Bryonolic Acid Blocks Cancer Cell Clonogenicity and Invasiveness through the Inhibition of Fatty Acid: Cholesteryl Ester Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Assays for Cholesterol Esterification (ACAT) Inhibition
2.3. Cell Culture
2.4. Assay for ACAT Activity in Intact Cells
2.5. Oestrogen Receptor Binding Assay
2.6. Cholesterol Epoxide Hydrolase (ChEH) Assays
2.7. Measurement of the Effect of Compounds on Cholesterol Esterification
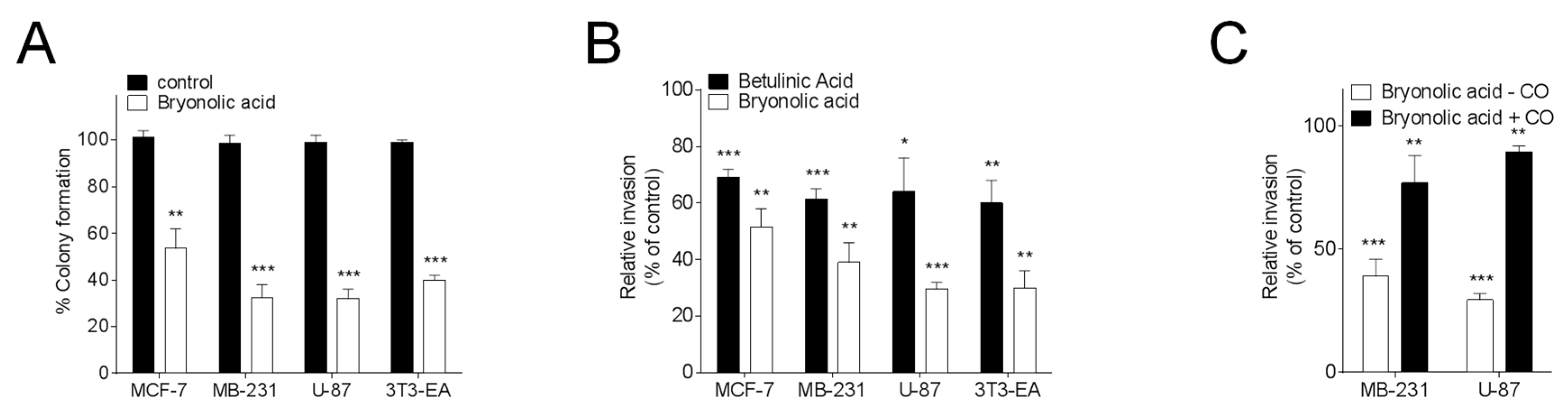
2.8. Clonogenic Assay
2.9. Cell Invasion Assays
2.10. Statistical Analysis
3. Results and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BrA | Bryonolic acid |
| Sah 058-035 | 3-[decyldimethylsilyl]-N-[2-(4-methylphenyl)-1-phenylethyl]-propanamide |
| ACAT | Acyl-CoA:cholesterol Acyl Transferase |
References
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Kondo, T.; Mizukami, H.; Ogihara, Y. Bryonolic acid production in hairy roots of Trichosanthes kirilowii Max. var Japonica Kitam. Transformed with Agrobacterium rhizogenes and its cytotoxic activity. Chem. Pharm. Bull. 1994, 42, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Lin, M.S.; Jiang, R.L.; Huang, S.C.; Ho, L.K. 20-Epibryonolic acid, phytosterols and ellagic acid from Coriaria intermedia. Phytochemistry 1996, 42, 559–560. [Google Scholar] [CrossRef]
- Khallouki, F.; Hull, W.E.; Owen, R.W. Characterization of a rare triterpenoid and minor phenolic compounds in the root bark of Anisophyllea dichostyla R. Br. Food Chem. Toxicol. 2009, 47, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Kongtun, S.; Jiratchariyakul, W.; Kummalue, T.; Tan-Ariya, P.; Kunnachak, S.; Frahm, A.W. Cytotoxic properties of root extract and fruit juice of Trichosanthes cucumerina. Planta Med. 2009, 75, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Gatbonton-Schwager, T.N.; Letterio, J.J.; Tochtrop, G.P. Bryonolic acid transcriptional control of anti-inflammatory and antioxidant genes in macrophages in vitro and in vivo. J. Nat. Prod. 2012, 75, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Ye, M.; Zhang, Y.; Xu, W.; Li, H.; Xu, W.; Chu, K. Bryonolic acid, a triterpenoid, protect against N-methyl-d-aspartate-induced neurotoxicity in PC12 Cells. Molecules 2016, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Doria, M.; Maugest, L.; Moreau, T.; Lizard, G.; Vejux, A. Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic. Biol. Med. 2016, 101, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B. The intracellular cholesterol landscape: Dynamic integrator of the immune response. Trends Immunol. 2016, 37, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Leignadier, J.; Dalenc, F.; Poirot, M.; Silvente-Poirot, S. Improving the efficacy of hormone therapy in breast cancer: The role of cholesterol metabolism in SERM-mediated autophagy, cell differentiation and death. Biochem. Pharmacol. 2017, 144, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kloudova, A.; Guengerich, F.P.; Soucek, P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 2017, 28, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Poirot, M. Cancer. Cholesterol and cancer, in the balance. Science 2014, 343, 1445–1446. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Poirot, M. Cholesterol metabolism and cancer: The good, the bad and the ugly. Curr. Opin. Pharmacol. 2012, 12, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Segala, G.; David, M.; de Medina, P.; Poirot, M.; Serhan, N.; Vergez, F.; Mougel, A.; Saland, E.; Carayon, K.; Leignadier, J.; et al. Dendrogenin A drives LXR to trigger lethal autophagy in cancers. Nat. Commun. 2017, 8, 1903. [Google Scholar] [CrossRef] [PubMed]
- Dalenc, F.; Poirot, M.; Silvente-Poirot, S. Dendrogenin A: A mammalian metabolite of cholesterol with tumor suppressor and neurostimulating properties. Curr. Med. Chem. 2015, 22, 3533–3549. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Paillasse, M.R.; Segala, G.; Voisin, M.; Mhamdi, L.; Dalenc, F.; Lacroix-Triki, M.; Filleron, T.; Pont, F.; Saati, T.A.; et al. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat. Commun. 2013, 4, 1840. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Paillasse, M.R.; Payre, B.; Silvente-Poirot, S.; Poirot, M. Synthesis of new alkylaminooxysterols with potent cell differentiating activities: Identification of leads for the treatment of cancer and neurodegenerative diseases. J. Med. Chem. 2009, 52, 7765–7777. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Genovese, S.; Paillasse, M.R.; Mazaheri, M.; Caze-Subra, S.; Bystricky, K.; Curini, M.; Silvente-Poirot, S.; Epifano, F.; Poirot, M. Auraptene is an inhibitor of cholesterol esterification and a modulator of estrogen receptors. Mol. Pharmacol. 2010, 78, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; de Medina, P.; Caze-Subra, S.; Bystricky, K.; Balaguer, P.; Poirot, M.; Silvente-Poirot, S. Molecular and biochemical analysis of the estrogenic and proliferative properties of Vitamin E compounds. Front. Oncol. 2015, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Li, J.; Traer, E.; Tyner, J.W.; Zhou, A.; Oh, S.T.; Cheng, J.X. Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia. PLoS ONE 2017, 12, e0179558. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Lopez-Vilaro, L.; Nasarre, L.; Perez-Olabarria, M.; Vazquez, T.; Escuin, D.; Badimon, L.; Barnadas, A.; Lerma, E.; Llorente-Cortes, V. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: A molecular and clinicopathological study. BMC Cancer 2015, 15, 460. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Li, J.; Lee, S.Y.; Lee, H.J.; Shao, T.; Song, B.; Cheng, L.; Masterson, T.A.; Liu, X.; Ratliff, T.L.; et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014, 19, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paillasse, M.R.; de Medina, P.; Amouroux, G.; Mhamdi, L.; Poirot, M.; Silvente-Poirot, S. Signaling through cholesterol esterification: A new pathway for the cholecystokinin 2 receptor involved in cell growth and invasion. J. Lipid Res. 2009, 50, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Payre, B.L.; Bernad, J.; Bosser, I.; Pipy, B.; Silvente-Poirot, S.; Favre, G.; Faye, J.C.; Poirot, M. Tamoxifen is a potent inhibitor of cholesterol esterification and prevents the formation of foam cells. J. Pharmacol. Exp. Ther. 2004, 308, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Gales, C.; Sanchez, D.; Poirot, M.; Pyronnet, S.; Buscail, L.; Cussac, D.; Pradayrol, L.; Fourmy, D.; Silvente-Poirot, S. High tumorigenic potential of a constitutively active mutant of the cholecystokinin 2 receptor. Oncogene 2003, 22, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Paillasse, M.R.; Segala, G.; Poirot, M.; Silvente-Poirot, S. Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc. Natl. Acad. Sci. USA 2010, 107, 13520–13525. [Google Scholar] [CrossRef] [PubMed]
- Segala, G.; de Medina, P.; Iuliano, L.; Zerbinati, C.; Paillasse, M.R.; Noguer, E.; Dalenc, F.; Payre, B.; Jordan, V.C.; Record, M.; et al. 5,6-Epoxy-cholesterols contribute to the anticancer pharmacology of tamoxifen in breast cancer cells. Biochem. Pharmacol. 2013, 86, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, D.; Lee, S.S.; Song, B.; Bandyopadhyay, S.; Chen, S.; Konieczny, S.F.; Ratliff, T.L.; Liu, X.; Xie, J.; et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 2016, 35, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediatedlipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Uda, S.; Accossu, S.; Spolitu, S.; Collu, M.; Angius, F.; Sanna, F.; Banni, S.; Vacca, C.; Murru, E.; Mulas, C.; et al. A lipoprotein source of cholesteryl esters is essential for proliferation of CEM-CCRF lymphoblastic cell line. Tumor Biol. 2012, 33, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in Hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid. Based Complement. Altern. Med. 2011, 2011, 801272. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Im, K.R.; Park, Y.D.; Sung, N.D.; Jeong, T.S. Human ACAT-1 and ACAT-2 inhibitory activities of pentacyclic triterpenes from the leaves of Lycopus lucidus TURCZ. Biol. Pharm. Bull. 2006, 29, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Chen, L.L.; Clader, J.W.; McPhail, A.T.; Burnett, D.A.; Bartner, P.; Das, P.R.; Pramanik, B.N.; Puar, M.S.; Feinmark, S.J.; et al. Rabbit and human liver contain a novel pentacyclic triterpene ester with acyl-CoA: Cholesterol acyltransferase inhibitory activity. J. Biol. Chem. 1990, 265, 8042–8051. [Google Scholar] [PubMed]
- Jordan, V.C. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat. Rev. Cancer 2007, 7, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Silvente-Poirot, S.; Poirot, M. Cholesterol epoxide hydrolase and cancer. Curr. Opin. Pharmacol. 2012, 12, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Kedjouar, B.; de Medina, P.; Oulad-Abdelghani, M.; Payre, B.; Silvente-Poirot, S.; Favre, G.; Faye, J.C.; Poirot, M. Molecular characterization of the microsomal tamoxifen binding site. J. Biol. Chem. 2004, 279, 34048–34061. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [PubMed]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]

| Compounds | IC50 in µM |
|---|---|
| sah 58-035 | 0.65 ± 0.22 |
| bryonolic acid | 12.6 ± 2.4 |
| betulinic acid | 18.5 ± 2.1 |
| ursolic acid | 71.4 ± 5.1 |
| MCF-7 | MB-231 | U-87 | 3T3-EA | |
|---|---|---|---|---|
| Compounds | IC50 in µM | |||
| sah 58-035 | 5.1 ± 0.5 | 9.1 ± 1.4 | 9.2 ± 2.2 | 7.4 ± 1.8 |
| bryonolic acid | 22.5 ± 3.7 | 29.5 ± 5.5 | 17.5 ± 4.8 | 19.4 ± 7.6 |
| betulinic acid | 53.7± 4.2 | 58.3 ± 8.1 | 61.4 ± 9.4 | 60.2 ± 8.0 |
| ursolic acid | n.m. | n.m. | n.m. | n.m |
| Compounds | ERα | ERβ | ChEH |
|---|---|---|---|
| IC50 in nM | |||
| E2 | 1.2 ± 0.5 | 1.7 ± 0.7 | n.m. |
| tamoxifen | 45.7 ± 5.1 | 58.4 ± 7.1 | 122.6 |
| bryonolic acid | n.m. | n.m. | n.m. |
| betulinic acid | n.m. | n.m. | n.m. |
| ursolic acid | n.m. | n.m. | n.m. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khallouki, F.; Owen, R.W.; Silvente-Poirot, S.; Poirot, M. Bryonolic Acid Blocks Cancer Cell Clonogenicity and Invasiveness through the Inhibition of Fatty Acid: Cholesteryl Ester Formation. Biomedicines 2018, 6, 21. https://doi.org/10.3390/biomedicines6010021
Khallouki F, Owen RW, Silvente-Poirot S, Poirot M. Bryonolic Acid Blocks Cancer Cell Clonogenicity and Invasiveness through the Inhibition of Fatty Acid: Cholesteryl Ester Formation. Biomedicines. 2018; 6(1):21. https://doi.org/10.3390/biomedicines6010021
Chicago/Turabian StyleKhallouki, Farid, Robert Wyn Owen, Sandrine Silvente-Poirot, and Marc Poirot. 2018. "Bryonolic Acid Blocks Cancer Cell Clonogenicity and Invasiveness through the Inhibition of Fatty Acid: Cholesteryl Ester Formation" Biomedicines 6, no. 1: 21. https://doi.org/10.3390/biomedicines6010021







