Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update
Abstract
:1. Introduction
2. Clinical Aspect of Actinic keratosis (AK)
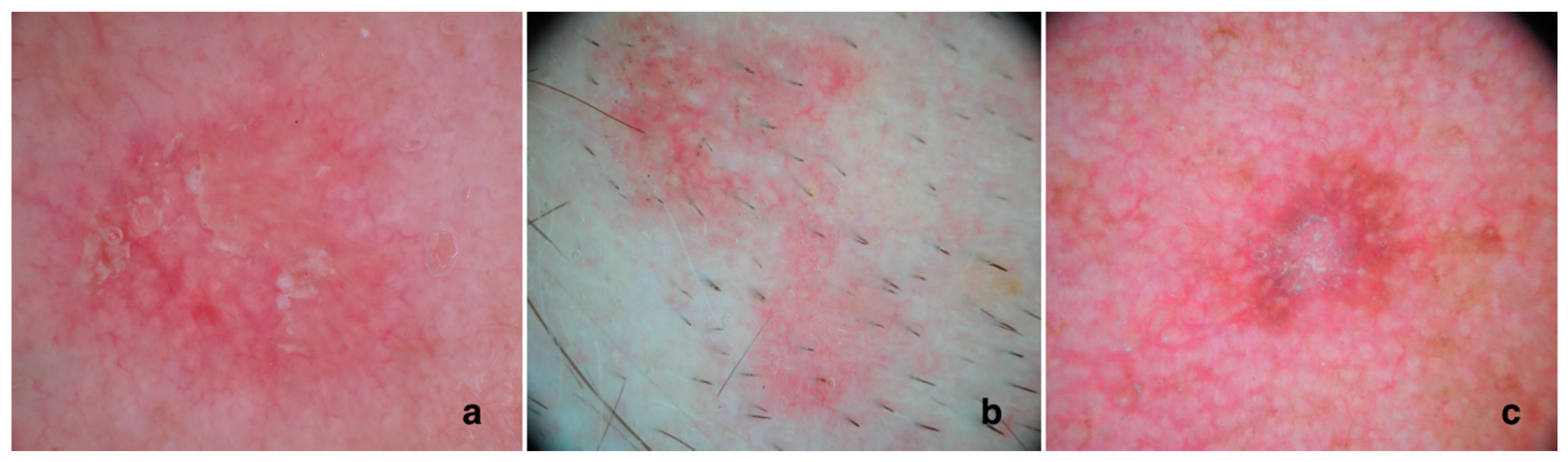
3. Dermoscopic Aspects of AKs
4. Fluorescence Techniques Detection in Skin Cancer
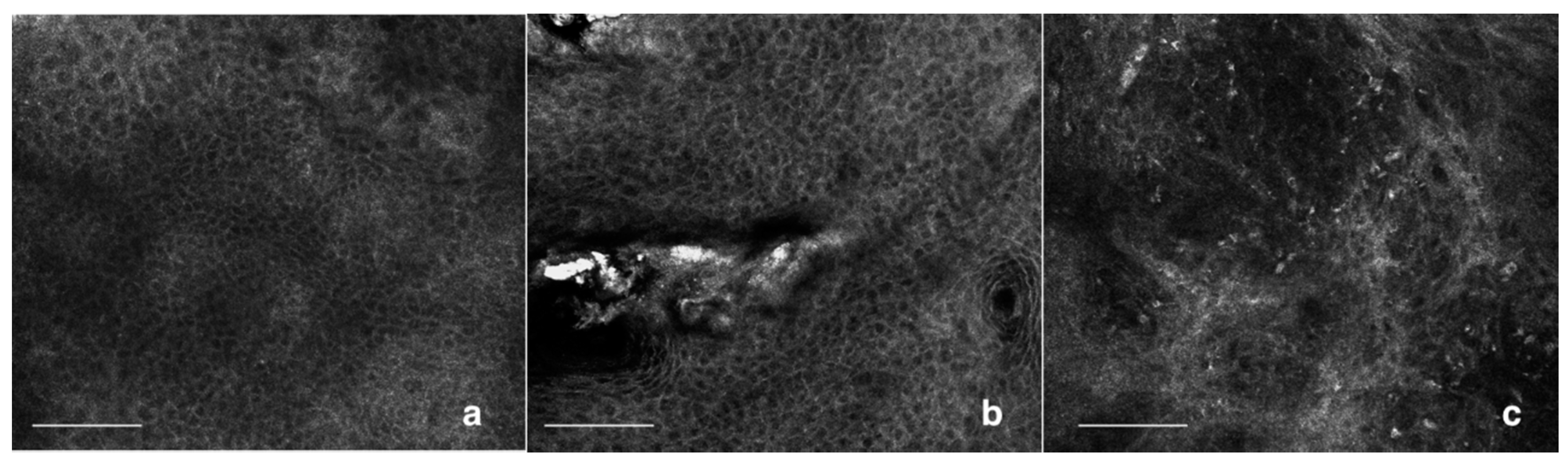
5. Confocal Aspects of AKs
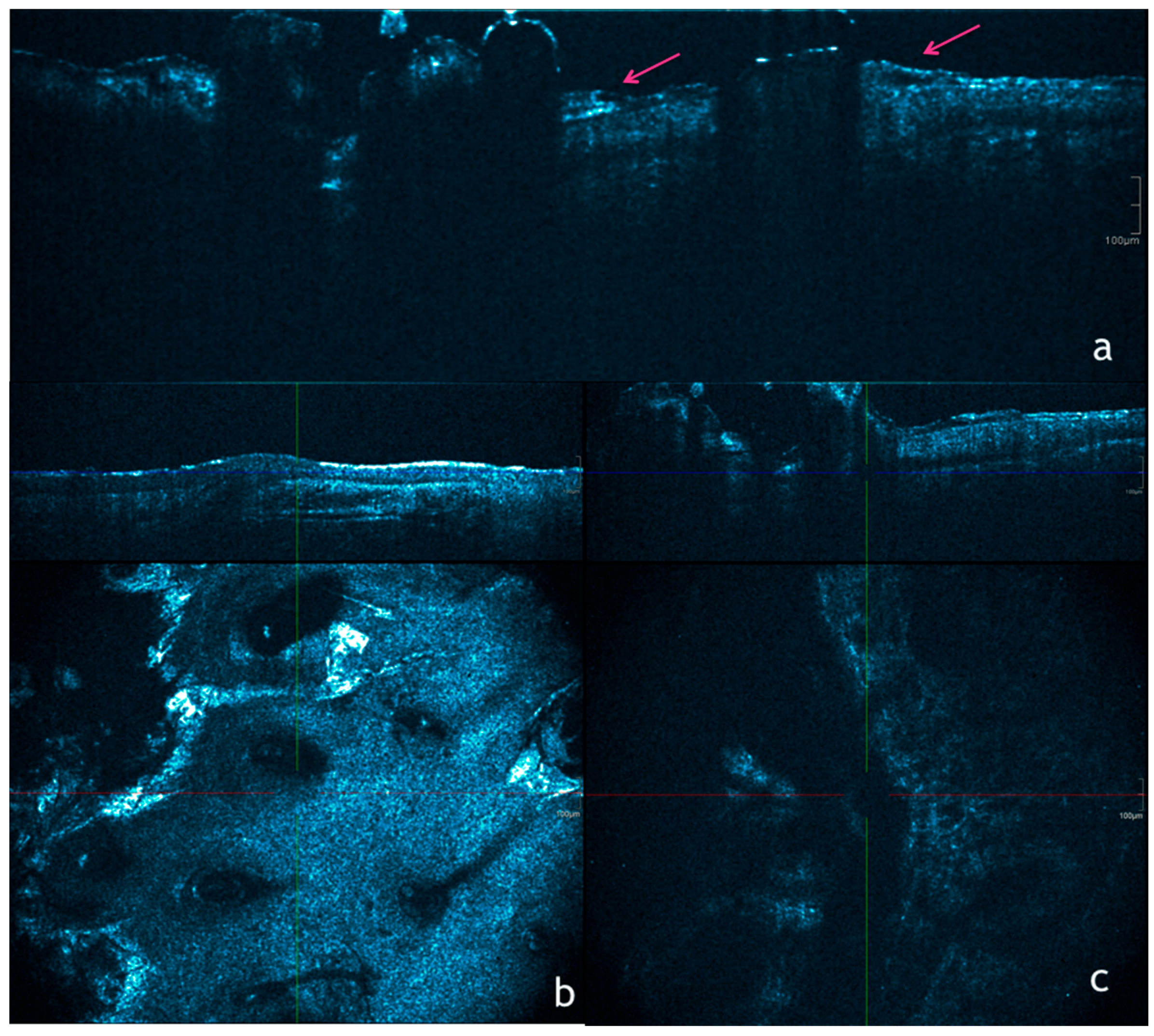
6. Optical Coherence Tomography Aspects of AKs
7. Conclusions
Conflicts of Interest
References
- Dubreuillh, W.A. DES hyperkeratosis circoscrites. Dermatol. Venereol. 1896, 27, 1158–1164. [Google Scholar]
- Marks, R. Non melanocitic skin cancer and solar keratoses. The quiet 20th century epidemic. Int. J. Dermatol. 1987, 26, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Foley, P.; Goodman, G.; Hage, B.H.; Selwood, T.S. Spontaneous remission of solar keratosis: The cse for conservative management. Br. J. Dermatol. 1986, 115, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Salasche, S.J. Epidemiology of actinic keratoses and squamous cell carcinoma. J. Am. Acad. Dermatol. 2000, 42, S4–S7. [Google Scholar] [CrossRef]
- Frost, C.; Williams, G.; Green, A. High incidence and regression rates of solar keratoses in a Queensland community. J. Investig. Dermatol. 2000, 115, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Fartasch, M.; Diepgen, T.L.; Schmitt, J.; Drexler, H. The relationship between occupational sun exposure and non-melanoma skin cancer: Clinical basics, epidemiology, occupational disease evaluation, and prevention. Dtsch. Arztebl. Int. 2012, 109, 715–720. [Google Scholar] [PubMed]
- Johannesdottir, S.A.; Chang, E.T.; Mehnert, F.; Schmidt, M.; Olesen, A.B.; Sørensen, H.T. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: A population-based case-control study. Cancer 2012, 118, 4768–4776. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Argenziano, G. Nonsteroidal anti-inflammatory drugs and the risk of keratinocyte skin cancer among women: Is there a link? Cancer 2013, 119, 1446. [Google Scholar] [CrossRef] [PubMed]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Rennie, G.; Selwood, T.S. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988, 8589, 795–797. [Google Scholar] [CrossRef]
- Green, A.; Battistutta, D. Incidence and determinants of skin cancer in a high-risk Australian population. Int. J. Cancer 1990, 46, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Mittelbronn, M.A.; Mullins, D.L.; Ramos-Caro, F.A.; Flowers, F.P. Frequency of pre- existing actinic keratosis in cutaneous squamous cell carcinoma. Int. J. Dermatol. 1998, 37, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, D.; Meehan, C.J.; Bruce, F.; Culjak, G. The majority of cutaneous squamous cell carcinomas arise in actinic keratoses. J. Cutan. Med. Surg. 2002, 6, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. The role of treatment of actinic keratoses in the prevention of morbidity and mortality due to squamous cell carcinoma. Arch. Dermatol. 1991, 127, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Rowert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic keratosis is an early in situ squamous cell carcinoma: A proposal for reclassification. Br. J. Dermatol. 2007, 156 (Suppl. S3), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Stockfleth, E.; Roewert-Huber, J.; Astner, S. Noninvasive diagnostic tools for nonmelanoma skin cancer. Br. J. Dermatol. 2007, 157 (Suppl. S2), 56–58. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Soyer, H.P.; Chimenti, S.; Talamini, R.; Corona, R.; Sera, F.; Binder, M.; Cerroni, L.; De Rosa, G.; Ferrara, G.; et al. Dermos- copy of pigmented skin lesions: Results of a consensus meeting via Internet. J. Am. Acad. Dermatol. 2003, 48, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Soyer, H.P.; De Giorgi, V.; Piccolo, D.; Carli, P.; Delfino, M.; Ferrari, A.; Hofmann-Wellenhof, R.; Massi, D.; Mazzocchetti, G.; et al. Interactive Atlas of Dermoscopy: A Tutorial (Book) and CD-ROM; Edra Medical Publishing & New Media: Milan, Italy, 2000. [Google Scholar]
- Zalaudek, I.; Giacomel, J.; Argenziano, G.; Hofmann-Wellenhof, R.; Micantonio, T.; Di Stefani, A.; Oliviero, M.; Rabinovitz, H.; Soyer, H.P.; Peris, K. Dermatoscopy of facial nonpigmented actinic keratosis. Br. J. Dermatol. 2006, 155, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Ferrara, G.; Leinweber, B.; Mercogliano, A.; D’Ambrosio, A.; Argenziano, G. Pitfalls in the clinical and dermoscopic diagnosis of pigmented actinic keratosis. J. Am. Acad. Dermatol. 2005, 53, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.N.; Kocyigit, P.; Heper, A.O.; Erdem, C. Dermatoscopy of flat pigmented facial lesions: Diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br. J. Dermatol. 2010, 163, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Baybay, H.; Moscarella, E.; Argenziano, G.; Gallouj, S.; Mernissi, F.Z. Dermoscopy of Pigmented Actinic Keratosis of the Face: A Study of 232 Cases. Actas Dermosifiliogr. 2017, 108, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Tschandl, P.; Kyrgidis, A.; Stolz, W.; Rabinovitz, H.; Cameron, A.; Gourhant, J.Y.; Giacomel, J.; Kittler, H.; Muir, J.; et al. Dermoscopic clues to differentiate facial lentigo maligna from pigmented actinic keratosis. Br. J. Dermatol. 2016, 174, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, F.; Vilalta, A.; Puig, S.; Palou, J.; Salerni, G.; Malvehy, J. New dermoscopic pattern in actinic keratosis and related conditions. Arch. Dermatol. 2009, 145, 732. [Google Scholar] [CrossRef] [PubMed]
- Kupsa, R.; Deinlein, T.; Woltsche, N.; Hofmann-Wellenhof, R.; Zalaudek, I. Dermoscopy of keratinocyte skin cancer. G. Ital. Dermatol. Venereol. 2016, 151, 649–662. [Google Scholar] [PubMed]
- Behera, B.; Thappa, D.M.; Chandrashekar, L. Floret or pseudorosette appearance under nonpolarized contact dermoscopy. J. Am. Acad. Dermatol. 2016, 75, e95–e96. [Google Scholar] [CrossRef] [PubMed]
- Schiffner, R.; Schiffner-Rohe, J.; Vogt, T.; Landthaler, M.; Wlotzke, U.; Cognetta, A.B.; Stolz, W. Improvement of early recognition of lentigo maligna using dermatoscopy. J. Am. Acad. Dermatol. 2000, 42, 25–32. [Google Scholar] [CrossRef]
- Sanmartin, O.; Guillen, C. Images in clinical medicine. Fluorescence diagnosis of subclinical actinic keratoses. N. Engl. J. Med. 2008, 8, 358. [Google Scholar]
- Rollakanti, K.R.; Kanick, S.C.; Davis, S.C.; Pogue, B.W.; Maytin, E.V. Techniques for fluorescence detection of protoporphyrin IX in skin cancers associated with photodynamic therapy. Photonics Lasers Med. 2013, 2, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Rishpon, A.; Kim, N.; Scope, A.; Porges, L.; Oliviero, M.C.; Braun, R.P.; Marghoob, A.A.; Fox, C.A.; Rabinovitz, H.S. Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch. Dermatol. 2009, 145, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Maltusch, A.; Rius-Diaz, F.; Rowert-Huber, J.; Gonzalez, S.; Sterry, W.; Stockfleth, E.; Astner, S. Clinical applicability of in vivo reflectance confocal microscopy for the diagnosis of actinic keratoses. Dermatol. Surg. 2008, 34, 610–619. [Google Scholar] [PubMed]
- Ulrich, M.; Zalaudek, I.; Welzel, J. Shining into the White: The Spectrum of Epithelial Tumors from Actinic Keratosis to Squamous Cell Carcinoma. Dermatol. Clin. 2016, 34, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Ulrich, M.; Casari, A.; Prow, T.W.; Cannillo, F.; Benati, E.; Losi, A.; Cesinaro, A.M.; Longo, C.; Argenziano, G.; et al. Grading keratinocyte atypia in actinic keratosis: A correlation of reflectance confocal microscopy and histopathology. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Pock, L.; Drlik, L.; Hercogova, J. Dermatoscopy of pigmented actinic keratosis: A striking similarity to lentigo maligna. Int. J. Dermatol. 2007, 46, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Guitera, P.; Pellacani, G.; Crotty, K.A.; Scolyer, R.A.; Li, L.X.; Bassoli, S.; Vinceti, M.; Rabinovitz, H.; Longo, C.; Menzies, S.W. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J. Investig. Dermatol. 2010, 130, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Maltusch, A.; Röwert-Huber, J.; Gonzalez, S.; Sterry, W.; Stockfleth, E.; Astner, S. Actinic keratoses: Non-invasive diagnosis for field cancerisation. Br. J. Dermatol. 2007, 156, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Jemec, G.B. Diagnosis of nonmelanoma skin cancer/keratinocyte carcinoma: A review of diagnostic accuracy of nonmelanoma skin cancer diagnostic tests and technologies. Dermatol. Surg. 2007, 33, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Moscarella, E.; Rabinovitz, H.; Zalaudek, I.; Piana, S.; Stanganelli, I.; Oliviero, M.C.; Lallas, A.; Ardigo, M.; Cota, C.; Catricalà, C.; et al. Dermoscopy and reflectance confocal microscopy of pigmented actinic keratoses: A morphological study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, P.; Pulitzer, M.P.; Scope, A.; Kovalyshyn, I.; Halpern, A.C.; Marghoob, A.A. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: A challenge for melanoma diagnosis. J. Am. Acad. Dermatol. 2012, 66, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Peppelman, M.; Hoogedoorn, L.; Van Erp, P.E.; Gerritsen, M.P. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: A systematic review. Eur. J. Dermatol. 2016, 26, 549–565. [Google Scholar]
- Aghassi, D.; Anderson, R.R.; Gonzalez, S. Confocal laser microscopic imaging of actinic keratoses in vivo: A preliminary report. J. Am. Acad. Dermatol. 2000, 43, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; González, S. Enlightening the Pink: Use of Confocal Microscopy in Pink Lesions. Dermatol. Clin. 2016, 34, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Farnetani, F.; Ciardo, S.; Cesinaro, A.M.; Moscarella, E.; Ponti, G.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Is confocal microscopy a valuable tool in diagnosing nodular lesions? A study of 140 cases. Br. J. Dermatol. 2013, 169, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Korde, V.R.; Bonnema, G.T.; Xu, W.; Krishnamurthy, C.; Ranger-Moore, J.; Saboda, K.; Slayton, L.D.; Salasche, S.J.; Warneke, J.A.; Alberts, D.S.; et al. Using optical coherence tomography to evaluate skin sun damage and precancer. Lasers Surg. Med. 2007, 39, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Gossage, K.W.; Xu, W.; Ranger-Moore, J.R.; Saboda, K.; Brooks, C.A.; Duckett, L.D.; Salasche, S.J.; Warneke, J.A.; Alberts, D.S. Investigating sun damaged skin and actinic keratosis with optical coherence tomography: A pilot study. Technol. Cancer Res. Treat. 2003, 2, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, J.M.; Warschaw, K.E.; Schmitt, J.M.; Swanson, D.L. Optical coherence tomography for the characterization of basal cell carcinoma in vivo: A pilot study. J. Am. Acad. Dermatol. 2006, 55, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.; Norrenberg, S.; Jemec, G.B.; Del, M.V. Imaging actinic keratosis by high-definition optical coherence tomography. Histomorphologic cor- relation: A pilot study. Exp. Dermatol. 2013, 22, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, M.; Jørgensen, T.M.; Nurnberg, B.M.; Morsy, H.A.; Thomsen, J.B.; Thrane, L.; Jemec, G.B. Assessment of optical coherence tomography imaging in the diagnosis of non- melanoma skin cancer and benign lesions versus normal skin: Observer-blinded evaluation by dermatologists and pathologists. Dermatol. Surg. 2009, 35, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.; Braun-Falco, M.; Laubender, R.P.; Ruzicka, T.; Berking, C. Actinic keratosis in the en-face and slice imaging mode of high-definition optical coherence tomography and comparison with histology. Br. J. Dermatol. 2013, 168, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Friis, K.B.E.; Themstrup, L.; Jemec, G.B.E. Optical coherence tomography in the diagnosis of actinic keratosis-A systematic review. Photodiagnosis Photodyn. Ther. 2017, 18, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Gill, M.; Halpern, A.C. Reflectance Confocal Microscopyof Cutaneous Tumors: An Atlas with Clinical, Dermoscopic and Histological Correlations; Informa Healthcare: London, UK, 2008. [Google Scholar]
- Terhorst, D.; Maltusch, A.; Stockfleth, E.; Lange-Asschenfeldt, S.; Sterry, W.; Ulrich, M.; Lange-Asschenfeldt, B. Reflectance confocal microscopy for the evaluation of acute epidermal wound healing. Wound Repair Regen. 2011, 19, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E. The importance of treating the field in actinic keratosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. S2), 8–11. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Suppa, M.; Pellacani, G.; Marneffe, A.; Miyamoto, M.; Alarcon, I.; Ruini, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Jemec, G.B. High-definition optical coherence tomography, algorithm for discrimination of basal cell carcinoma from clinical bcc imitators and differentiation between common subtypes. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.; Suppa, M.; Marneffe, A.; Miyamoto, M.; Jemec, G.B.; Del Marmol, V. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, C.J.; Wharton, J.R. New histopathological classification of actinic keratosis (incipient intraepidermal squamous cell carcinoma). J. Drugs Dermatol. 2005, 4, 462–467. [Google Scholar] [PubMed]
- Dreno, B.; Amici, J.M.; Basset-Seguin, N.; Cribier, B.; Claudel, J.P.; Richard, M.A. Management of actinic keratosis: A practical report and treatment algorithm from AKTeam expert clinicians. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casari, A.; Chester, J.; Pellacani, G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines 2018, 6, 8. https://doi.org/10.3390/biomedicines6010008
Casari A, Chester J, Pellacani G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines. 2018; 6(1):8. https://doi.org/10.3390/biomedicines6010008
Chicago/Turabian StyleCasari, Alice, Johanna Chester, and Giovanni Pellacani. 2018. "Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update" Biomedicines 6, no. 1: 8. https://doi.org/10.3390/biomedicines6010008