Carcinoembryonic Antigen Serum Levels in Nonmelanoma Skin Cancer
Abstract
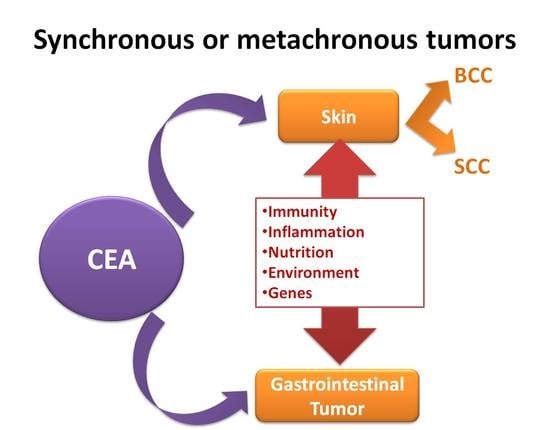
:1. Introduction
2. Methods and Materials
2.1. Patients
2.2. Ethics
2.3. Methods
2.4. Laboratory Analysis
2.5. Tumour Marker Assay
3. Statistical Analysis
4. Results
4.1. Basal Characteristic of NMSC
4.1.1. CEA in the Squamous Cell Carcinoma
4.1.2. CEA in the Basal Cell Carcinoma
4.1.3. CEA in the Matched Control
Comparison with Groups
Comparison with SCC and Dermatitis
Comparison between BCC to Dermatitis
Comparison between SCC to BCC
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Latteri, S.; Teodoro, M.; Malaguarnera, M.; Mannino, M.; Currò, G.; La Greca, G. Abdominal perineal resection or wilde local excision in primary anorectal malignant melanoma. Case report and review. Ann. Med. Surg. 2016, 19, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- La Greca, G.; Santangelo, A.; Primo, S.; Sofia, M.; Latteri, S.; Russello, D.; Magro, G. Clinical and diagnostic problems of desmoid-type fibromatosis of the mesentery: Case report and review of the literature. Ann. Ital. Chir. 2014, 85. pii: S2239253X14023226. [Google Scholar]
- Kwa, R.E.; Campana, K.; Moy, R.L. Biology of cutaneous squamous cell carcinoma. J. Am. Acad. Dematol. 1992, 26, 1–26. [Google Scholar] [CrossRef]
- Levi, F.; La Vecchia, C.; Te, V.C.; Randimbison, L.; Erler, G. Incidence of invasive cancers following basal cell skin cancer. Am. J. Epidemiol. 1998, 147, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.G.; Weinstock, M.A. Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J. Investig. Dermatol. 2007, 127, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Fleischer Jr, A.B.; Smith, E.D.; Kancler, C.; Goldman, N.D.; Williford, P.M.; Feldman, S.R. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol. Surg. 2001, 27, 1035–1038. [Google Scholar] [PubMed]
- Levi, F.; Randimbison, L.; La Vecchia, C.; Erler, G.; Te, V.C. Incidence of invasive cancers following squamous cell skin cancer. Am. J. Epidemiol. 1997, 146, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Aiad, H.A.; Hanout, H.M. Immunohistochemical Expression of CD10 in Cutaneous Basal and Squamous Cell Carcinomas. J. Egypt Natl. Cancer Inst. 2007, 19, 195–201. [Google Scholar]
- Rajabi, P.; Aboutalebdokht, M.; Heidarpour, M.; Asilian, A.; Rajabi, F. Evaluation of diagnostic values of EMA and Ber-Ep4 in distinction between basal cell carcinoma and squamous cell carcinoma of the skin. Iran. J. Pathol. 2007, 2, 7–10. [Google Scholar]
- Malaguarnera, G.; Giordano, M.; Cappellani, A.; Berretta, M.; Malaguarnera, M.; Perrotta, R.E. Skin cancers in elderly patients. Anticancer Agents Med. Chem. 2013, 13, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Catania, V.; Consoli, A.; Cavallaro, A.; Liardo, R.L.; Malaguarnera, M. The neo-adjuvant treatment in gastrointestinal stromal tumor. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 727–730. [Google Scholar] [PubMed]
- Nap, M.; Mollgard, K.; Burtin, P.; Fleuren, G.J. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumor Biol. 1988, 9, 145–153. [Google Scholar] [CrossRef]
- Rees, J.R.; Zens, M.S.; Gui, J.; Celaya, M.O.; Riddle, B.L.; Karagas, M.R. Non melanoma skin cancer and subsequent cancer risk. PLoS ONE 2014, 9, e99674. [Google Scholar] [CrossRef] [PubMed]
- La Greca, G.; Sofia, M.; Lombardo, R.; Latteri, S.; Ricotta, A.; Puleo, S.; Russello, D. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: influence of bilirubin and C-reactive protein. World J. Gastroenterol. 2012, 18, 4150–4155. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Battisti, L.; Betta, A.; Palma, P.D.; Paci, E.; Piffer, S.; Pojer, A.; Polla, E.; Zappa, M. The cytological screening turned out effective also for adenocarcinoma: a population-based case-control study in Trento, Italy. Eur. J. Cancer Prev. 2007, 16, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Wheless, L.; Black, J.; Alberg, A.J. Nonmelanoma skin cancer and the risk of second primary cancers: A systematic review. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Fischer, A.H. Is a personal history of nonmelanoma skin cancer associated with increased or decreased risk of other cancers? Cancer Epidemiol. Biomark. Prev. 2014, 23, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Madeddu, R.; Catania, V.E.; Bertino, G.; Morelli, L.; Perrotta, R.E.; Drago, F.; Malaguarnera, M.; Latteri, S. Anorectal mucosal melanoma. Oncotarget 2018, 9, 8785–8800. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cristaldi, E.; Romano, G.; Malaguarnera, L. Autoimmunity in the elderly: Implications for cancer. J. Cancer Res. Ther. 2012, 8, 520–527. [Google Scholar] [PubMed]
- Metze, D.; Luger, T.A. Ultrastructural localization of carcinoembryonic antigen (CEA) glycoproteins and epithelial membrane antigen (EMA) in normal and neoplastic sweat glands. J. Cutan. Pathol. 1996, 23, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Chisari, G.; Rampello, L.; Chisari, E.M.; Catania, V.E.; Greco, C.; Stagni, E.; Chisari, C.G. Microbiology synergism between tear substitutes and symbiotic treatment of patients with irritable bowel syndrome. Acta Med. Mediterr. 2016, 32, 865–870. [Google Scholar]
- Malaguarnera, M.; Vacante, M.; Condorelli, G.; Leggio, F.; Di Rosa, M.; Motta, M.; Malaguarnera, G.; Alessandria, I.; Rampello, L.; Chisari, G. Probiotics and prebiotics in the management of constipation in the elderly. Acta Med. Mediterr. 2013, 29, 791. [Google Scholar]
- Thompson, J.A.; Grunert, F.; Zimmermann, W. Carcinoembryonic antigen gene family: Molecular biology and clinical perspectives. J. Clin. Lab. Anal. 1991, 5, 344–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Latteri, S.; Malaguarnera, G.; Mannino, M.; Pesce, A.; Currò, G.; Tamburrini, S.; Scuderi, M. Ultrasound as point of care in management of polytrauma and its complication. J. Ultrasound 2017, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6. [Google Scholar] [CrossRef]
- Piccolo, D.; Kostaki, D. Photodynamic Therapy Activated by Intense Pulsed Light in the Treatment of Nonmelanoma Skin Cancer. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Pugliese, L.; Botti, M.; Peri, A.; Cavazzi, E.; Latteri, S.; Auricchio, F.; Pietrabissa, A. Value of 3D printing for the comprehension of surgical anatomy. Surg. Endosc. 2017, 31, 4102–4110. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Vacante, M.; Giordano, M.; Motta, M.; Bertino, G.; Pennisi, M.; Neri, S.; Malaguarnera, M.; Volti, G.L.; Galvano, F. L-carnitine supplementation improves hematological pattern in patients affected by HCV treated with Peg interferon-α 2b plus ribavirin. World J. Gastroenterol. 2011, 17, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Donati, M.; Didona, D.; Mercuri, S.R.; Cantisani, C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Casari, A.; Chester, J.; Pellacani, G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines 2018, 6. [Google Scholar] [CrossRef]
- Cantisani, C.; Paolino, G.; Melis, M.; Faina, V.; Romaniello, F.; Didona, D.; Cardone, M.; Calvieri, S. Actinic Keratosis Pathogenesis Update and New Patents. Recent Pat. Inflamm. Allergy. Drug Discov. 2016, 10, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L.; Cristaldi, E.; Malaguarnera, M. The role of immunity in elderly cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Efird, J.T.; Friedman, G.D.; Habel, L.; Tekawa, I.S.; Nelson, L.M. Risk of subsequent cancer following invasive or in situ squamous cell skin cancer. Ann. Epidemiol. 2002, 12, 469–475. [Google Scholar] [CrossRef]
- Frisch, M.; Hjalgrim, H.; Olsen, J.H.; Melbye, M. Risk for subsequent cancer after diagnosis of basal-cell carcinoma. A population-based, epidemiologic study. Ann. Intern. Med. 1996, 125, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.; Freedman, S.O. Demonstration of tumor specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.F.; Greig, J.D.; Seth, J.; Sturgeon, C.; Nixon, S.J. Raised carcinoembryonic antigen level as an indicator of recurrent disease in follow up of patients with colorectal cancer. Br. J. Gen. Pract. 1995, 45, 287–288. [Google Scholar] [PubMed]
- Ramezani, M.; Mohamadzaheri, E.; Khazaei, S.; Najafi, F.; Vaisi-Raygani, A.; Rahbar, M.; Sadeghi, M. Comparison of EMA,CEA, CD10 and Bcl-2 Biomarkers by Immunohistochemistry in Squamous Cell Carcinoma and Basal Cell Carcinoma of the Skin. Asian Pac. J. Cancer Prev. 2016, 17, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Firnhaber, J.M. Diagnosis and treatment of Basal cell and squamous cell carcinoma. Am. Fam. Physician 2012, 86, 161–168. [Google Scholar] [PubMed]
- Zhang, S.Y.; Lin, M.; Zhang, H.B. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9404–9409. [Google Scholar] [PubMed]
- Malaguarnera, G.; Latteri, S.; Catania, V.E.; Malaguarnera, M. Reduction of cardiovascular risk in subjects with high lipoprotein (a) levels. J. Thorac. Dis. 2017, 9, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Andriulli, A.; Gindro, T.; Piantino, P. Prospective evaluation of the diagnostic efficacy of CA 19-9 assay as a marker for gastrointestinal cancers. Digestion 1986, 33, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Ardiri, A.M.; Calvagno, G.S.; Malaguarnera, G.; Interlandi, D.; Vacante, M.; Bertino, N.; Lucca, F.; Madeddu, R.; Motta, M. Carbohydrate 19.9 antigen serum levels in liver disease. Biomed. Res. Int. 2013, 2013, 531640. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Dermatitis (n. 566) | Group 1 SCC (n. 304) | Group 2 BCC (n. 262) | Controls vs. Group 1 | Controls vs. Group 2 | Group 1 vs. Group 2 | Controls vs. Group 1 + 2 |
|---|---|---|---|---|---|---|---|
| Sex (Male/Female) | 210/256 (45.06%/54.94%) | 148/156 (48.68%/51.32%) | 113/149 (43.13%/56.87%) | -- | -- | -- | -- |
| Age (years) | 65–81 | 65–81 | 65–81 | -- | -- | -- | -- |
| Race | Caucasian | Caucasian | Caucasian | -- | -- | -- | -- |
| Body-mass index (kg/m2) | 24.10 ± 2.40 | 24.20 ± 2.10 | 24.50 ± 2.20 | N.S. | 0.027 | 0.098 | N.S. |
| Systolic blood pressure (mmHg) | 134.20 ± 8.10 | 138.00 ± 7.80 | 137.00 ± 8.10 | <0.001 | <0.001 | N.S. | <0.001 |
| Diastolic blood pressure (mmHg) | 85.60 ± 9.00 | 88.00 ± 9.50 | 86.00 ± 8.90 | <0.001 | N.S. | 0.010 | 0.010 |
| Weight (kg) | 69.10 ± 2.40 | 68.00 ± 2.30 | 71.20 ± 2.80 | <0.001 | <0.001 | <0.001 | 0.014 |
| Aspartate Transaminase (AST) (IU/l) (n.v. 8–18) | 18.20 ± 2.40 | 24.10 ± 2.20 | 23.10 ± 2.00 | <0.001 | <0.001 | <0.001 | <0.001 |
| Alanine Transaminase (ALT) (IU/l) (n.v. 8–18) | 16.60 ± 2.10 | 21.90 ± 2.40 | 21.80 ± 2.60 | <0.001 | <0.001 | N.S. | <0.001 |
| Gamma-glutamyltransferase (γGT) (IU/l) (n.v. 2–30) | 24.90 ± 4.80 | 22.70 ± 3.40 | 22.80 ± 3.20 | <0.001 | <0.001 | N.S. | <0.001 |
| Alkaline Phosphatase (IU/l) (n.v. 35–100) | 36.80 ± 2.40 | 35.10 ± 2.40 | 35.40 ± 2.90 | <0.001 | <0.001 | N.S. | <0.001 |
| Total bilirubin (mg/dL) (n.v. 0.2–1.2) | 0.96 ± 0.20 | 1.04 ± 0.40 | 1.07 ± 0.30 | N.S. | <0.001 | 0.320 | <0.001 |
| Fasting glucose (mg/dL) (n.v. 74–106) | 81.40 ± 7.80 | 87.80 ± 8.10 | 83.20 ± 9.60 | <0.001 | <0.001 | 0.006 | <0.001 |
| Serum urea (mg/dL) (n.v. 6–40) | 31.40 ± 3.10 | 36.00 ± 3.10 | 34.00 ± 3.30 | <0.001 | <0.001 | <0.001 | <0.001 |
| Serum creatinine (mg/dL) (n.v. 0.7–1.3) | 0.80 ± 0.40 | 0.80 ± 0.30 | 0.60 ± 0.30 | N.S. | <0.001 | <0.001 | <0.001 |
| CEA (ng/mL) (n.v. <5) | 4.20 ± 1.20 | 96.10 ± 30.10 | 68.00 ± 30.30 | <0.001 | <0.001 | <0.001 | <0.001 |
| BCC | SCC | Dermatitis | ||||
|---|---|---|---|---|---|---|
| % | CI 95% (LL-UL) | % | CI 95% (LL-UL) | % | CI 95% (LL-UL) | |
| Sensitivity (SE) | 84.2 | (79.1–88.3) | 80.6 | (75.7–84.8) | 40 | (33-47.2) |
| Specificity (SP) | 63.4 | (57.3–69.2) | 62.6 | (56.8–68) | 98.3 | (96.5-99.2) |
| Positive Predective Value (PPV) | 48.5 | (42.3–54.7) | 48.7 | (43–54.5) | 20 | (16.5–24) |
| Negative Predective Value (NPV) | 90.8 | (86.4–93.9) | 88 | (83.7-91.3) | 99.3 | (97.9–99.8) |
| Positive Likelihood Ratio (LR+) | 2.303 | (1.990–2.666) | 2.154 | (1.865–2.488) | 23.050 | (4.399–13.666) |
| Negative Likelihood Ratio (LR−) | 0.249 | (0.215–0.288) | 0.309 | (0.268–0.357) | 0.611 | (0.209–1.787) |
| Prevalence | 29 | (23.7–35) | 30.6 | (25.5–36.2 | 1.1 | (0.4–2.6) |
| Odds pre-test − | 0.409 | -- | 0.441 | -- | 0.011 | -- |
| Odds post-test + | 0.941 | -- | 0.949 | -- | 0.250 | -- |
| Odds post-test − | 0.102 | -- | 0.136 | -- | 0.007 | -- |
| P pre-test | 0.290 | (0.190–0.396) | 0.306 | (0.214–0.403) | 0.011 | (−0.076–0.105) |
| P post-test + | 0.485 | (0.397–0.573) | 0.487 | (0.406–0.568) | 0.200 | (0.121–0.284) |
| P post-test - | 0.092 | (−0.018–0.214) | 0.120 | (0.019–0.231) | 0.007 | (−0.080–0.101) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latteri, S.; Catania, V.E.; Malaguarnera, G.; Peri, A.; Bertino, G.; Frazzetto, G.; Borzì, A.M.; Biondi, A.; Perrotta, R.E.; Malaguarnera, M. Carcinoembryonic Antigen Serum Levels in Nonmelanoma Skin Cancer. Biomedicines 2018, 6, 24. https://doi.org/10.3390/biomedicines6010024
Latteri S, Catania VE, Malaguarnera G, Peri A, Bertino G, Frazzetto G, Borzì AM, Biondi A, Perrotta RE, Malaguarnera M. Carcinoembryonic Antigen Serum Levels in Nonmelanoma Skin Cancer. Biomedicines. 2018; 6(1):24. https://doi.org/10.3390/biomedicines6010024
Chicago/Turabian StyleLatteri, Saverio, Vito Emanuele Catania, Giulia Malaguarnera, Andrea Peri, Gaetano Bertino, Giuseppe Frazzetto, Antonio Maria Borzì, Antonio Biondi, Rosario Emanuele Perrotta, and Michele Malaguarnera. 2018. "Carcinoembryonic Antigen Serum Levels in Nonmelanoma Skin Cancer" Biomedicines 6, no. 1: 24. https://doi.org/10.3390/biomedicines6010024