Is There a Causal Relationship between Intussusception and Food Allergy?
Abstract
:1. Introduction
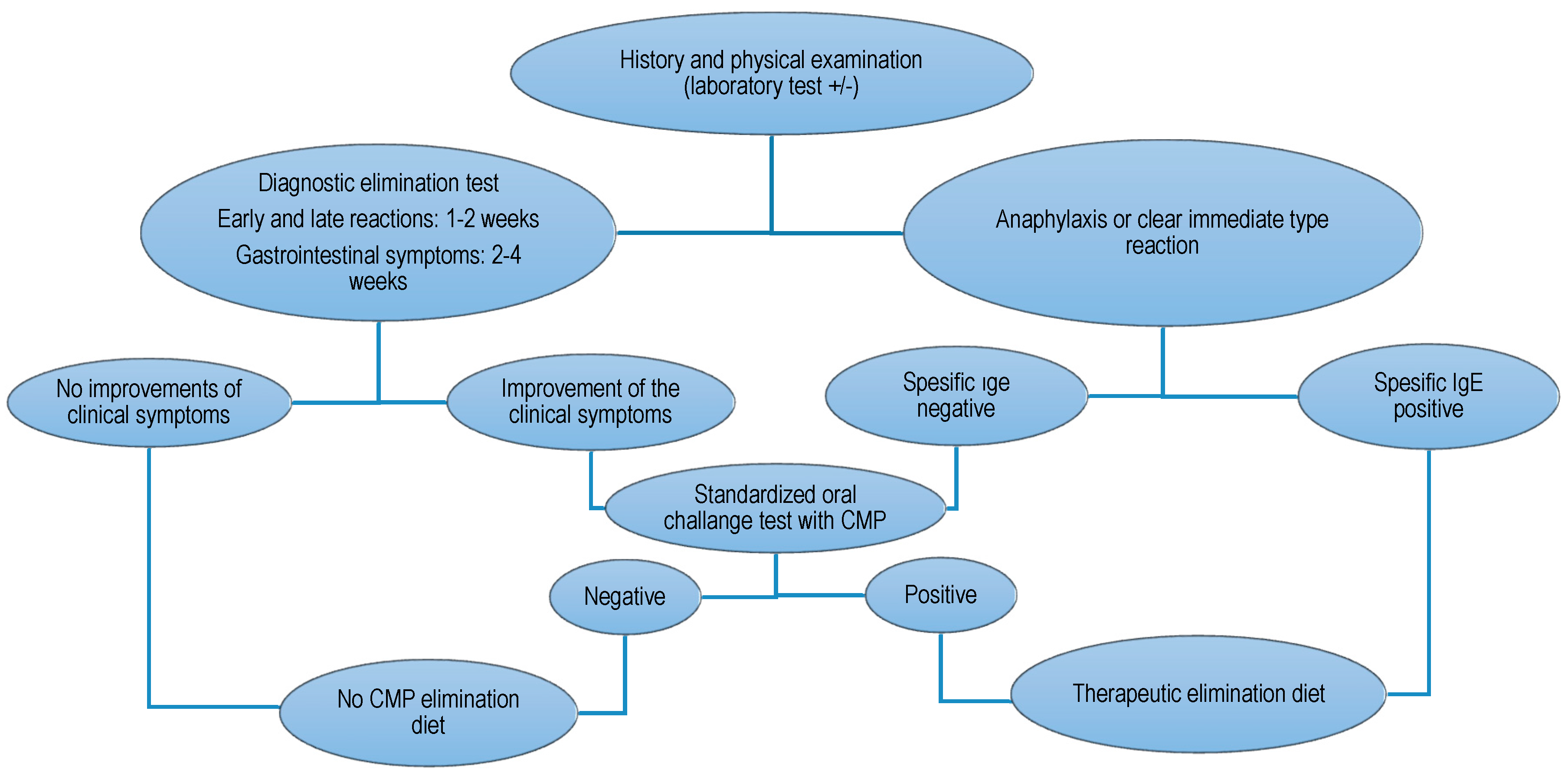
2. Materials and Methods
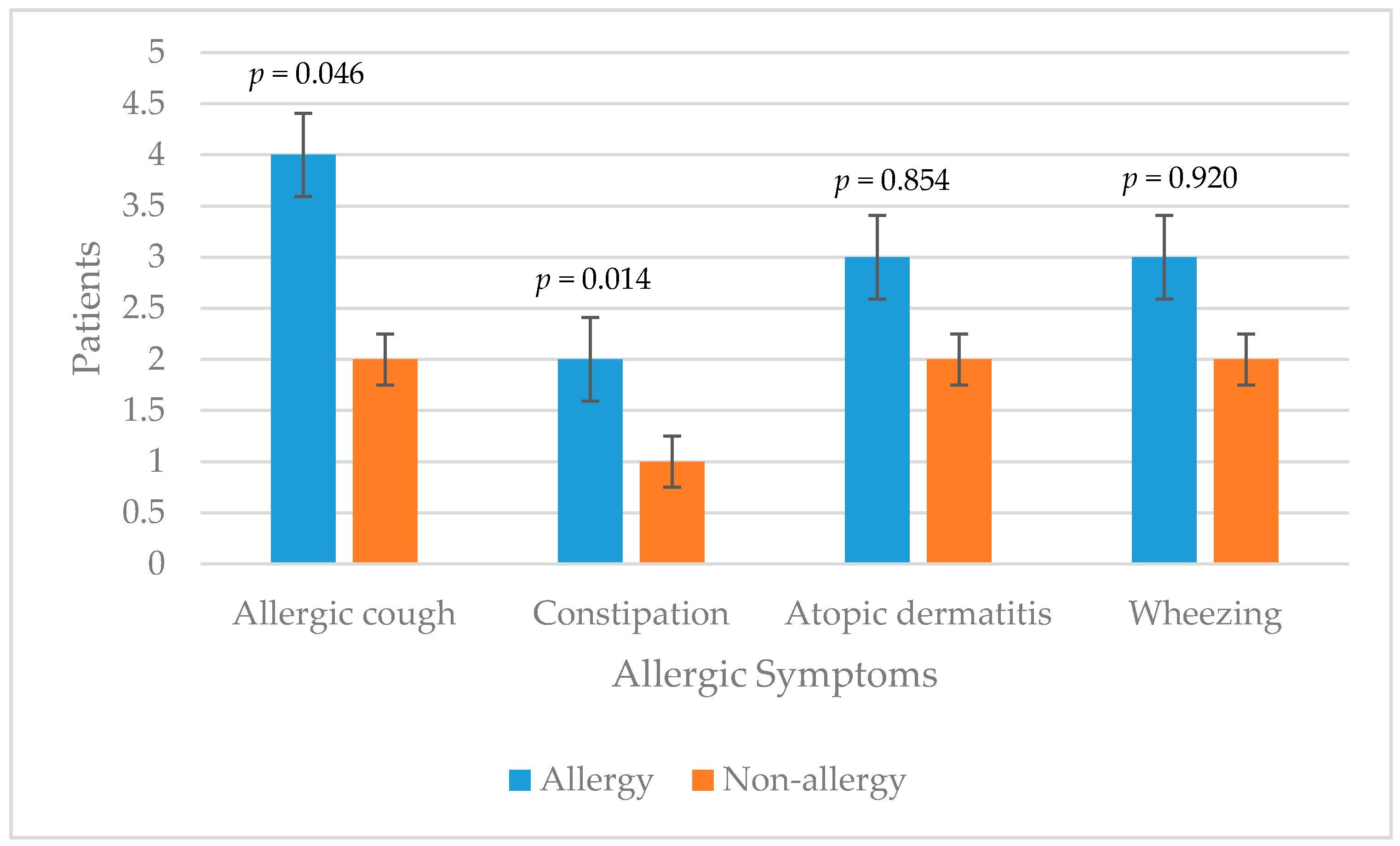
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- DiFiore, J.W. Intussusception. Semin. Pediatr. Surg. 1999, 8, 214–220. [Google Scholar] [CrossRef]
- Aydin, E. One Month Ongoing Intussusception: Case Report. Open J. Pediatr. 2016, 6, 14–16. [Google Scholar] [CrossRef]
- Zavras, N.; Tsilikas, K.; Vaos, G. Chronic Intussusception Associated with Malrotation in a Child: A Variation of Waugh’s Syndrome? Case Rep. Surg. 2016, 2016, 5638451. [Google Scholar] [CrossRef] [PubMed]
- Bines, J.E.; Ivanoff, B.; Justice, F.; Mulholland, K. Clinical Case Definition for the Diagnosis of Acute Intussusception. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Khorana, J.; Patumanond, J.; Ukarapol, N.; Laohapensang, M.; Visrutaratna, P.; Singhavejsakul, J. Clinical prediction rules for failed nonoperative reduction of intussusception. Ther. Clin. Risk Manag. 2016, 12, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Lau, C.H.; Dyer, A.A.; Sohn, M.W.; Altshuler, B.A.; Kaye, B.A.; Necheles, J. Food allergy diagnosis and management practices among pediatricians. Clin. Pediatr. (Phila.) 2014, 53, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. Prevalence of challenge-proven IgE-mediated food allergy using population based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, T.A.; Peterson, S.; Lau, C.H.; Saltoun, C.A.; Gupta, R.S.; Bryce, P.J. Prevalence and characteristics of adult-onset food allergy. J. Allergy Clin. Immunol. Pract. 2015, 3, 114–115. [Google Scholar] [CrossRef] [PubMed]
- NIAID-Sponsored Expert Panel; Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, 1–58. [Google Scholar]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Klemola, T.; Vanto, T.; Juntunen-Backman, K.; Kalimo, K.; Korpela, R.; Varjonen, E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: A prospective, randomized study with a follow-up to the age of 2 years. J. Pediatr. 2002, 140, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Eggesbø, M.; Botten, G.; Halvorsen, R.; Magnus, P. The prevalence of CMA/CMPI in young children: The validity of parentally perceived reactions in a population-based study. Allergy 2001, 56, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Beser, O.F. Food allergy: A rare cause of recurrent intussusception. APSP J. Case Rep. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, K.; Jolles, S.; Huddart, S.; Tuthill, D.P. Successful dietary treatment of recurrent intussusception. Arch. Dis. Child. 2009, 94, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Yamada, K.; Fujinami, H. Colonoscopic reduction of colo-colic intussusception in an adult with immunoglobulin A vasculitis (Henoch-Schönlein purpura). Dig. Endosc. 2016, 28, 101. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Viboud, C.; Elixhauser, A.; Taylor, R.J.; Kapikian, A.Z. More on RotaShield and intussusception: The role of age at the time of vaccination. J. Infect. Dis. 2005, 192, 36–43. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee; WHO Technical Report Series 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Hidvégi, E.; Cserháti, E.; Kereki, E.; Arató, A. Higher serum eosinophil cationic protein levels in children with cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.M.; Catto Smith, A.G.; Gall, D.G.; Trevenen, C.; Scott, R.B. Allergic colitis in infancy: Clinical and pathologic aspects. J. Pediatr. Gastroenterol. Nutr. 1994, 19, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Kazatsky, A.M.; Wood, R.A. Classification of Food Allergens and Cross-Reactivity. Curr. Allergy Asthma Rep. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Baldi, F.; Bendandi, B.; Calzone, L.; Marani, M.; Pasquinelli, P. Cow’s milk protein allergy in children: A practical guide. Ital. J. Pediatr. 2010, 36, 5. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Alarcon, P.; Alliet, P.; De Greef, E.; De Ronne, N.; Hoffman, I.; Van Winckel, M.; Hauser, B. Algorithms for managing infant constipation, colic, regurgitation and cow’s milk allergy in formula-fed infants. Acta Paediatr. 2015, 104, 449–457. [Google Scholar] [CrossRef] [PubMed]
| Allergy | Non-Allergy | p-Value | |
|---|---|---|---|
| Age | 4.36 ± 3.08 | 4.44 ± 3.10 | 0.955 |
| Gender | 0.864 | ||
| Male | 4 (50%) | 7 (53.85%) | |
| Female | 4 (50%) | 6 (46.15%) | |
| Consanguineous marriage | 4 (50%) | 3 (23.08%) | 0.204 |
| Total breastfeeding | |||
| <6 months | 2 (25%) | 2 (15.38%) | 0.586 |
| Supplementary feeding | |||
| <6 months | 5 (62.50%) | 5 (38.46%) | 0.284 |
| Mean z-score | |||
| Weight for height | 0.19 ± 0.50 | 0.28 ± 0.61 | 0.816 |
| Weight for age | −0.48 ± 1.14 | −0.22 ± 0.95 | 0.578 |
| Height for age | −1.01 ± 1.58 | −0.93 ± 1.29 | 0.900 |
| BMI for age | −0.11 ± 0.91 | 0.47 ± 0.66 | 0.103 |
| Allergy | Non-Allergy | p-Value | |
|---|---|---|---|
| Physical examination | |||
| Normal | 2 (25%) | 4 (30.77%) | 0.776 |
| X-ray | |||
| Normal | 2 (25%) | 4 (30.77%) | 0.776 |
| Ultrasonography | |||
| Intussusception | 8 (100%) | 8 (100%) | 0.324 |
| Level | |||
| Ileoileal | 2 (25%) | 5 (38.46%) | 0.525 |
| Intervention | 7 (87.50%) | 9 (69.23%) | 0.034 |
| Number of intussusception attacks | 1.63 ± 1.19 | 1 ± 0.0 | 0.039 |
| Allergy | Non-Allergy | p-Value | |
|---|---|---|---|
| Skin prick test | 3 (37.50%) | 1 (7.69%) | 0.004 |
| Eosinophils | 0.34 ± 0.29 | 0.16 ± 0.19 | 0.014 |
| Total IgE | 416.42 ± 846.06 | 102.74 ± 173.52 | 0.205 |
| ECP | 58.31 ± 44.15 | 28.47 ± 18.55 | 0.043 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, E.; Beşer, O.F.; Ozek, E.; Sazak, S.; Duras, E. Is There a Causal Relationship between Intussusception and Food Allergy? Children 2017, 4, 89. https://doi.org/10.3390/children4100089
Aydin E, Beşer OF, Ozek E, Sazak S, Duras E. Is There a Causal Relationship between Intussusception and Food Allergy? Children. 2017; 4(10):89. https://doi.org/10.3390/children4100089
Chicago/Turabian StyleAydin, Emrah, Omer F. Beşer, Esra Ozek, Soner Sazak, and Ensar Duras. 2017. "Is There a Causal Relationship between Intussusception and Food Allergy?" Children 4, no. 10: 89. https://doi.org/10.3390/children4100089





