The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature
Abstract
:1. Introduction
2. Fetal Pulmonary Vascular Resistance
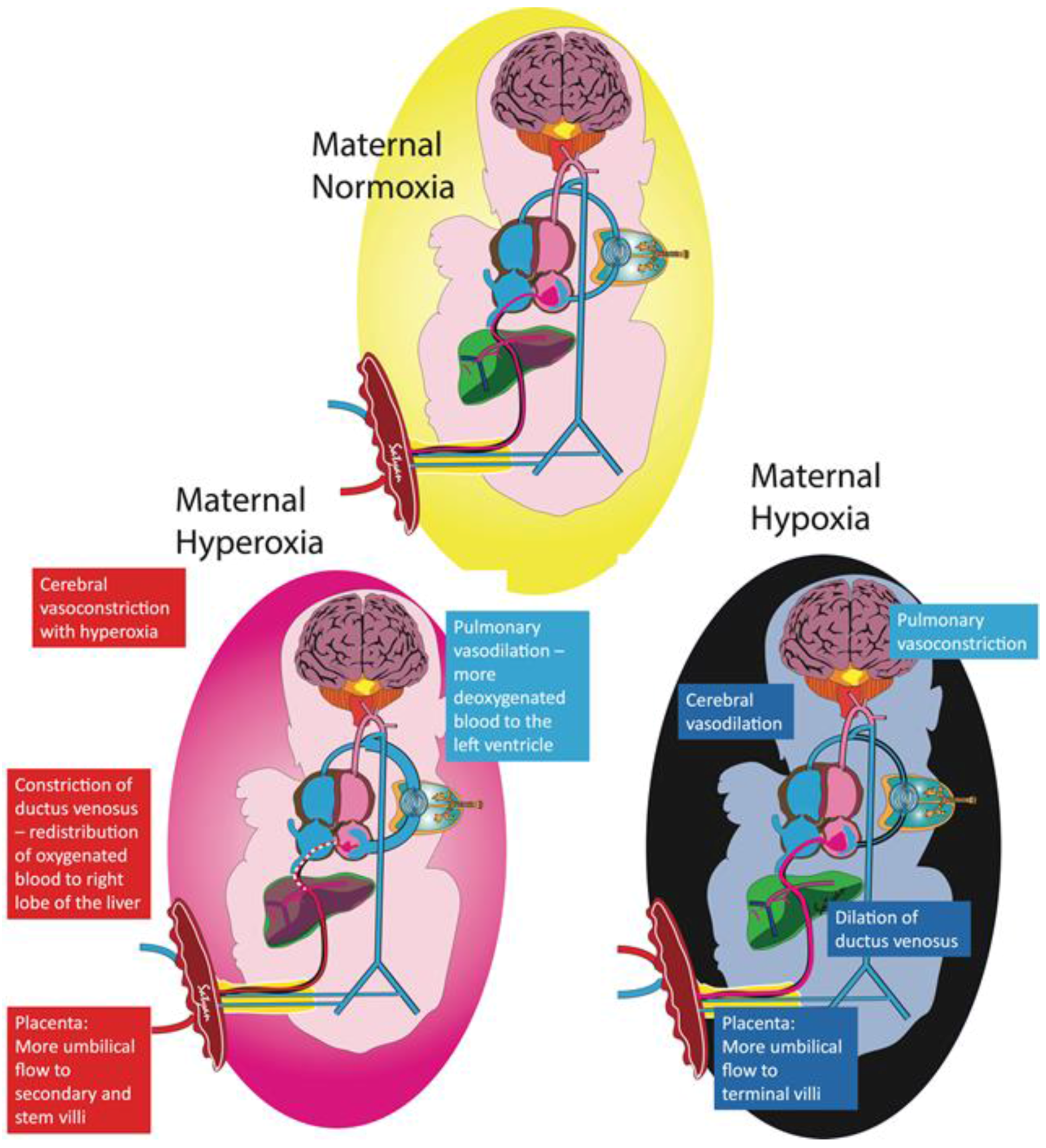
2.1. Effects of Oxygen and Fetal PVR
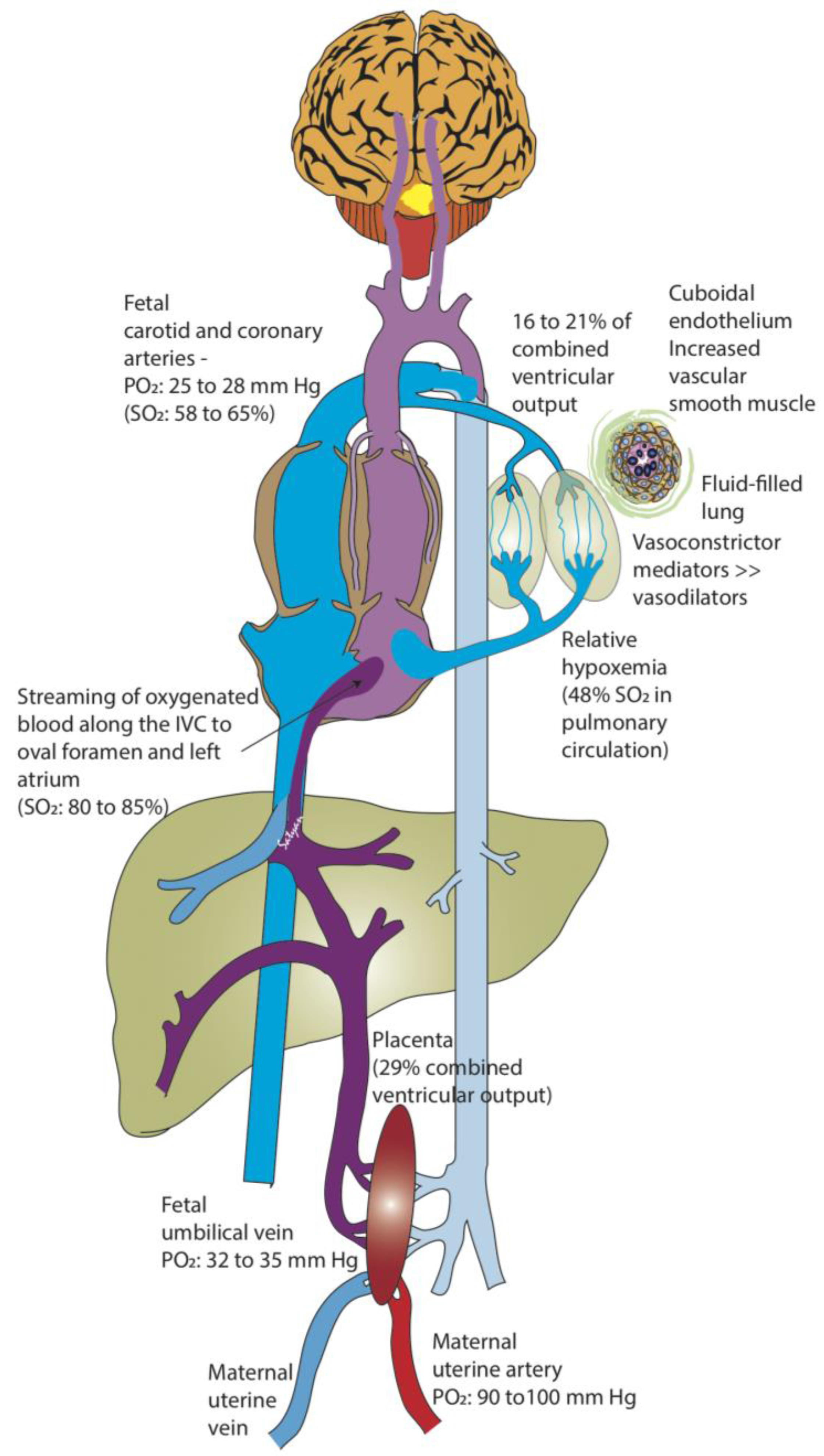
2.2. Fetal Circulation and the Role of the Lungs
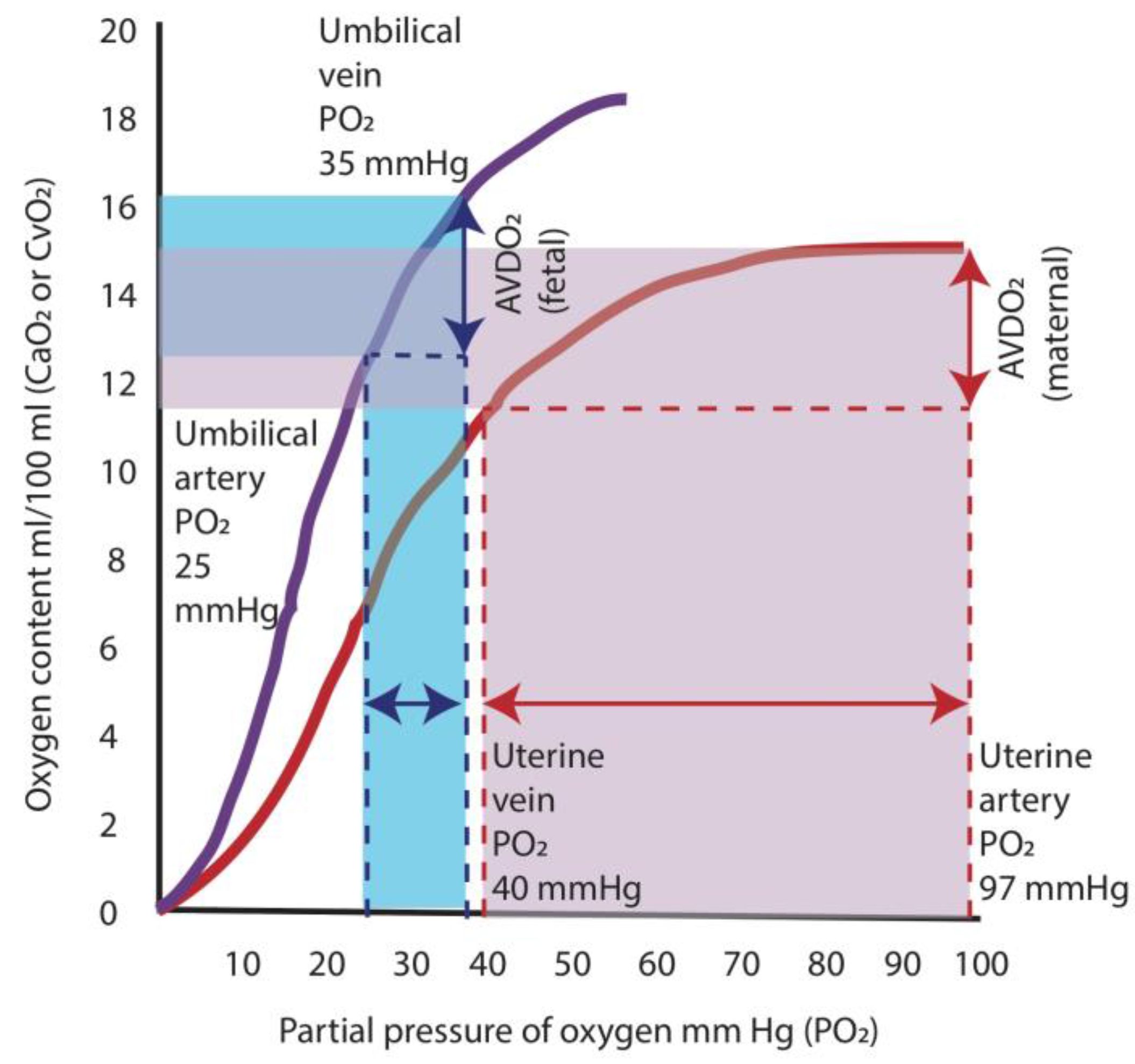
2.3. Fetal Oxygenation
3. Transition at Birth and PPHN
The Role of Oxygen in Treating PPHN
4. Oxygen Use for PPHN in the Neonatal Intensive Care Unit (NICU)
Determining the Optimal Oxygenation Range
5. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gersony, W.; Duc, G.; Sinclair, J. “Pfc syndrome” (persistence of the fetal circulation). Circulation 1969, 87–94. [Google Scholar]
- Aschner, J.L.; Gien, J.; Ambalavanan, N.; Kinsella, J.P.; Konduri, G.G.; Lakshminrusimha, S.; Saugstad, O.D.; Steinhorn, R.H. Challenges, priorities and novel therapies for hypoxemic respiratory failure and pulmonary hypertension in the neonate. J. Perinatol. 2016, 36, S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, R.H. Advances in neonatal pulmonary hypertension. Neonatology 2016, 109, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Konduri, G.G.; Steinhorn, R.H. Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates. J. Perinatol. 2016, 36, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef] [PubMed]
- Walsh-Sukys, M.C.; Tyson, J.E.; Wright, L.L.; Bauer, C.R.; Korones, S.B.; Stevenson, D.K.; Verter, J.; Stoll, B.J.; Lemons, J.A.; Papile, L.A.; et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics 2000, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.H.; Hutchison, A.A.; Lakshminrusimha, S.; Morin, F.C.; Wynn, R.J.; Ryan, R.M. Characteristics of pulmonary hypertension in preterm neonates. J. Perinatol. 2007, 27, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Konduri, G.G.; Vohr, B.; Robertson, C.; Sokol, G.M.; Solimano, A.; Singer, J.; Ehrenkranz, R.A.; Singhal, N.; Wright, L.L.; Van Meurs, K.; et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: Neurodevelopmental follow-up. J. Pediatr. 2007, 150, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Porta, N.F.; Steinhorn, R.H. Pulmonary vasodilator therapy in the NICU: Inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin. Perinatol. 2012, 39, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Hislop, A.; Reid, L. Intra-pulmonary arterial development during fetal life-branching pattern and structure. J. Anat. 1972, 113, 35–48. [Google Scholar] [PubMed]
- Levin, D.L.; Rudolph, A.M.; Heymann, M.A.; Phibbs, R.H. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation 1976, 53, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hislop, A.; Reid, L. Pulmonary arterial development during childhood: Branching pattern and structure. Thorax 1973, 28, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Fineman, J.R.; Soifer, S.J.; Heymann, M.A. Regulation of pulmonary vascular tone in the perinatal period. Annu. Rev. Physiol. 1995, 57, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Steinhorn, R.H. Pulmonary vascular biology during neonatal transition. Clin. Perinatol. 1999, 26, 601–619. [Google Scholar] [PubMed]
- Von Euler, U.; Liljestrand, G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol. Scand. 1946, 12, 301–320. [Google Scholar] [CrossRef]
- Lewis, A.B.; Heymann, M.A.; Rudolph, A.M. Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ. Res. 1976, 39, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Morin, F.C.; Egan, E.A. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J. Appl. Physiol. (1985) 1992, 73, 213–218. [Google Scholar]
- Morin, F.C.; Egan, E.A.; Ferguson, W.; Lundgren, C.E. Development of pulmonary vascular response to oxygen. Am. J. Physiol. 1988, 254, H542–H546. [Google Scholar] [PubMed]
- Rasanen, J.; Wood, D.C.; Debbs, R.H.; Cohen, J.; Weiner, S.; Huhta, J.C. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: A randomized study. Circulation 1998, 97, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A. The fetal circulation. In Congenital Diseases of the Heart: Clinical-Physiological Considerations, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–24. [Google Scholar]
- Rasanen, J.; Wood, D.C.; Weiner, S.; Ludomirski, A.; Huhta, J.C. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation 1996, 94, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Prsa, M.; Sun, L.; van Amerom, J.; Yoo, S.J.; Grosse-Wortmann, L.; Jaeggi, E.; Macgowan, C.; Seed, M. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ. Cardiovasc. Imaging 2014, 7, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.P.; Ivy, D.D.; Abman, S.H. Ontogeny of no activity and response to inhaled no in the developing ovine pulmonary circulation. Am. J. Physiol. 1994, 267, H1955–H1961. [Google Scholar] [PubMed]
- Lakshminrusimha, S. The pulmonary circulation in neonatal respiratory failure. Clin. Perinatol. 2012, 39, 655–683. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.; Holm, D.; Pedersen, M.; Tietze, A.; Stausbol-Gron, B.; Duus, L.; Uldbjerg, N. Left-right difference in fetal liver oxygenation during hypoxia estimated by bold mri in a fetal sheep model. Ultras. Obstet. Gynecol. 2011, 38, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Konduri, G.G.; Gervasio, C.T.; Theodorou, A.A. Role of adenosine triphosphate and adenosine in oxygen-induced pulmonary vasodilation in fetal lambs. Pediatr. Res. 1993, 33, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.H.; Alexander, G. Supplementary observations on the oxygen pressure gradient between the maternal and fetal bloods of sheep. Yale J. Biol. Med. 1952, 25, 61–66. [Google Scholar] [PubMed]
- Soothill, P.W.; Nicolaides, K.H.; Rodeck, C.H.; Campbell, S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal. Ther. 1986, 1, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Teitel, D.F.; Iwamoto, H.S.; Rudolph, A.M. Changes in the pulmonary circulation during birth-related events. Pediatr. Res. 1990, 27, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.L.; Thornburg, K.L. Pulmonary pressure-flow relationships in the fetal lamb during in utero ventilation. J. Appl. Physiol. (1985) 1990, 69, 1630–1636. [Google Scholar]
- Shaul, P.W.; Farrar, M.A.; Zellers, T.M. Oxygen modulates endothelium-derived relaxing factor production in fetal pulmonary arteries. Am. J. Physiol. 1992, 262, H355–H364. [Google Scholar] [PubMed]
- Kato, M.; Staub, N.C. Response of small pulmonary arteries to unilobar hypoxia and hypercapnia. Circ. Res. 1966, 19, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, R.; Michelakis, E.D.; Archer, S.L. Hypoxic pulmonary vasoconstriction. J. Appl. Physiol. (1985) 2005, 98, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M.; Yuan, S. Response of the pulmonary vasculature to hypoxia and h+ ion concentration changes. J. Clin. Investig. 1966, 45, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M. Fetal and neonatal pulmonary circulation. Annu. Rev. Physiol. 1979, 41, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Swartz, D.D.; Gugino, S.F.; Ma, C.X.; Wynn, K.A.; Ryan, R.M.; Russell, J.A.; Steinhorn, R.H. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr. Res. 2009, 66, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Steinhorn, R.H.; Wedgwood, S.; Savorgnan, F.; Nair, J.; Mathew, B.; Gugino, S.F.; Russell, J.A.; Swartz, D.D. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J. Appl. Physiol. (1985) 2011, 111, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Russell, J.A.; Steinhorn, R.H.; Ryan, R.M.; Gugino, S.F.; Morin, F.C.; Swartz, D.D.; Kumar, V.H. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr. Res. 2006, 59, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Byrns, R.E.; Buga, G.M.; Wood, K.S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ. Res. 1987, 61, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N. Engl. J. Med. 1997, 336, 597–604. [Google Scholar]
- Davidson, D.; Barefield, E.S.; Kattwinkel, J.; Dudell, G.; Damask, M.; Straube, R.; Rhines, J.; Chang, C.T. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: A randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics 1998, 101, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Kueser, T.J.; Walker, M.W.; Southgate, W.M.; Huckaby, J.L.; Perez, J.A.; Roy, B.J.; Keszler, M.; Kinsella, J.P. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 2000, 342, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Fineman, J.R.; Morin, F.C.; Shaul, P.W.; Rimar, S.; Schreiber, M.D.; Polin, R.A.; Zwass, M.S.; Zayek, M.M.; Gross, I.; et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 1997, 336, 605–610. [Google Scholar] [CrossRef] [PubMed]
- The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics 1997, 99, 838–845. [Google Scholar]
- Brennan, L.A.; Steinhorn, R.H.; Wedgwood, S.; Mata-Greenwood, E.; Roark, E.A.; Russell, J.A.; Black, S.M. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: A role for nadph oxidase. Circ. Res. 2003, 92, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Russell, J.A.; Wedgwood, S.; Gugino, S.F.; Kazzaz, J.A.; Davis, J.M.; Steinhorn, R.H. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am. J. Respir. Crit. Care. Med. 2006, 174, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Didion, S.P. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Sanderud, J.; Norstein, J.; Saugstad, O.D. Reactive oxygen metabolites produce pulmonary vasoconstriction in young pigs. Pediatr. Res. 1991, 29, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Belik, J.; Jankov, R.P.; Pan, J.; Yi, M.; Chaudhry, I.; Tanswell, A.K. Chronic o2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane. J. Appl. Physiol. (1985) 2004, 96, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Farrow, K.N.; Groh, B.S.; Schumacker, P.T.; Lakshminrusimha, S.; Czech, L.; Gugino, S.F.; Russell, J.A.; Steinhorn, R.H. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ. Res. 2008, 102, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sanderud, J.; Oroszlàn, G.; Bjøro, K.; Kumlin, M.; Saugstad, O.D. D-penicillamine inhibits the action of reactive oxygen species in the pig pulmonary circulation. J. Perinat. Med. 1995, 23, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Russell, J.A.; Steinhorn, R.H.; Swartz, D.D.; Ryan, R.M.; Gugino, S.F.; Wynn, K.A.; Kumar, V.H.; Mathew, B.; Kirmani, K.; et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr. Res. 2007, 62, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Aune, D. Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Manja, V.; Lakshminrusimha, S.; Cook, D.J. Oxygen saturation target range for extremely preterm infants: A systematic review and meta-analysis. JAMA Pediatr. 2015, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.J.; Lakshminrusimha, S.; Polin, R.A. Oxygen-saturation targets in preterm infants. N. Eng. J. Med. 2016, 375, 186–187. [Google Scholar]
- Alapati, D.; Jassar, R.; Shaffer, T.H. Management of supplemental oxygen for infants with persistent pulmonary hypertension of newborn: A survey. Am. J. Perinatol. 2017, 34, 276–282. [Google Scholar] [PubMed]
- Nakwan, N.; Chaiwiriyawong, P. An international survey on persistent pulmonary hypertension of the newborn: A need for an evidence-based management. J. Neonatal. Perinatal. Med. 2016, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.S.; Chalak, L.F.; DuPont, T.L.; Rollins, N.K.; Brion, L.P.; Wyckoff, M.H. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Stiris, T.; Odden, J.P.; Hansen, T.W.; Hall, C.; Bratlid, D. The effect of arterial pco2-variations on ocular and cerebral blood flow in the newborn piglet. Pediatr. Res. 1989, 25, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Shankaran, S.; Laptook, A.R.; Langer, J.C.; Bara, R.; Ehrenkranz, R.A.; Goldberg, R.N.; Das, A.; Higgins, R.D.; Tyson, J.E.; et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 2011, 158, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vali, P.; Lakshminrusimha, S. The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature. Children 2017, 4, 67. https://doi.org/10.3390/children4080067
Vali P, Lakshminrusimha S. The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature. Children. 2017; 4(8):67. https://doi.org/10.3390/children4080067
Chicago/Turabian StyleVali, Payam, and Satyan Lakshminrusimha. 2017. "The Fetus Can Teach Us: Oxygen and the Pulmonary Vasculature" Children 4, no. 8: 67. https://doi.org/10.3390/children4080067




