Clinical Profile Associated with Adverse Childhood Experiences: The Advent of Nervous System Dysregulation
Abstract
:1. Introduction
2. Methods
Statistical Analysis
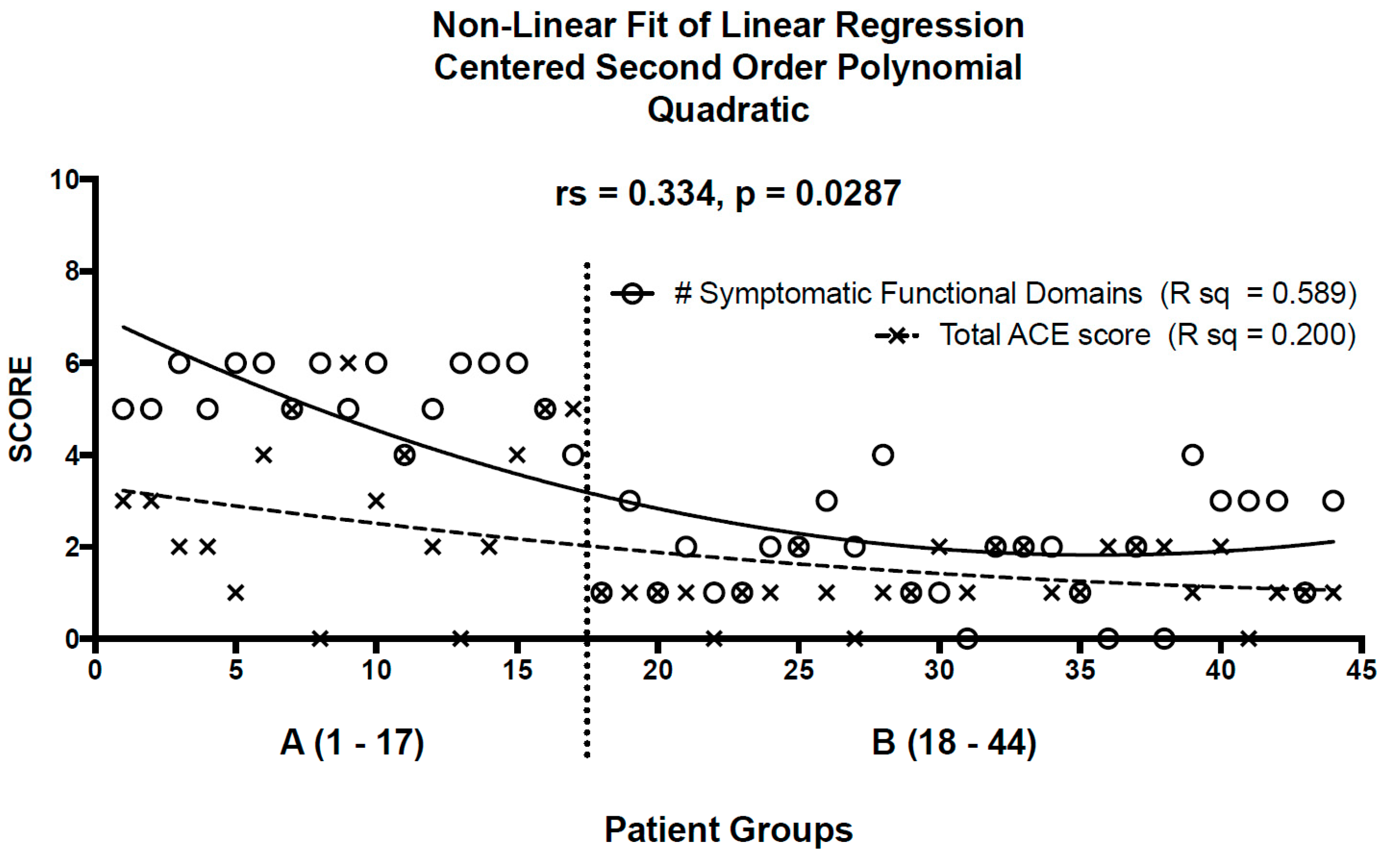
3. Results
4. Discussion
5. Future Directions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | Adverse Childhood Experience |
| ANS | autonomic nervous system |
| HPA | hypothalamic-pituitary-adrenal |
| PTSD | post-traumatic stress disorder |
References
- Kimbrel, N.A.; DeBeer, B.B.; Meyer, E.C.; Silvia, P.J.; Beckham, J.C.; Young, K.A.; Morissette, S.B. An examination of the broader effects of warzone experiences on returning Iraq/Afghanistan veterans’ psychiatric health. Psychiatry Res. 2015, 226, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, T.S.; Newnham, E.A.; Layne, C.M.; Kim, S.; Steinberg, A.M.; Ellis, H.; Birman, D. Trauma history and psychopathology in war-affected refugee children referred for trauma-related mental health services in the United States. J. Trauma Stress 2012, 25, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, C.; Barnow, S.; Gau, K.; Freyberger, H.J.; Grabe, H.J. Childhood maltreatment in patients with somatization disorder. Aust. N. Z. J. Psychiatry 2008, 42, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Dwivedi, A.K.; Privitera, M.D.; Isaacs, K.; Hughes, C.; Bowman, M. Comparisons of childhood trauma, alexithymia, and defensive styles in patients with psychogenic non-epileptic seizures vs. epilepsy: Implications for the etiology of conversion disorder. J. Psychosom. Res. 2013, 75, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Waldinger, R.J.; Schulz, M.S.; Barsky, A.J.; Ahern, D.K. Mapping the road from childhood trauma to adult somatization: the role of attachment. Psychosom. Med. 2006, 68, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.E.; Brandes, J.L.; Peterlin, B.L.; Eloff, A.; Dafer, R.M.; Stein, M.R.; Drexler, E.; Martin, V.T.; Hutchinson, S.; Aurora, S.K.; et al. Childhood maltreatment and migraine (part I). Prevalence and adult revictimization: A multicenter headache clinic survey. Headache 2010, 50, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Shonkoff, J.P.; Garner, A.S.; The Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics; Siegel, B.S.; Dobbins, M.I.; Earls, M.F.; Garner, A.S.; McGuinn, L.; Pascoe, J.; Wood, D.L. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012, 129, e232–e246. [Google Scholar] [CrossRef] [PubMed]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Kertes, D.A.; Gunnar, M.R.; Madsen, N.J.; Long, J.D. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Dev. Psychopathol. 2008, 20, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Sioen, I.; Clays, E.; De Buyzere, M.; Ahrens, W.; Huybrechts, I.; Vanaelst, B.; De Henauw, S. Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biol. Psychol. 2013, 94, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics. Early childhood adversity, toxic stress, and the role of the pediatrician: Translating developmental science into lifelong health. Pediatrics 2012, 129, e224–e231. [Google Scholar]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, M.; Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA 2009, 301, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Marques, S.S.; Oh, D.; Harris, N.B. Toxic stress in children and adolescents. Adv. Pediatr. 2016, 63, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Benjamin, J.; Geva, A.B.; Matar, M.A.; Kaplan, Z.; Kotler, M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: Application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000, 96, 1–13. [Google Scholar] [CrossRef]
- Peres, M.F.; Sanchez del Rio, M.; Seabra, M.L.; Tufik, S.; Abucham, J.; Cipolla-Neto, J.; Silberstein, S.D.; Zukerman, E. Hypothalamic involvement in chronic migraine. J. Neurol. Neurosurg. Psychiatry 2001, 71, 747–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, J.; Williams, D.P.; Kemp, A.H.; Thayer, J.F. Vagally mediated heart rate variability in headache patients-a systematic review and meta-analysis. Cephalalgia 2016, 36, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Yi, H.A.; Lee, H. Recent advances in orthostatic hypotension presenting orthostatic dizziness or vertigo. Neurol. Sci. 2015, 36, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. The neurobiology of stress and gastrointestinal disease. Gut 2000, 47, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Burke, N.J.; Hellman, J.L.; Scott, B.G.; Weems, C.F.; Carrion, V.G. The impact of adverse childhood experiences on an urban pediatric population. Child Abuse Negl. 2011, 35, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Burke, G.; Davis, P.M.; Rzepski, B.; Andrulonis, P.A. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J. Pediatr. 1996, 129, 220–226. [Google Scholar] [CrossRef]
- Fink, P.; Steen Hansen, M.; Sondergaard, L. Somatoform disorders among first-time referrals to a neurology service. Psychosomatics 2005, 46, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.; Spinhoven, P. Trauma and medically unexplained symptoms towards an integration of cognitive and neuro-biological accounts. Clin. Psychol. Rev. 2007, 27, 798–820. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Zisk, A. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Spottiswoode, B.S.; Carey, P.D.; Stein, D.J.; Seedat, S. Relationship between neurocognition and regional brain volumes in traumatized adolescents with and without posttraumatic stress disorder. Neuropsychobiology 2012, 66, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Scheeringa, M.S.; Zeanah, C.H.; Myers, L.; Putnam, F. Heart period and variability findings in preschool children with posttraumatic stress symptoms. Biol. Psychiatry 2004, 55, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Huss, D.; Derefinko, K.; Milich, R.; Farzam, F.; Baumann, R. Examining the stress response and recovery among children with migraine. J. Pediatr. Psychol. 2009, 34, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Vasko, R.; Buysse, D.; Ombao, H.; Chen, Q.; Cashmere, J.D.; Kupfer, D.; Thayer, J.F. Acute stress affects heart rate variability during sleep. Psychosom. Med. 2004, 66, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Nilsson, K.W.; Nyberg, F.; Hogmark, A.; Lindblad, F. Cortisol levels in children with attention-deficit/hyperactivity disorder. J. Psychiatr. Res. 2012, 46, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Larson, M.G.; O’Donnell, C.J.; Wilson, P.F.; Tsuji, H.; Lloyd-Jones, D.M.; Levy, D. Association of hyperglycemia with reduced heart rate variability (The Framingham heart study). Am. J. Cardiol. 2000, 86, 309–312. [Google Scholar] [CrossRef]
- Tsuji, H.; Larson, M.G.; Venditti, F.J., Jr.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham heart study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Venditti, F.J., Jr.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham heart study. Circulation 1994, 90, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Grosberg, B.M.; Mauskop, A.; Cady, R.; Simmons, K.A. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia 2014, 34, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Carreno, F.R.; Frazer, A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 2017, 14, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Chaudhry, H.; Williams, K.; Christo, P.J. Review of the uses of vagal nerve stimulation in chronic pain management. Curr. Pain Headache Rep. 2015, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B.; Bradley, R.G.; Liu, W.; Epstein, M.P.; Deveau, T.C.; Mercer, K.B.; Tang, Y.; Gillespie, C.F.; Heim, C.M.; Nemeroff, C.B.; et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 2008, 299, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Fichna, M.; Krzysko-Pieczka, I.; Zurawek, M.; Skowronska, B.; Januszkiewicz-Lewandowska, D.; Fichna, P. FKBP5 polymorphism is associated with insulin resistance in children and adolescents with obesity. Obes. Res. Clin. Pract. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Cai, J.; Brancati, F.L.; Folsom, A.; Barnes, R.W.; Tyroler, H.A.; Heiss, G. Association of vagal tone with serum insulin, glucose, and diabetes mellitus--The ARIC study. Diabetes Res. Clin. Pract. 1995, 30, 211–221. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
| Patient (n = 17) | Age (Years) | Sex | Medically-Unexplained Symptoms | Other Medical Problems | Duration of Medical Symptoms | Total ACE Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Executive Dysfunction | Sleep Problems | Autonomic Symptoms | Emotional Dysregulation | Digestive/Urinary Problems | Somatization | ||||||
| 1 | 14 | F | Attention problems | Insomnia | None | Anxiety, panic attacks | Constipation, frequent urination | Tremor, weakness | Asthma, chronic fatigue | 6 years | 3 |
| 2 | 8 | F | Poor memory and attention | Nightmares | Dizziness | Cries easily, temper tantrums | None | Headache, blurred vision | 4-months | 3 | |
| 3 | 14 | M | Poor attention | Insomnia | Dizziness, syncope | Anxiety, depression, self-harm, suicidality | Diarrhea | Headache weakness, paresthesias | Sinus bradycardia, chronic fatigue | 8-months | 2 |
| 4 | 15 | F | Poor memory and attention | Insomnia, frequent night wakings | Dizziness, syncope, | Anxiety, depression, panic attacks | Constipation | Headache, weakness, paresthesias, abdominal pain | Asthma, cardiac rhythm disturbance, chronic fatigue | 4-months | 2 |
| 5 | 15 | F | Poor concentration | Insomnia | Dizziness | Anxiety, OCD, depression, panic attacks | Reflux, diarrhea | Headache, paresthesias, abdominal pain | Asthma | 18-months | 1 |
| 6 | 7 | M | Poor attention | Insomnia, frequent night wakings | Dizziness | Anxiety, fearfulness | Constipation, nocturnal enuresis | Headaches, blurred vision, paresthesias, weakness | Chronic fatigue | 3-years | 4 |
| 7 | 13 | F | Poor memory and attention | Insomnia, frequent night wakings | Dizziness | Anxiety, depression, panic attacks, self-harm, suicidality | None | Headaches, blurred vision, PNES | Eating disorder, chronic fatigue | 4-years | 5 |
| 8 | 13 | F | Poor attention | Insomnia, nightmares | Migraine | Anxiety, depression, rage and panic attacks | Constipation, vomiting, frequent urination | Abdominal pain | Asthma, tics | 8-years | 0* |
| 9 | 5 | M | Poor attention | Frequent night wakings, nightmares | None | Anxiety, fear, temper tantrums | Diarrhea, nocturnal enuresis | Headaches, abdominal pain | Asthma, chronic fatigue | 2-years | 6 |
| 10 | 17 | F | Poor memory and attention | Insomnia, night sweats | Dizziness, syncope | Anxiety, depression, anorexia, self-harm | Diarrhea, constipation | Headaches, weakness, PNES | Eating disorder, POTS, sleep apnea, chronic fatigue | 3-years | 3 |
| 11 | 5 | M | Poor memory and attention | Insomnia, frequent night wakings | None | Anxiety, fear, OCD, separation anxiety | None | Headaches | Sleep apnea, tics | 3-years | 4 |
| 12 | 17 | F | Poor memory, slow processing | Insomnia | Dizziness | Anxiety, depression, panic attacks | None | Headaches, blurred vision, unsteady gait, chest pain, tinnitus | Frequent ear infections, chronic fatigue | 2-years | 2 |
| 13 | 15 | F | Poor memory, slow processing | Insomnia | Dizziness | Anxiety, depression, panic attacks | Diarrhea, constipation | Headaches, numbness, pelvic pain | Asthma, chronic fatigue | 1-year | 0* |
| 14 | 15 | F | Poor memory | Insomnia, frequent night wakings | Dizziness, syncope | Anxiety, depression | Nausea | Headaches, numbness, neck pain, unsteady gait | POTS, chronic fatigue | 18-months | 2 |
| 15 | 13 | M | Poor attention | Insomnia | Dizziness | Panic attacks, rage attacks | Nausea, reflux | Headaches, blurred vision, paresthesias | Derealization, depersonalization, | 10-months | 4 |
| 16 | 16 | F | None | Insomnia, nightmares | Dizziness, migraine | Anxiety, depression, panic and rage attacks, suicidality | Diarrhea, dysuria | Neck pain, back pain | Asthma, eating disorder, heart palpitations, chronic fatigue | 5-years | 5 |
| 17 | 10 | F | Poor attention | Insomnia, nightmares | None | Rage attacks, panic attacks | None | Headaches, abdominal pain, blurred vision | Heart palpitations, shortness of breath, tics | 5-years | 5 |
| Patient (n = 27) | Age (Years) | Sex | Medically-Unexplained Symptoms | Other Medical Problems | Duration of Medical Symptoms | Total ACE Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Executive Dysfunction | Sleep Problems | Autonomic Symptoms | Emotional Dysregulation | Digestive/Urinary Problems | Somatization | ||||||
| 1 | 16 | M | Cognitive dysfunction | None | None | None | None | None | Sickle cell disease, stroke, moyamoya syndrome | 2 years | 1 |
| 2 | 10 | F | None | None | Dizziness, syncope | Depression | None | Headaches, leg pain | None | 2-months | 1 |
| 3 | 14 | F | None | None | None | Depression | None | None | Generalized epilepsy | 1-year | 1 |
| 4 | 14 | F | None | None | Migraine | Depression | None | None | Epilepsy | 2-years | 1 |
| 5 | 7 | M | None | None | None | None | None | Daily headache | None | 1 year | 0 * |
| 6 | 11 | M | Attention problems | None | None | None | None | None | Tics | 5 years | 1 |
| 7 | 12 | F | None | Insomnia | None | Anxiety, depression, OCD | None | None | Trichotillomania tics, asthma, eczema | 7 years | 1 |
| 8 | 17 | M | Attention problems | None | Migraine | None | None | None | None | 5 years | 2 |
| 9 | 15 | M | Cognitive dysfunction | Insomnia | None | Depression, anxiety | None | None | Epilepsy | 3 years | 1 |
| 10 | 13 | F | None | Insomnia | None | None | None | Headache | None | 1 year | 0 * |
| 11 | 9 | M | None | Insomnia | None | Depression | None | Headache | Congenital heart disease | 3-years | 1 |
| 12 | 14 | F | None | None | None | None | None | Headache | Breast discharge | 1-year | 1 |
| 13 | 15 | F | None | None | Migraine | None | None | None | None | 7-years | 1 |
| 14 | 17 | M | None | None | None | None | None | None | Epilepsy | 1-month | 1 |
| 15 | 7 | M | Cognitive dysfunction, attention problems | None | None | None | Constipation | None | Developmental delay | 7 years | 2 |
| 16 | 8 | F | None | None | Migraines | Anxiety | None | None | None | 3-months | 2 |
| 17 | 5 | F | None | None | None | None | Constipation | Headache, abdominal pain | None | 8-months | 1 |
| 18 | 8 | F | None | None | Migraine | None | None | None | None | 3-months | 1 |
| 19 | 5 | F | None | None | None | None | None | None | Febrile seizures | 2 years | 2 |
| 20 | 11 | M | Cognitive dysfunction | Insomnia | None | None | None | None | Epilepsy | 1-year | 2 |
| 21 | 15 | F | None | None | No | None | None | None | Epilepsy, Moyamoya disease | 3-years | 2 |
| 22 | 8 | M | None | Insomnia | Migraine | Anxiety | None | None | Cerebral dysgenesis | 7-years | 1 |
| 23 | 15 | M | Cognitive problems, memory problems | Insomnia | None | Anxiety | None | None | Epilepsy | 5-months | 2 |
| 24 | 13 | M | Learning problems, attention problems | Insomnia | Migraine | None | None | None | Neuro-fibromatosis Type-1 | 6 years | 0 * |
| 25 | 15 | F | Cognitive difficulties | None | Dizziness | Anxiety, depression, panic attacks | None | None | None | 1-year | 1 |
| 26 | 10 | F | None | None | None | None | None | Headache | Arnold Chiari malformation | 1-year | 1 |
| 27 | 10 | M | Cognitive dysfunction | Insomnia | None | None | None | Back pain | Cerebral palsy | 6-months | 1 |
| Adverse Childhood Experience | Total (%) N = 100 | Group A (%) n = 17 | Group B (%) n = 27 | OR (95% CI) [Comparing Group A to Group B] | p-Value |
|---|---|---|---|---|---|
| Physical abuse | 2 (2%) | 2 (12%) | 0 | indeterminate | |
| Emotional/verbal abuse | 15 (15%) | 10 (59%) | 5 (19%) | 6.29 (1.60–24.73) | 0.009 * |
| Sexual abuse | 5 (5%) | 5 (29%) | 0 | indeterminate | |
| Physical or emotional neglect | 4 (4%) | 3 (18%) | 1 (4%) | 5.57 (0.53–58.69) | 0.28 |
| Incarcerated household member | 2 (2%) | 2 (12%) | 0 | indeterminate | |
| Exposure to domestic violence | 5 (5%) | 4 (24%) | 1 (4%) | 8 (0.81–79.02) | 0.06 |
| Substance user in the home | 7 (7%) | 7 (41%) | 0 | indeterminate | |
| Mental illness in a household member | 6 (6%) | 4 (24%) | 2 (7%) | 3.70 (0.59–22.94) | 0.19 |
| One or no parents/divorce | 37 (37%) | 14 (82%) | 23 (85%) | 0.81 (0.16–4.17) | 1.0 |
| Other traumatic events | homeless, foster care, near-drowning accident | motor vehicle accident, death of a loved one | |||
| Median Total ACE score (interquartile range) | 0 (0–6) | 3 (0–6) | 1 (0–2) | N/A | 0.0001 ‡ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbers, J.; Rovnaghi, C.R.; Golianu, B.; Anand, K.J.S. Clinical Profile Associated with Adverse Childhood Experiences: The Advent of Nervous System Dysregulation. Children 2017, 4, 98. https://doi.org/10.3390/children4110098
Elbers J, Rovnaghi CR, Golianu B, Anand KJS. Clinical Profile Associated with Adverse Childhood Experiences: The Advent of Nervous System Dysregulation. Children. 2017; 4(11):98. https://doi.org/10.3390/children4110098
Chicago/Turabian StyleElbers, Jorina, Cynthia R. Rovnaghi, Brenda Golianu, and Kanwaljeet J. S. Anand. 2017. "Clinical Profile Associated with Adverse Childhood Experiences: The Advent of Nervous System Dysregulation" Children 4, no. 11: 98. https://doi.org/10.3390/children4110098





