Parent Cardiac Response in the Context of Their Child’s Completion of the Cold Pressor Task: A Pilot Study
Abstract
:1. Introduction
1.1. The Role of Parents in Children’s Pain Experience
1.2. Heart Rate and Heart Rate Variability
1.3. Current Study
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.2.1. Cold Pressor Task
2.2.2. Electrocardiogram
2.2.3. Video
2.3. Procedure
2.4. Measures
2.4.1. Demographics
2.4.2. Self-Assessment Manikin
2.5. Data Preparation
3. Results
3.1. Pre-Analysis
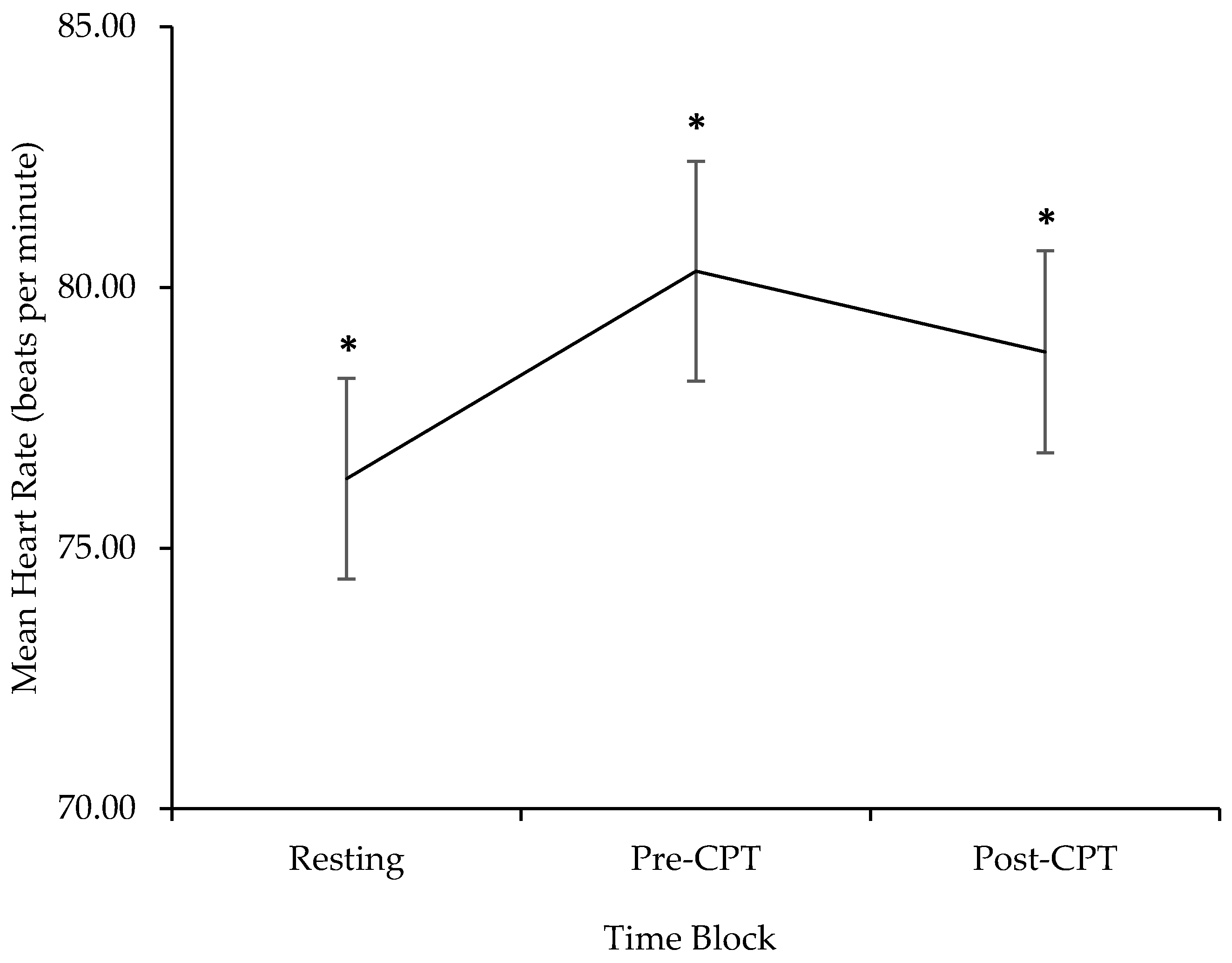
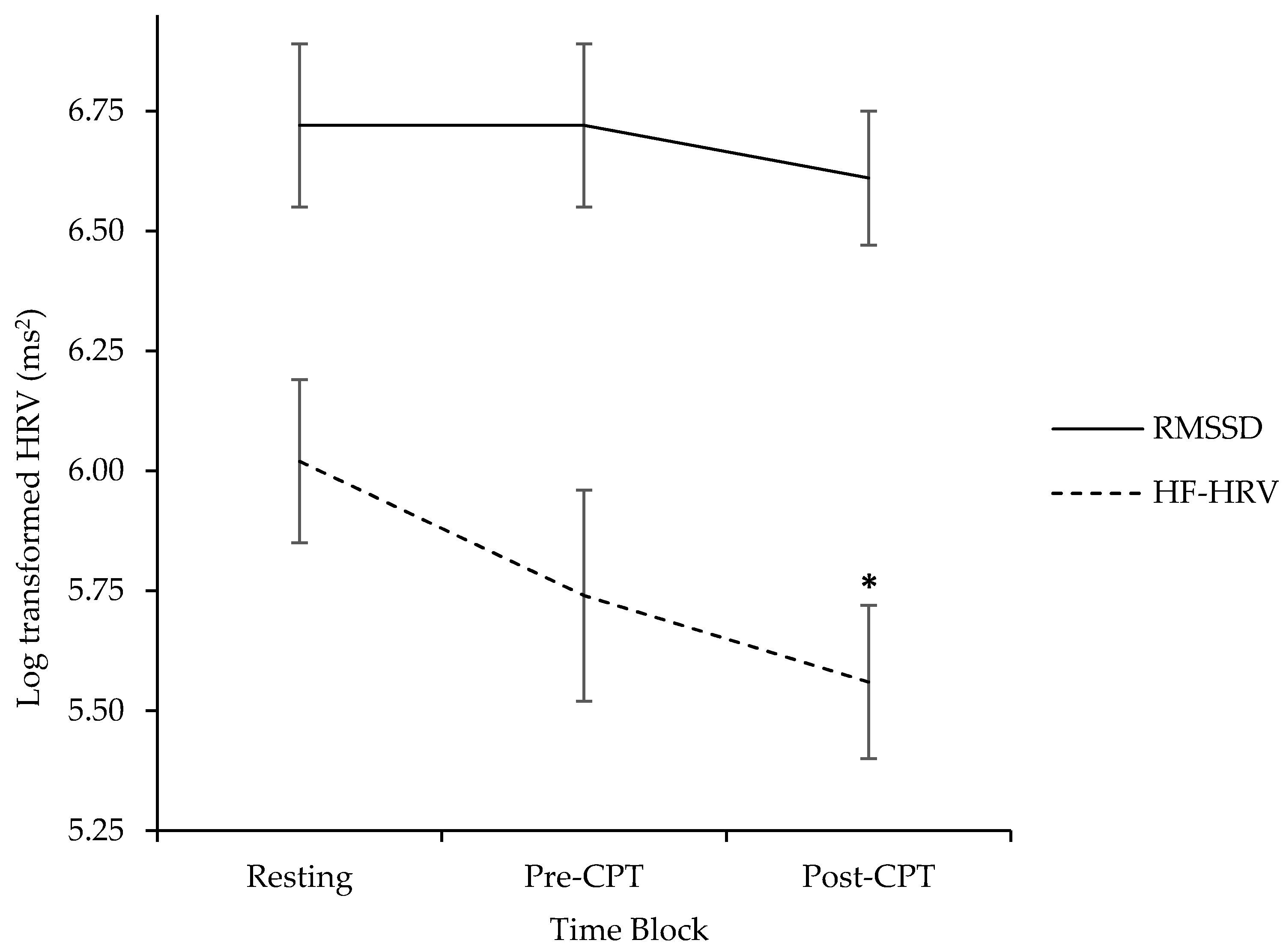
3.2. Exploring Parent Cardiac Response during Resting, Pre-CPT, and Post-CPT
3.3. Factors Posited to Affect HR and HRV across the Three Phases of the Study
3.3.1. Resting HRV and Its Association with Phasic and Recovery HRV
3.3.2. Children’s Typical Response to Needle Pain
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merskey, H.; Bogduk, N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms; IASP Press: Seattle, WA, USA, 1994; ISBN 0-931092-05-1. [Google Scholar]
- Taddio, A. Needle procedures. In Oxford Textbook of Paediatric Pain; McGrath, P.J., Stevens, B.J., Walker, S.M., Zempsky, W.T., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 184–193. ISBN 978-0-19-964265-6. [Google Scholar]
- Taddio, A.; Ipp, M.; Thivakaran, S.; Jamal, A.; Parikh, C.; Smart, S. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012, 30, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M.; Pillai Riddell, R.; Taddio, A.; Racine, N.; Asmundson, G.J.; Noel, M.; Chambers, C.T.; Shah, V.; HELPinKids & Adults Team. Far from “just a poke”: Common painful needle procedures and the development of needle fear. Clin. J. Pain 2015, 31, S3–S11. [Google Scholar] [CrossRef]
- Craig, K.D. Putting the social back in the biopsychosocial model of pain. In Psychology Serving Humanity: Proceedings of the 30th International Congress of Psychology; Cooper, S., Ratele, K., Eds.; Psychology Press: New York, NY, USA, 2014; pp. 127–139. [Google Scholar]
- Craig, K.D. The social communication model of pain. Can. Psychol. 2009, 50, 22–32. [Google Scholar] [CrossRef]
- Vervoort, T.; Trost, Z. Examining affective-motivational dynamics and behavioural implications within the interpersonal context of pain. J. Pain 2017, 18, 1174–1183. [Google Scholar] [CrossRef]
- Kain, Z.N.; Caldwell-Andrews, A.A.; Mayes, L.C.; Wang, S.M.; Krivutza, D.M.; LoDolce, M.E. Parental presence during induction of anesthesia: Physiological effects on parents. Anesthesiology 2003, 98, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Shah, V.; Goldman, R.; Taddio, A. Caregivers’ responses to pain in their children in the emergency department. Arch. Pediatr. Adolesc. Med. 2007, 161, 578–582. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M. Pediatric needle procedures: Parent-child interactions, child fear, and evidence-based treatment. Can. Psychol. 2013, 54, 75–79. [Google Scholar] [CrossRef]
- Constantin, K.; Moline, R.; McMurtry, C.M. The role of non-verbal features of caregiving behaviour. In Social and Interpersonal Dynamics in Pain: We Don’t Suffer Alone; Prkachin, K., Vervoort, T., Trost, Z., Karos, K., Eds.; Springer: Berlin, Germany, 2017. [Google Scholar]
- Constantin, K.; McMurtry, C.M.; Bailey, H.N. Parental cardiac response in the context of pediatric acute pain: Current knowledge and future directions. Pain Manag. 2016, 7, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Goubert, L.; Craig, K.D.; Vervoort, T.; Morley, S.; Sullivan, M.J.L.; Williams, A.C.; Cano, A.; Crombez, G. Facing others in pain: The effects of empathy. Pain 2005, 118, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.B.; Camras, L.A.; Pelphrey, K.A. Physiology and functioning: Parents’ vagal tone, emotion socialization, and children’s emotion knowledge. J. Exp. Child Psychol. 2008, 100, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kreibig, S.D. Autonomic nervous system activity in emotion: A review. Biol. Psychol. 2010, 84, 394–421. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Doussard-Roosevelt, J.; Maita, A.K. Vagal tone and the physiological regulation of emotion. Monogr. Soc. Child Dev. 1994, 59, 167–186. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Park, G.; Vasey, M.W.; Van Bavel, J.J.; Thayer, J.F. When tonic cardiac vagal tone predicts changes in phasic vagal tone: The role of fear and perceptual load. Psychophysiology 2014, 51, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.C.M.; Vennewald, N.; Kubiak, T.; Weber, H. The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Personal. Individ. Differ. 2010, 49, 723–728. [Google Scholar] [CrossRef]
- Geisler, F.C.M.; Kubiak, T.; Siewert, K.; Weber, H. Cardiac vagal tone is associated with social engagement and self-regulation. Biol. Psychol. 2013, 93, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.H. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Koval, P.; Ogrinz, B.; Kuppens, P.; Van den Bergh, O.; Tuerlinckx, F.; Sütterlin, S. Affective instability in daily life is predicted by resting heart rate variability. PLoS ONE 2013, 8, e81536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervoort, T.; Trost, Z.; Sütterlin, S.; Caes, L.; Moors, A. Emotion regulatory function of parent attention to child pain and associated implications for parental pain control behaviour. Pain 2014, 155, 1453–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Baeyer, C.L.; Piira, T.; Chambers, C.T.; Trapanotto, M.; Zeltzer, L.K. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. J. Pain 2005, 6, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Birnie, K.A.; Noel, M.; Chambers, C.T.; von Baeyer, C.L.; Fernandez, C.V. The cold pressor task: Is it an ethically acceptable pain research method in children? J. Pediatr. Psychol. 2010, 36, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Bigger, J.T.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Lipponen, J.; Niskanen, J.; Ranta-aho, P.O. Kubios HRV: User’s Guide. Available online: http://www.kubios.com/downloads/Kubios_HRV_Users_Guide.pdf (accessed on 12 May 2017).
- Tarvainen, M.P.; Niskanen, J.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Heathers, J.A.J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014, 5, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paret, L.; Bailey, H.N.; Roche, J.; Bureau, J.; Moran, G. Preschool ambivalent attachment associated with a lack of vagal withdrawal in response to stress. Attach. Hum. Dev. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- National Geographic. Dazzling Time-Lapse Reveals America’s Great Spaces. Available online: https://www.youtube.com/watch?v=9d8wWcJLnFI&feature=youtu.be (accessed on 15 September 2016).
- Morris, J.D. SAM: The self-assessment manikin. An efficient cross-cultural measurement of emotional response. J. Advert. Res. 1995, 35, 63–68. [Google Scholar]
- Allen, J.J.; Chambers, A.S.; Towers, D.N. A Pragmatic Manual for Getting Started with the QRSTool: Reducing EKG Data and Obtaining Many Metrics with CMetX. Available online: http://jallen.faculty.arizona.edu/resources_and_downloads (accessed on 22 October 2016).
- Peltola, M.A. Role of editing of R-R intervals in the analysis of heart rate variability. Front. Physiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, A.D.; van der Sluis, S.; Houtveen, J.H.; Willemsen, G.; de Geus, E.J. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology 2007, 44, 203–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Werner, G.G.; Ford, B.Q.; Mauss, I.B.; Schabus, M.; Blechert, J.; Wilhelm, F.H. High cardiac vagal control is related to better subjective and objective sleep quality. Biol. Psychol. 2015, 106, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lumma, A.L.; Kok, B.E.; Singer, T. Is mediation always relaxing? Investigating heart rate, heart rate variability, experienced effort and likeability during training of three types of mediation. Int. J. Psychophysiol. 2015, 97, 38–45. [Google Scholar] [CrossRef]
- Frazier, T.W.; Strauss, M.E.; Steinhauer, S.R. Respiratory sinus arrhythmia as an indicator of emotional response in young adults. Psychophysiology 2004, 41, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hadjistavropoulos, T.; Craig, K.D.; Duck, S.; Cano, A.; Goubert, L.; Jackson, P.L.; Mogil, J.S.; Rainville, P.; Sullivan, M.J.L.; Williams, A.C.; et al. A biopsychosocial formulation of pain communication. Psychol. Bull. 2011, 137, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Resting | Pre-CPT | Post-CPT a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | M | SD | Range | |
| HR | 76.33 | 9.59 | 61.83–97.05 | 80.31 | 10.53 | 61.90–103.99 | 78.76 | 9.52 | 64.31–96.31 |
| HF-HRV | 6.02 | 0.84 | 4.55–7.36 | 5.74 | 1.11 | 3.26–7.24 | 5.57 | 0.80 | 3.90–7.26 |
| RMSSD | 6.72 | 0.86 | 4.94–8.06 | 6.72 | 0.86 | 4.39–8.50 | 6.61 | 0.68 | 5.34–7.79 |
| Phasic = Pre-CPT − Resting | Recovery a = Post-CPT − Resting | |||||
|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |
| ΔHR | 4.00 | 4.48 | −6.15–14.22 | 1.96 | 3.05 | −2.83 to 8.07 |
| ΔHF-HRV | −0.28 | 0.85 | −2.52 to 1.02 | −0.41 | 0.78 | −1.64 to 1.39 |
| ΔRMSSD | 0.00 | 0.67 | −2.00 to 1.00 | −0.06 | 0.70 | −1.62 to 1.75 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, K.; Moline, R.L.; McMurtry, C.M.; Bailey, H.N. Parent Cardiac Response in the Context of Their Child’s Completion of the Cold Pressor Task: A Pilot Study. Children 2017, 4, 100. https://doi.org/10.3390/children4110100
Constantin K, Moline RL, McMurtry CM, Bailey HN. Parent Cardiac Response in the Context of Their Child’s Completion of the Cold Pressor Task: A Pilot Study. Children. 2017; 4(11):100. https://doi.org/10.3390/children4110100
Chicago/Turabian StyleConstantin, Kaytlin, Rachel L. Moline, C. Meghan McMurtry, and Heidi N. Bailey. 2017. "Parent Cardiac Response in the Context of Their Child’s Completion of the Cold Pressor Task: A Pilot Study" Children 4, no. 11: 100. https://doi.org/10.3390/children4110100






