; Nambiar, P.; Horwitz, B.; Fox, J.; Erdman, S. Proinflammatory CD4+ CD45RBhi lymphocytes promote mammary and intestinal carcinogenesis in apcMin/+ mice. Cancer Res. 2006, 66, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.; Hu, B.; Jin, C.; Flavell, R. Inflammation induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.; Gloor, G.; Baban, C.; Reid, G. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.; Chung, A.; Di Nome, M.; Chung, M.; Sieling, P.; Lee, D. Microbial dysbiosis is associated with human breast cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Watters, C.; Koenig, L.; Youn, E.; Olmos, A.; Li, G.; Williams, S.C.; Rumbaugh, K. Human transcriptome analysis reveals a potential role for active transport in the metabolism of pseudomonas aeruginosa autoinducers. Microbes Infect. 2010, 12, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bryan, J.; Kumar, S. Bacterial quorum sensing molecule N-3-oxododecanoyl-l-homoserine lactone causes direct cytotoxicity and reduced cell motility in human pancreatic carcinoma cells. PLoS ONE 2014, 9, e106480. [Google Scholar] [CrossRef]
- Jing, M.; Mao, X.; Li, C.; Wei, J.; Liu, C.; Jin, F. Estrogen receptor-alpha promoter methylation in sporadic basal-like breast cancer of Chinese women. Tumour Biol. 2011, 32, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Reading, N.; Kasper, D. The starting lineup: Key microbial players in intestinal immunity and homeostasis. Front. Microbiol. 2011, 2, 148. [Google Scholar] [CrossRef] [PubMed]
- Shapira, I.; Sultan, K.; Lee, A.; Taioli, E. Evolving concepts: How diet and the intestinal microbiome act as modulators of breast malignancy. ISRN Oncol. 2013, 2013, 693920. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.; Wang, H.; Lane, K.; Scott, J.; Orans, J.; Koo, J.; Venkatesh, M.; Jobin, C.; Yeh, L.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, E.; Fernandez, L.; Maldonado, A.; Martin, R.; Olivares, M.; Xaus, J.; Rodriguez, J.M. Oral Administration of Lactobacillus Strains Isolated from Breast Milk as an Alternative for the reatment of Infectious Mastitis Microbiology. Appl. Environ. Microbiol. 2008, 74, 4650–4655. [Google Scholar] [CrossRef] [PubMed]
- Maroof, H.; Hassan, Z.M.; Mobarez, A.M.; Mohamadabadi, M.A. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model. J. Clin. Immunol. 2012, 32, 1353–1359. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagarajan, D.; McArdle, S.E.B. Immune Landscape of Breast Cancers. Biomedicines 2018, 6, 20. https://doi.org/10.3390/biomedicines6010020
Nagarajan D, McArdle SEB. Immune Landscape of Breast Cancers. Biomedicines. 2018; 6(1):20. https://doi.org/10.3390/biomedicines6010020
Chicago/Turabian StyleNagarajan, Divya, and Stephanie E. B. McArdle. 2018. "Immune Landscape of Breast Cancers" Biomedicines 6, no. 1: 20. https://doi.org/10.3390/biomedicines6010020