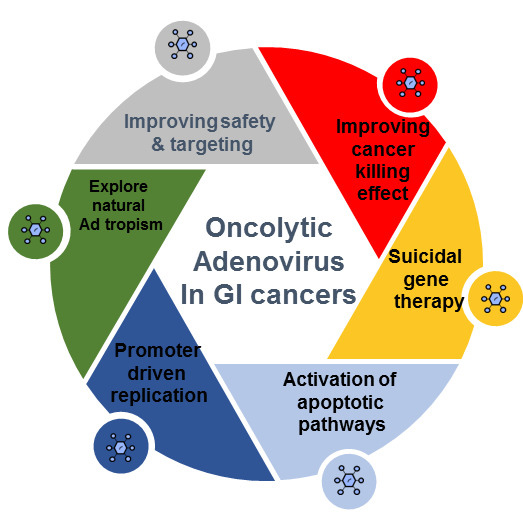
Oncolytic Adenoviruses in Gastrointestinal Cancers
Abstract
:1. Introduction
2. Oncolytic Adenoviral Platform Development
3. Preclinical Perspectives in Gastrointestinal Cancers
3.1. Esophageal Cancer
3.2. Gastric Cancer
3.3. Liver and Biliary Cancers
3.4. Pancreatic Cancer
3.5. Colorectal Cancer
4. Clinical Trials and Translational Period of Research
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017.
- Alemany, R. Oncolytic Adenoviruses in Cancer Treatment. Biomedicines 2014, 2, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.P.; Seto, J.; Liu, E.B.; Dehghan, S.; Hudson, N.R.; Lukashev, A.N.; Ivanova, O.; Chodosh, J.; Dyer, D.W.; Jones, M.S.; et al. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J. Clin. Microbiol. 2011, 49, 3482–3490. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Old, M.O.; Wise-Draper, T.; Wright, C.L.; Kaur, B.; Teknos, T. The current status of oncolytic viral therapy for head and neck cancer. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lahdeaho, M.L.; Lehtinen, M.; Rissa, H.R.; Hyoty, H.; Reunala, T.; Maki, M. Antipeptide antibodies to adenovirus E1b protein indicate enhanced risk of celiac disease and dermatitis herpetiformis. Int. Arch. Allergy Immunol. 1993, 101, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Operario, D.J.; Platts-Mills, J.A.; Nadan, S.; Page, N.; Seheri, M.; Mphahlele, J.; Praharaj, I.; Kang, G.; Araujo, I.T.; Leite, J.P.G.; et al. Etiology of Severe Acute Watery Diarrhea in Children in the Global Rotavirus Surveillance Network Using Quantitative Polymerase Chain Reaction. J. Infect. Dis. 2017, 216, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, S.; Hagbom, M.; Rajan, A.; Loitto, V.; Persson, B.D.; Allard, A.; Nordgren, J.; Sharma, S.; Magnusson, K.E.; Arnberg, N.; et al. Interaction of human enterochromaffin cells with human enteric adenovirus 41 leads to serotonin release and subsequent activation of enteric glia cells. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Matsuoka, J.; Yamatsuji, T.; Maeda, Y.; Durbin, M.L.; Naomoto, Y. Adenovirus-mediated cancer gene therapy and virotherapy. Int. J. Mol. Med. 2010, 25, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.A.; Davydova, J.G.; Adachi, Y.; Takayama, K.; Barker, S.D.; Reynolds, P.N.; Krasnykh, V.N.; Kunisaki, C.; Shimada, H.; Curiel, D.T.; et al. Promoter-controlled infectivity-enhanced conditionally replicative adenoviral vectors for the treatment of gastric cancer. J. Gastroenterol. 2005, 40, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pin, R.H.; Reinblatt, M.; Fong, Y. Utilizing alpha-fetoprotein expression to enhance oncolytic viral therapy in hepatocellular carcinoma. Ann. Surg. 2004, 240, 659–665. [Google Scholar] [PubMed]
- Wang, Y.; Liu, T.; Huang, P.; Zhao, H.; Zhang, R.; Ma, B.; Chen, K.; Huang, F.; Zhou, X.; Cui, C.; et al. A novel Golgi protein (GOLPH2)-regulated oncolytic adenovirus exhibits potent antitumor efficacy in hepatocellular carcinoma. Oncotarget 2015, 6, 13564–13578. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Tazawa, H.; Hashimoto, Y.; Shirakawa, Y.; Kuroda, S.; Nishizaki, M.; Kishimoto, H.; Uno, F.; Nagasaka, T.; Urata, Y.; et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells in S/G2/M phases. Clin. Cancer Res. 2013, 19, 6495–6505. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, W.; Hu, H.; Ma, J.; Li, X.; Mei, W.; Xu, Y.; Hu, H.; Yan, Y.; Song, Q.; et al. Survivin promoter-regulated oncolytic adenovirus with Hsp70 gene exerts effective antitumor efficacy in gastric cancer immunotherapy. Oncotarget 2014, 5, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.Y.; Wang, K.L.; Fang, F.F.; Gu, W.; Huang, F.; Wang, F.Z.; Li, B.; Wang, L.N. Triple-controlled oncolytic adenovirus expressing melittin to exert inhibitory efficacy on hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 10403–10411. [Google Scholar] [PubMed]
- Araki, Y.; Fujiwara, H.; Inada, S.; Atsuji, K.; Yamagishi, H. [An antitumor effect of oncolytic adenovirus capable of selectively replicating in CEA-expressing cancer cells and its enhancement by 5-FU]. Gan Kagaku Ryoho 2006, 33, 1754–1755. [Google Scholar]
- Zhou, X.; Xie, G.; Wang, S.; Wang, Y.; Zhang, K.; Zheng, S.; Chu, L.; Xiao, L.; Yu, Y.; Zhang, Y.; et al. Potent and specific antitumor effect for colorectal cancer by CEA and Rb double regulated oncolytic adenovirus harboring ST13 gene. PLoS ONE 2012, 7, e47566. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Karli, E.; Kipar, A.; Escutenaire, S.; Kanerva, A.; Hemminki, A. Mutation of the fiber shaft heparan sulphate binding site of a 5/3 chimeric adenovirus reduces liver tropism. PLoS ONE 2013, 8, e60032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. Cancer Stat. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Moriyama, H.; Yasuda, H.; Hara, K.; Maniwa, Y.; Hamada, H.; Yokono, K.; Nagata, M. Modification of the Rb-binding domain of replication-competent adenoviral vector enhances cytotoxicity against human esophageal cancers via NF-κB activity. Hum. Gene Ther. 2007, 18, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Komaki, R.; Wang, L.; Fang, B.; Chang, J.Y. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin. Cancer Res. 2008, 14, 2813–2823. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Kawamura, K.; Li, Q.; Okamoto, S.; Suzuki, N.; Kobayashi, H.; Liang, M.; Tada, Y.; Tatsumi, K.; Hiroshima, K.; et al. Combinatory cytotoxic effects produced by E1B-55kDa-deleted adenoviruses and chemotherapeutic agents are dependent on the agents in esophageal carcinoma. Cancer Gene Ther. 2010, 17, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T. A novel molecular therapy using bioengineered adenovirus for human gastrointestinal cancer. Acta Med. Okayama 2011, 65, 151–162. [Google Scholar] [PubMed]
- Zheng, H.; Li, M.S.; Zhao, G.Q.; Dong, Z.M. [Effect of CEA gene regulation on the anti-tumor activity of oncolytic adenovirus H101 to esophageal carcinoma]. Zhonghua Zhong Liu Za Zhi 2011, 33, 822–826. [Google Scholar] [PubMed]
- He, D.; Sun, L.; Li, C.; Hu, N.; Sheng, Y.; Chen, Z.; Li, X.; Chi, B.; Jin, N. Anti-tumor effects of an oncolytic adenovirus expressing hemagglutinin-neuraminidase of Newcastle disease virus in vitro and in vivo. Viruses 2014, 6, 856–874. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Jiang, K.; Al-Diffhala, S.; Centeno, B.A. Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification. Cancer Control 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, D.C.; Chen, Y.; Amin, P.; Zhang, H.; Nguyen, N.; Henderson, D.R. A hepatocellular carcinoma-specific adenovirus variant, CV890, eliminates distant human liver tumors in combination with doxorubicin. Cancer Res 2001, 61, 6428–6436. [Google Scholar] [PubMed]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; McCormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sham, J.; Yang, J.; Su, C.; Xue, H.; Chua, D.; Sun, L.; Zhang, Q.; Cui, Z.; Wu, M.; et al. Potent antitumor efficacy of an E1B 55kDa-deficient adenovirus carrying murine endostatin in hepatocellular carcinoma. Int. J. Cancer 2005, 113, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.C.; Zhang, Q.; Yang, Y.; Lu, M.Q.; Li, H.; Xu, C.; Chen, G.H. p53-expressing conditionally replicative adenovirus CNHK500-p53 against hepatocellular carcinoma in vitro. World J. Gastroenterol. 2007, 13, 683–691. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Lei, W.; Wang, S.; Xiao, R.; Guo, K.; Xia, Y.; Zhou, X.; Zhang, K.; Liu, X.; Wang, Y. Overexpression of tumor suppressor TSLC1 by a survivin-regulated oncolytic adenovirus significantly inhibits hepatocellular carcinoma growth. J. Cancer Res. Clin. Oncol. 2012, 138, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.W.; Zhong, S.Y.; Liu, B.S.; Liu, J.; Cai, R.; Wang, Y.G.; Liu, X.Y.; Qian, C. Enhanced sensitivity of hepatocellular carcinoma cells to chemotherapy with a Smac-armed oncolytic adenovirus. Acta Pharmacol. Sin. 2007, 28, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.L.; Lee, C.H.; Teo, M.L.; Lin, Y.J.; Huang, Y.S.; Wu, C.L.; Shiau, A.L. Transthyretin-driven oncolytic adenovirus suppresses tumor growth in orthotopic and ascites models of hepatocellular carcinoma. Cancer Sci. 2009, 100, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lv, S.; Yang, J.; Wang, X.; Hu, H.; Su, C.; Zhou, C.; Li, J.; Huang, Y.; Li, L.; et al. Use of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cells. PLoS ONE 2011, 6, e21307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Sun, B.; Ji, W.; Peng, Z.; Xu, Y.; Wu, M.; Su, C. An Artificially Designed Interfering lncRNA Expressed by Oncolytic Adenovirus Competitively Consumes OncomiRs to Exert Antitumor Efficacy in Hepatocellular Carcinoma. Mol. Cancer Ther. 2016, 15, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.C.; Cao, X.; Gui, J.H.; Zhou, X.M.; Zhong, D.; Yan, Q.L.; Huang, W.D.; Qian, Q.J.; Zhao, F.L.; Liu, X.Y. Augmenting the antitumor effect of TRAIL by SOCS3 with double-regulated replicating oncolytic adenovirus in hepatocellular carcinoma. Hum. Gene Ther. 2011, 22, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Wei, X.; Cui, Q.; Lou, W.; Wang, G.; Hu, X.; Qian, C. Potent antitumor activity of oncolytic adenovirus-mediated SOCS1 for hepatocellular carcinoma. Gene Ther. 2013, 20, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Li, L.; Wu, H.; Wang, C.; Yan, Z.; Wang, Y.; Su, C.; Jin, H.; Zhou, F.; et al. The combination of an oxygen-dependent degradation domain-regulated adenovirus expressing the chemokine RANTES/CCL5 and NK-92 cells exerts enhanced antitumor activity in hepatocellular carcinoma. Oncol. Rep. 2013, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Ma, B.; Wang, Y.; Xiao, R.; Kong, Y.; Zhou, X.; Xia, D. Targeting gene-virus-mediated manganese superoxide dismutase effectively suppresses tumor growth in hepatocellular carcinoma in vitro and in vivo. Cancer Biother. Radiopharm. 2014, 29, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Zhou, X.; Huang, P.; Zhang, R.; Liu, T.; Cui, C.; Liu, X.; Wang, Y. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J. Cancer Res. Clin. Oncol. 2015, 141, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, M.; Hamada, H.; Motoi, F.; Oonuma, M.; Abe, H.; Saitoh, Y.; Hoshida, T.; Ottomo, S.; Omura, N.; Matsuno, S. Oncolytic virotherapy as a novel strategy for pancreatic cancer. Pancreas 2004, 28, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.O.; Barton, K.N.; Brown, S.L.; Narra, V.; Zhang, Y.; Tyson, D.; Nall, C.; Lu, M.; Ajlouni, M.; Movsas, B.; et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol. Ther. 2007, 15, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Sobrevals, L.; Miguel Camacho-Sanchez, J.; Huch, M.; Andreu, N.; Ayuso, E.; Navarro, P.; Alemany, R.; Fillat, C. Intraductal delivery of adenoviruses targets pancreatic tumors in transgenic Ela-myc mice and orthotopic xenografts. Oncotarget 2013, 4, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Rovira-Rigau, M.; Luna, J.; Gimenez-Alejandre, M.; Vaquero, E.; Garcia de la Torre, B.; Andreu, D.; Alemany, R.; Fillat, C. A genetic fiber modification to achieve matrix-metalloprotease-activated infectivity of oncolytic adenovirus. J. Control Release 2014, 192, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hiraoka, N.; Goto, N.; Rin, Y.; Miura, K.; Narumi, K.; Uchida, H.; Tagawa, M.; Aoki, K. A targeting ligand enhances infectivity and cytotoxicity of an oncolytic adenovirus in human pancreatic cancer tissues. J. Control Release 2014, 192, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Green, N.K.; Reynolds, G.M.; Flavell, J.R.; Mautner, V.; Kerr, D.J.; Young, L.S.; Searle, P.F. Enhanced efficacy of Escherichia coli nitroreductase/CB1954 prodrug activation gene therapy using an E1B-55K-deleted oncolytic adenovirus vector. Gene Ther. 2004, 11, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Schepelmann, S.; Ogilvie, L.M.; Hedley, D.; Friedlos, F.; Martin, J.; Scanlon, I.; Chen, P.; Marais, R.; Springer, C.J. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res 2007, 67, 4949–4955. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Kagawa, S.; Nishizaki, M.; Mizuguchi, H.; Hayakawa, T.; Kyo, S.; Nagai, K.; Urata, Y.; Tanaka, N.; Fujiwara, T. Enhanced oncolysis by a tropism-modified telomerase-specific replication-selective adenoviral agent OBP-405 (‘Telomelysin-RGD’). Oncogene 2005, 24, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, J.; Zhao, L.; He, L.; Qian, W.; Wang, J.; Wang, Y.; Qian, Q.; Qian, C.; Wu, J.; et al. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res 2006, 66, 4291–4298. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liu, H.; Zhang, Z.; Basnet, S.; Dai, Z.; Li, S.; Wang, Y.; Xu, B.; Ge, H. A dual-regulated oncolytic adenovirus carrying TAp63 gene exerts potent antitumor effect on colorectal cancer cells. Am. J. Transl. Res. 2017, 9, 2966–2974. [Google Scholar] [PubMed]
- Sato-Dahlman, M.; Miura, Y.; Huang, J.L.; Hajeri, P.; Jacobsen, K.; Davydova, J.; Yamamoto, M. CD133-targeted oncolytic adenovirus demonstrates anti-tumor effect in colorectal cancer. Oncotarget 2017, 8, 76044–76056. [Google Scholar] [CrossRef] [PubMed]
- Yokoda, R.; Nagalo, B.M.; Vernon, B.; Oklu, R.; Albadawi, H.; DeLeon, T.T.; Zhou, Y.; Egan, J.B.; Duda, D.G.; Borad, M.J. Oncolytic virus delivery: From nano-pharmacodynamics to enhanced oncolytic effect. Oncolytic Virother. 2017, 6, 39–49. [Google Scholar] [CrossRef] [PubMed]
| Subgroup | Serotype | Attachment Receptors | Natural Tropism |
|---|---|---|---|
| A | 12 | CAR | Gastrointestinal |
| B1 | 35 | CD46 | Respiratory |
| B2 | 3 | DSG-2 | Renal |
| B3 | 11 | CD46 and DSG-2 | Renal |
| C | 2 and 5 | CAR | Respiratory |
| D | 19 | CAR and sialic acid | Ocular |
| E | 4 | CAR | Respiratory and Ocular |
| F | 40 and 41 | CAR | Gastrointestinal |
| G | 52 | unknown | Gastrointestinal |
| Virus Promoter | GI Cancer | Ref. |
|---|---|---|
| Cytomegalovirus (CMV) | Ubiquitous | [10] |
| Midkine (MK) | Gastric | [11] |
| Cyclooxygenase-2 (Cox-2M) and (Cox-2L) | Gastric | [11] |
| Alpha-fetoprotein (AFP) | HCC | [12] |
| Golgi protein 73 | HCC | [13] |
| Human telomerase reverse transcriptase (hTERT) | Cancer cells in general | [14] |
| Survivin | Cancer cells in general | [15] |
| Chimeric: Hypoxia-response element (HRE) and Alpha-fetoprotein (AFP) | HCC | [16] |
| Carcinoembryonic antigen (CEA) | Gastric cancer cells and Colon cancer cells | [17,18] |
| Viral Construct Name | In Vitro Cell Line | In Vivo Model | Vector Modifications | Conclusion | Ref. |
|---|---|---|---|---|---|
| AxE1AdB | EC-GI-10 T.Tn. | CB17 scid mouse CDX Heterotopic subcutanous transplant | E1A gene abolish binding to pRB | Enhanced apoptosis, and cytotoxicity against p53-mutant cells | [21] |
| Ad/TRAIL-E1 | Seg-1 Seg-1 with Radioresistance (R) TE-2, and TE-2R | Nude mice CDX Heterotopic subcutanous transplant | hTERT promoter controlling E1A | Ad/TRAIL-E1 preferentially targeted radioresistant-cells | [22] |
| Ad-delE1B55 | TE-1 TE-2 TE-10 TE-11 YES-2 YES-4 YES-5 YES-6 T. Tn | Nude mice CDX Heterotopic subcutanous transplant | CMV Promoter Deleted a part of E3 region and 55 kDa-encoding E1B region | The combinatory antitumor effect depends on the chemotherapy agent | [23] |
| Telomelysin (OBP-301) | A549 H1299 | Nude mice PDX Orthotopic transplant | hTERT Promoter Deleted a part of E3 region and 55 kDa-encoding E1B region | A substantial anti-tumor effect was achieved when radiation followed the intratumoral injection | [24] |
| Ad-hTERTp-E1a-HN | EC109 | Nude mice CDX Heterotopic subcutaneous transplant | hTERT Promoter Deleted a part of E3 region and 55 kDa-encoding E1B region Expressing HN from NDV | Suppression in tumor volume in both delivery modes IT and IVComplete response to vector IT injection | [26] |
| Viral Construct Name | In Vitro Cell Line | In Vivo Model | Vector Modifications | Conclusion | Ref. |
|---|---|---|---|---|---|
| AdCEAp/Rep | MKN-45 MKN-74 | - | CEA promoter | Cytotoxicity against CEA producing cells was dose-dependent | [17] |
| Telomelysin (OBP-301) | MKN-45 MKN-74 | NOD/SCID Mice CDX Heterotopic transplant | hTERT promoter | Cell death in quiescent CD133+ cells | [14] |
| E1B 55-kDa-attenuated Ad | AGS MGc80-3 | C57Bl6/J Mice CDX Heterotopic transplant | E1B 55-kDa-deficient Ad expressing Endostatin | Synergistic effect | [27] |
| AdSurp-Hsp70 | SGC-7901 BCG-823 MNK-45 | Nude mice CDX Heterotopic transplant | Survivin promoter Vector expressing chaperone Hsp-70 | Selective replication in survivin-positive tumor cells | [15] |
| Viral Construct Name | In Vitro Cell Line | In Vivo Model | Vector Modifications | Conclusion | Ref. |
|---|---|---|---|---|---|
| CV890 | HepG2 Huh7 Hep3B PLC/PRF/5 SNU449 | Nude mice CDX Heterotopic subcutaneous transplant | AFP TRE to control an artificial E1A-IRES-E1B bicistronic cassette in an adenovirus 5 vector | Volume of distant xenografts dropped below baseline at 4 weeks | [29] |
| CNH500-p53 | Hep3B HepG2 SMMC-7721 | - | hTERT promoter and HRE promoter | Higher oncolytic effect | [32] |
| ZD55-Smac | Bel-7404 SMMC7721 Huh-7 | - | Incorporation of therapeutic gene: ZD-Smac Under CMV promoter | ZD55-Smac was superior to ONYX-015 | [34] |
| AFP-D55-SOC3 | Hep3B PLC HepG2 Huh-7 LM6 BEL7404 | Nude mice CDX Heterotopic subcutaneous transplant | SOCS3 downregulate Cyclin D1 and anti-apoptotic proteins such as XIAP, Survivin, Bcl-xL, and Mcl-1 | Restoration of SOCS3 antagonize HCC therapeutic resistance to TRAIL | [38] |
| SD55-TSLC1 | Huh-7 | Nude mice CDX Heterotopic subcutaneous transplant | Expression of TSLC1 a tumor suppressor gene | Caspase pathways provide antitumor effect | [33] |
| AdCN305-SOCS1 | Bel-7404 Hep3B Huh-7 SMMC7721 | Nude mice CDX Heterotopic subcutaneous transplant | Expression of an SOCS1 a negative regulator of STAT3 | Inhibition of STAT3 phosphorylation and downregulation of survivin, cyclin-D1, Bcl-xL, and C-myc | [39] |
| AdCN205-IL-24-miR-34a | PLC/PRF/5 Huh7 Bel-7404 | Nude mice CDX Heterotopic subcutaneous transplant | Co-expression of miRNA-34a and IL-24 | Complete tumor regression | [36] |
| QG511-HA-Melittin | Hep3B SMMC7721 HepG2 | Nude mice CDX Heterotopic subcutaneous transplant | hybrid promoter, hypoxia-response element and alpha-fetoprotein (HRE)-AFP | Inhibit the growth of HCC xenografts | [16] |
| AdSVPE1a-lncR | Huh-7 HepG2 SMMC7721 Hep3B L02 | Nude mice CDX Heterotopic subcutaneous transplant | Long noncoding RNA expression under a surviving promoter | Competitively consumes OncomiRs (oncogenic miRNAs) promoting tumor shrinkage | [37] |
| Viral Construct Name | In Vitro Cell Line | In Vivo Model | Vector Modifications | Conclusion | Ref. |
|---|---|---|---|---|---|
| Ad5-yCD/mutTKSR39rep-ADP | Panc 1 MiaPaCa-2 | Nude mice CDX Heterotopic subcutaneous transplant; GEMM, CDX Orthotopic transplant | Contains a bacterial cytosine deaminase (CD) and wild-type herpes simplex virus thymidine kinase (HSV-1 TK) gene under CMV promoter | Improved the effectiveness of radiotherapy without excessive toxicity | [44,45] |
| AdTATMMP | RWP1 Panc1 PSC-21 CAF-25 CAF-28 | GEMM CDX orthotopic transplant | AdTATMMP transduction is activated by matrix metalloproteases MMP2 and MMP9 | In comparison to Ad5 wild type, there was increased antitumor activity | [46] |
| AdSur-SYE | AsPC-1 BxPC-3 Panc-1 MIAPaCa-2 | Nude mice CDX Heterotopic subcutaneous transplant | Displays a pancreatic cancer targeting sequence SYENFSA on the fiber knob; survivin promoter | High infectivity in human pancreatic cancer tissues | [47] |
| Viral Construct Name | In Vitro Cell Line | In Vivo Model | Vector Modifications | Conclusion | Ref |
|---|---|---|---|---|---|
| CRAd-NTR(PS1217H6) | SW480 | Nude mice CDX Heterotopic subcutaneous transplant | Vector E1B-55-KDa-deleted expressing prodrug-activating enzyme nitroreductase (NTR) | Greater sensitization to the prodrug CB1954 | [48] |
| Telomelysin OBP-405 | SW620 | Nude miceCDX Heterotopic subcutaneous transplant | Vector has mutant fiber containing the RGD peptide, CDCRGDCFC, in the HI loop of the fiber knob | Increased infection property | [50] |
| ZD55-MnSOD ZD55-TRAIL | SW620 | Nude mice CDX Heterotopic Random tumor inoculation | Vector with the E1B 55-kDa gene deletion and expressing Manganese superoxide dismutase (MnSOD) | Effective oncolysis | [51] |
| AdV.hTERT-CPG2 | SW620 SW480 HCT116 LS174T LoVo DLD-1 HT-29 Caco-2 Colo205 | Nude mice CDX Heterotopic subcutaneous transplant | Delivery of gene for the prodrug-activating enzyme carboxypeptidase G2 (CPG2) to tumors | significant bystander effects in vivo | [49] |
| Ad·(ST13)·CEA·E1A(Δ24) | SW620 HT29 HCT116 | Nude mice CDX Heterotopic subcutaneous transplant | Vector with CEA promoter expressing suppression of ST13 | Induced tumor apoptosis through the mitochondrial-mediated apoptosis pathway | [18] |
| Ad-survivin-ZD55-TAp63 | HCT116 | Nude mice CDX Heterotopic subcutaneous transplant | Tap63 expressing cassette in Adenovirus under surviving | in vitro inhibition of cell proliferation | [52] |
| AdML-TYML | LoVo LS174T | Nude mice CDX Heterotopic subcutaneous transplant | A TYMLSRN peptide motif in place of the primary CAR-binding domains in AB-loop of fiber knob | Selective virus for CSC | [53] |
| GI Cancer | Vector Construct | Phase | Country | Clinicaltrials.gov Number |
|---|---|---|---|---|
| Pancreatic cancer | Ad5-yCD/mutTKSR39rep-hIL12 (Oncolytic adenovirus expressing two suicide genes and human IL-12) | I | USA | NCT03281382 |
| Pancreatic cancer | LOAd703 Oncolytic adenovirus serotype 5/35 encoding TMZ-CD40L and 4-1BBL | I/II | Sweden | NCT03225989 |
| Pancreatic cancer | LOAd703 | I/II | USA | NCT02705196 |
| Pancreatic cancer | VCN-1 expressing PH20 hyaluronidase | I | Spain | NCT02045602 |
| Pancreatic cancer | VCN-1 expressing PH20 hyaluronidase | I | Spain | NCT02045589 |
| Hepatocellular carcinoma | Telomesyn OBP-301 | I/II | Korea & Taiwan | NCT02293850 |
| Hepatocellular carcinoma | Recombinant Ad5 | III | China | NCT01869088 |
| Liver Cancer | Ad5-CMV-p53 | I | USA | NCT00003147 |
| Colorectal cancer | LOAd703 | I/II | Sweden | NCT03225989 |
| Colorectal cancer | Ad11/Ad3 Enadenotucirev (previously ColoAd1) | I/II | Belgium & Spain | NCT02028442 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoda, R.T.; Nagalo, B.M.; Borad, M.J. Oncolytic Adenoviruses in Gastrointestinal Cancers. Biomedicines 2018, 6, 33. https://doi.org/10.3390/biomedicines6010033
Yokoda RT, Nagalo BM, Borad MJ. Oncolytic Adenoviruses in Gastrointestinal Cancers. Biomedicines. 2018; 6(1):33. https://doi.org/10.3390/biomedicines6010033
Chicago/Turabian StyleYokoda, Raquel T., Bolni M. Nagalo, and Mitesh J. Borad. 2018. "Oncolytic Adenoviruses in Gastrointestinal Cancers" Biomedicines 6, no. 1: 33. https://doi.org/10.3390/biomedicines6010033