1. Introduction
Connexins are a large family of six-subunit transmembrane hemi-channels. A total of 21 connexin genes have been described in humans, and 20 in mice [
1,
2]. Individual hemi-channels (connexons) as part of a gap junction channel allow for the diffusion of ions and small molecules between the extracellular space and the cytosol, and gap junction channels facilitate the diffusion of ions, metabolites, and signalling molecules between cells [
3,
4].
Lymphedema is an incurable condition resulting from obstruction of the lymphatic system, characterised by localised fluid retention, swelling, and susceptibility to infection. The condition is sub-classified into two varieties: the so-called primary lymphedema is inherited, resulting from mutations in various genes essential for lymphatic development and function, whilst secondary lymphedema is generally a post-operative complication of surgery, usually affecting women undergoing treatment for breast cancer [
5,
6,
7]. The estimates of the proportion of patients affected range from 2–80%, no doubt partly reflecting differences in measurement and diagnostic criteria [
2]. Several other medical factors, such as the stage of cancer at the time of diagnosis, the pathological involvement of lymph nodes, the number of dissected lymph nodes during breast cancer surgery, the type and extent of surgery, and also the extent and method of radio- and chemotherapy are considered important in the development of secondary lymphedema in breast cancer patients. Additionally, patient age, body mass index, and degree of physical activity have all been suggested to influence the risk of developing secondary lymphedema [
7,
8].
Intriguingly, there is also some evidence for genetic predisposition to secondary lymphedema [
9,
10]. For example, mutations in hepatocyte growth factor/high affinity hepatocyte growth factor receptor/mesenchymal-epithelial transition (
HGF/
MET) have been reported in both primary and secondary lymphedema [
9]. This protein is expressed in lymphatic endothelial cells and has functions in cell growth, mobility, differentiation, and intercellular junctions [
9]. Another set of mutations associated with secondary lymphedema affect the connexin Cx47 [
8]. Similar mutations are also associated with Pelizaues–Merzbacher-like disease (PMLD) [
11], spastic paraplegia [
12], and primary lymphedema [
11,
12]. It has been shown that Cx43 is abundantly expressed in the ventricular myocardium and in cardiac neural crest cells and plays an important role in human congenital heart disease [
13].
Connexins adopt complex tertiary structures achieved through the coordination of six subunits, representing a “connexon”, which is capable of generating a gap junction by docking to another connexon on an adjacent cell [
14]. This suggests a general model in which a genetic predisposition to form inappropriate cellular junctions may explain the development of some secondary lymphedemas.
Here, we demonstrate that polymorphisms in another connexin, Cx37, are differentially distributed in patients with and without secondary lymphedema, following surgery for breast cancer. Cx37 is a good candidate marker because it is expressed in the lymphatic system and endothelial cells [
15]. Furthermore, single nucleotide polymorphisms (SNPs) in
GJA4 (the gene that codes for Cx37) have previously been shown to be associated with myocardial infarction and atherosclerosis, suggesting (by analogy with the wide-ranging effects of mutations in
HGF/
MET and Cx47), that Cx37 could have a role in secondary lymphedema [
16].
4. Discussion
The results presented in this paper indicate that two SNPs in the 3’ UTR of the
GJA4 gene are associated with an increased risk of secondary lymphedema in patients being treated for breast cancer.
GJA4 was chosen because it encodes Cx37; other studies have already described that two genes (GJC2 encoding connexin 47 and MET gene) also involved in junction formation have mutations associated with the predisposition to secondary lymphedema [
20,
21]. The results thus provide strong support for the hypothesis that secondary lymphedema is caused at least partly by genetic factors that presumably lead to inappropriate formation of cellular junctions and, consequently, blockage of the lymphatic system. This has important implications for the diagnosis and treatment of lymphedema.
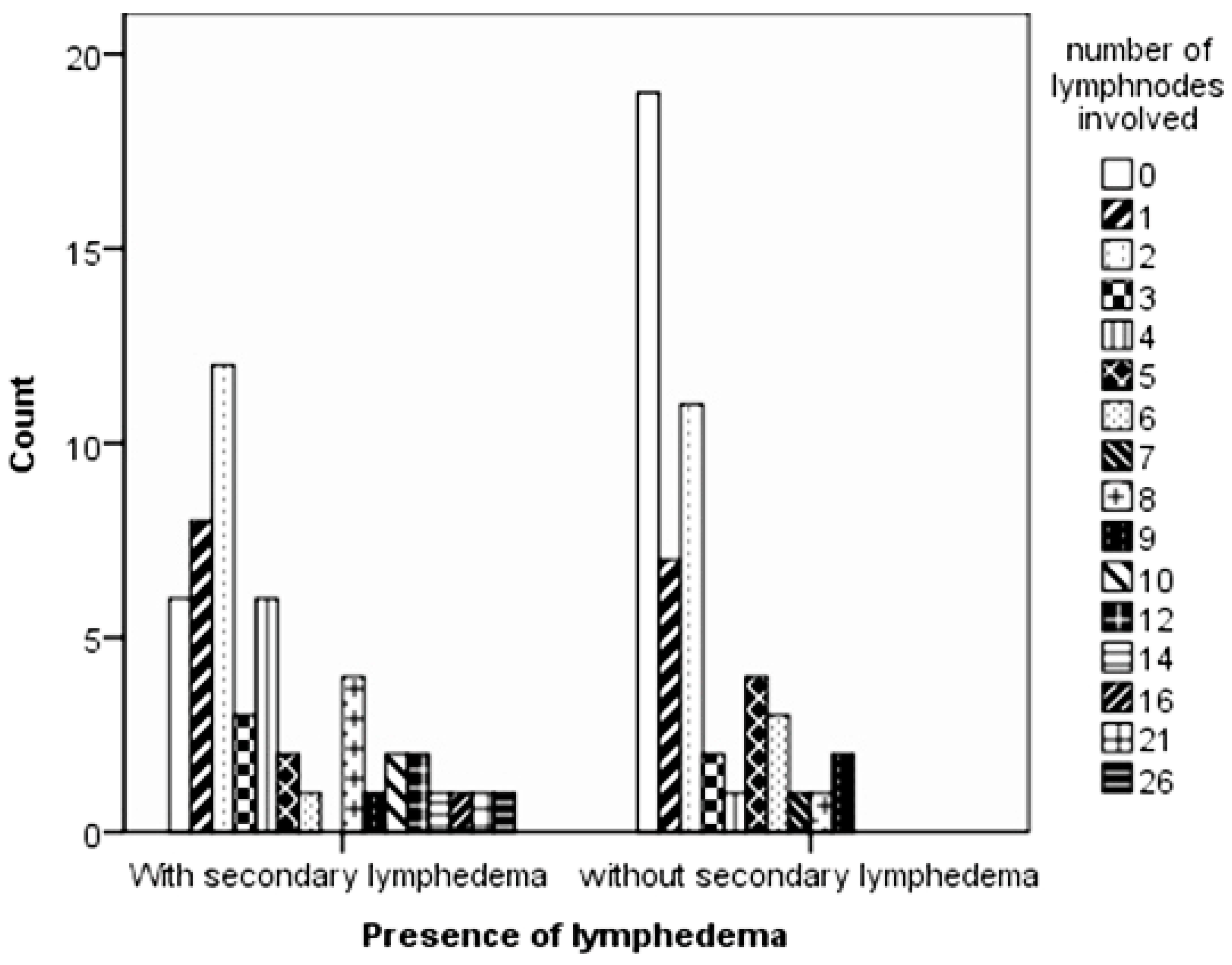
In comparison to the BCS procedure, although statistically not significant, MRM surgical procedure seemed to increase the odds of secondary lymphedema (odds ratio = 2.766,
p = 0.075,
Table 4). Tumour size, number of lymph nodes removed during the surgery, and number of lymph nodes being invaded by the tumour had little impact on the presence of lymphedema (odds ratio close to 1 and
p > 0.05).
The Wald statistic did not indicate that the β coefficients for the genotypes were statistically significantly different from 0 (p > 0.05), however the odds ratios for rs3543 (CC) and in particular for rs705193 (CC) showed their odds in favour of without lymphedema (internal value without lymphedema 0, with lymphedema 1).
It is important to note that the SNPs detected are in a region annotated as a 3’UTR, meaning that a direct effect on the protein sequence is unlikely (albeit we have not shown directly that the protein sequence is actually unaffected by the variation, and there remains a possibility that the annotation of this region may be erroneous). Likely, therefore, the mutation associated with secondary lymphedema affects the post-transcriptional fate of the mRNA through effects on stability, as several microRNAs have already been shown to target other connexin family members [
22].
Alternatively, there might be effects on transcription through long-range interactions. Finally, it is possible that the variation is functionally insignificant and rather an artefact of linkage or some other confounding variable. Though possible, we consider this latter unlikely in view of the fact that other secondary lymphedema-associated mutations also affect junction-forming proteins [
23].
From the molecular pathological point of view, the results presented here suggest that a fruitful approach to secondary lymphedema may be to characterise the cell–cell junctions in healthy and pathological tissues, with the aim of determining, for example, whether the problem is fundamentally linked to junctions that are too tight or too loose [
15,
16]. Given that the 3’UTR of genes is often involved in RNA stability, we may speculate that the mutations result in loss of function, i.e., less RNA and therefore less protein, which would probably manifest as “too loose” junctions. Alternatively, if the mutations remove a microRNA target, the effect would be increased translation, possibly manifesting as “too tight” junctions. This fundamental and essential work is however beyond the scope of the present study.
The lymphatic drainage pathways of the breast (axillary, internal mammary, and supraclavicular nodal groups) are the regional areas most likely to be involved with metastatic breast cancer, and it has been shown that patients who undergo more extensive surgery, have many lymph nodes removed, or have radiation therapy to the axilla or groin after surgery are more likely to develop lymphedema [
24].
The next step of our research, also to increase the strength of our results and conclusion, will be to increase the sample size and to collect similar samples from different geographical areas and other ethnic groups. It is important to notice the importance of ethnicity on the genetic variations and of the sample size, because too big or too small sample sizes have limitations that can compromise the conclusions drawn from studies.
5. Conclusions
The results in this study confirm that the number of lymph nodes being invaded by breast tumours had a statistically significant impact on the presence of lymphedema and that increased lymph node invasion correlated with an increased probability of secondary lymphedema.
Significantly, we have discovered a novel predictive biomarker for the predisposition to secondary lymphedema in breast cancer patients, following surgical intervention. Testing for the condition-associated allele should help inform the treatment and post-operative care of patients, with desirable outcomes for the management of breast cancer. Further study of genes involved in junction formation may reveal additional secondary lymphedema-associated polymorphisms, and hence extra biomarkers, offering an exciting new area of breast cancer research.