Preparation and Characterization of the TiO2 Immobilized Polymeric Photocatalyst for Degradation of Aspirin under UV and Solar Light
Abstract
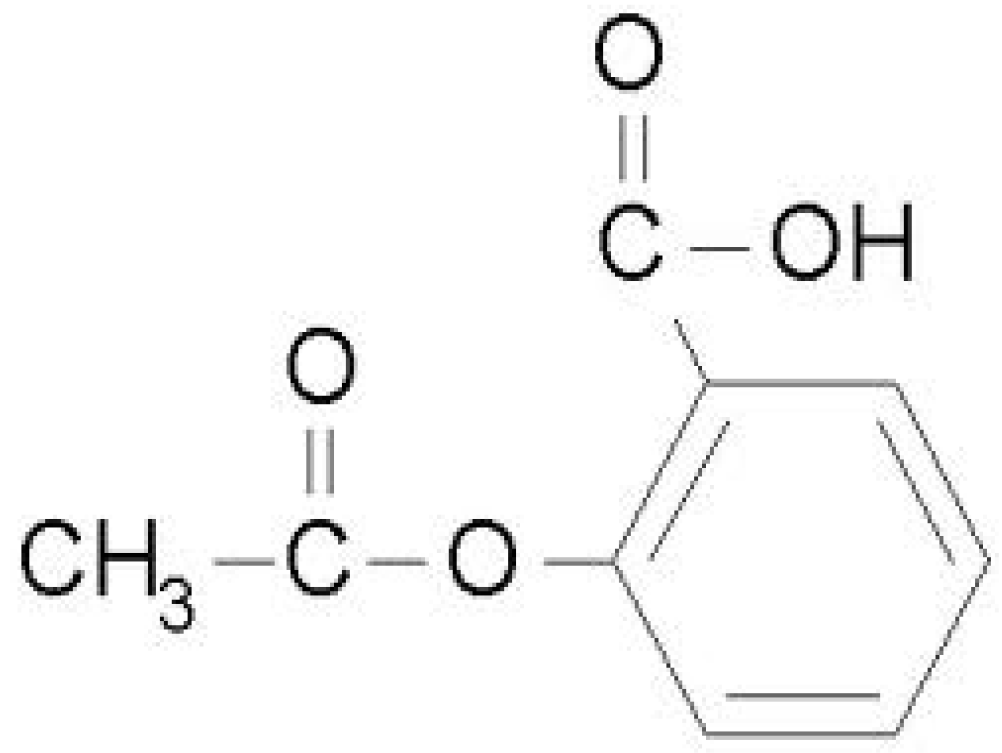
:1. Introduction
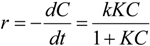
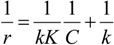
2. Experimental Section
2.1. TiO2 Immobilization Procedure
2.1.1. Preparation of Polymeric/TiO2 Membranes
2.1.2. Physical Cross-Linking Methods
2.1.2.1. Freeze Drying
2.1.2.2. Heat Treatment
2.1.3. Chemical Cross-Linking Methods
2.1.3.1. Acetaldehyde Treatment
2.1.3.2. UV Treatment
2.1.3.3. Freeze-Dried and UV Treated
3. Characterization and Degradation of Aspirin
4. Results and Discussions
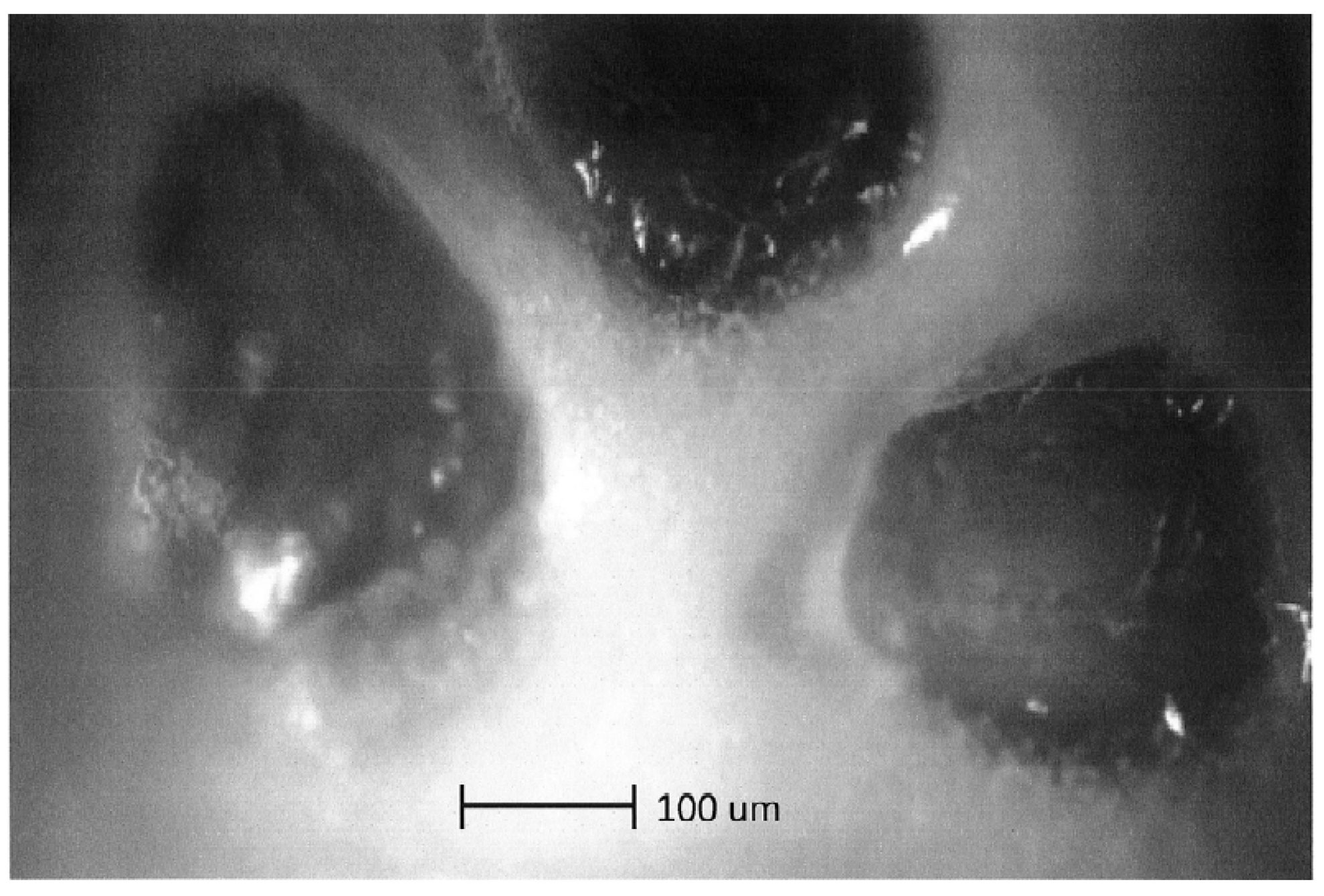
4.1. Optical Microscopy
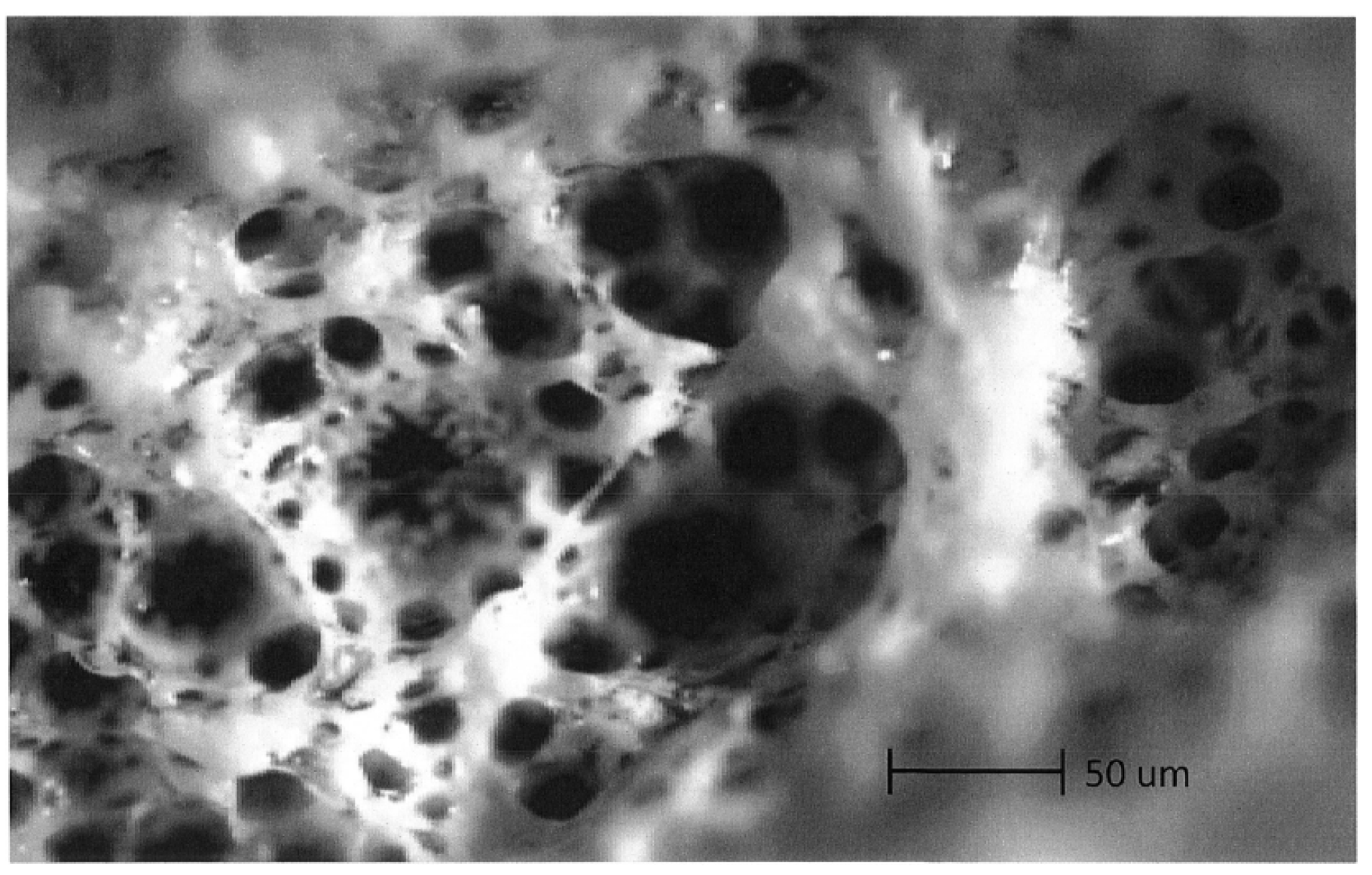
4.2. Scanning Electron Microscopy (SEM) Study
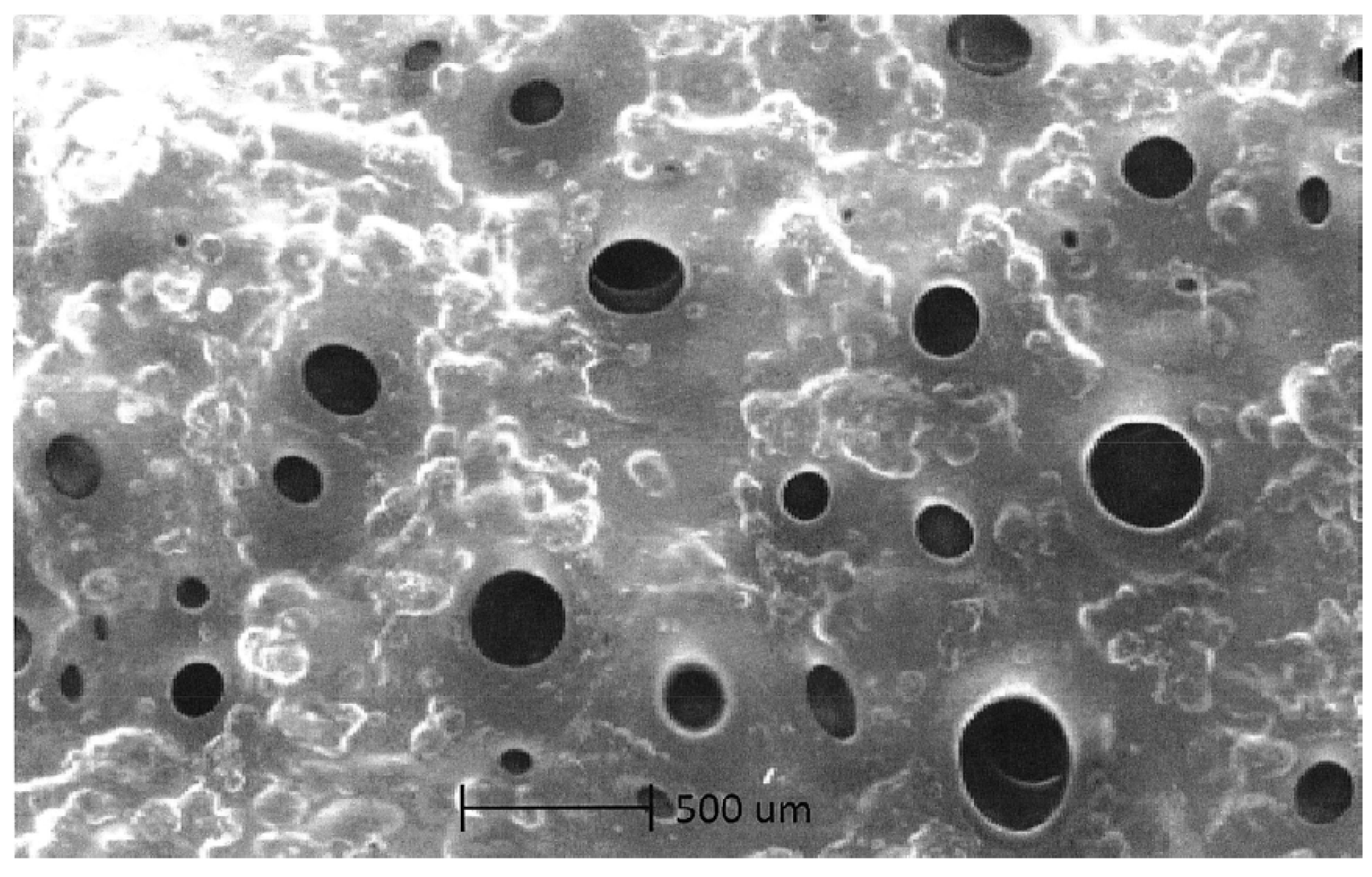


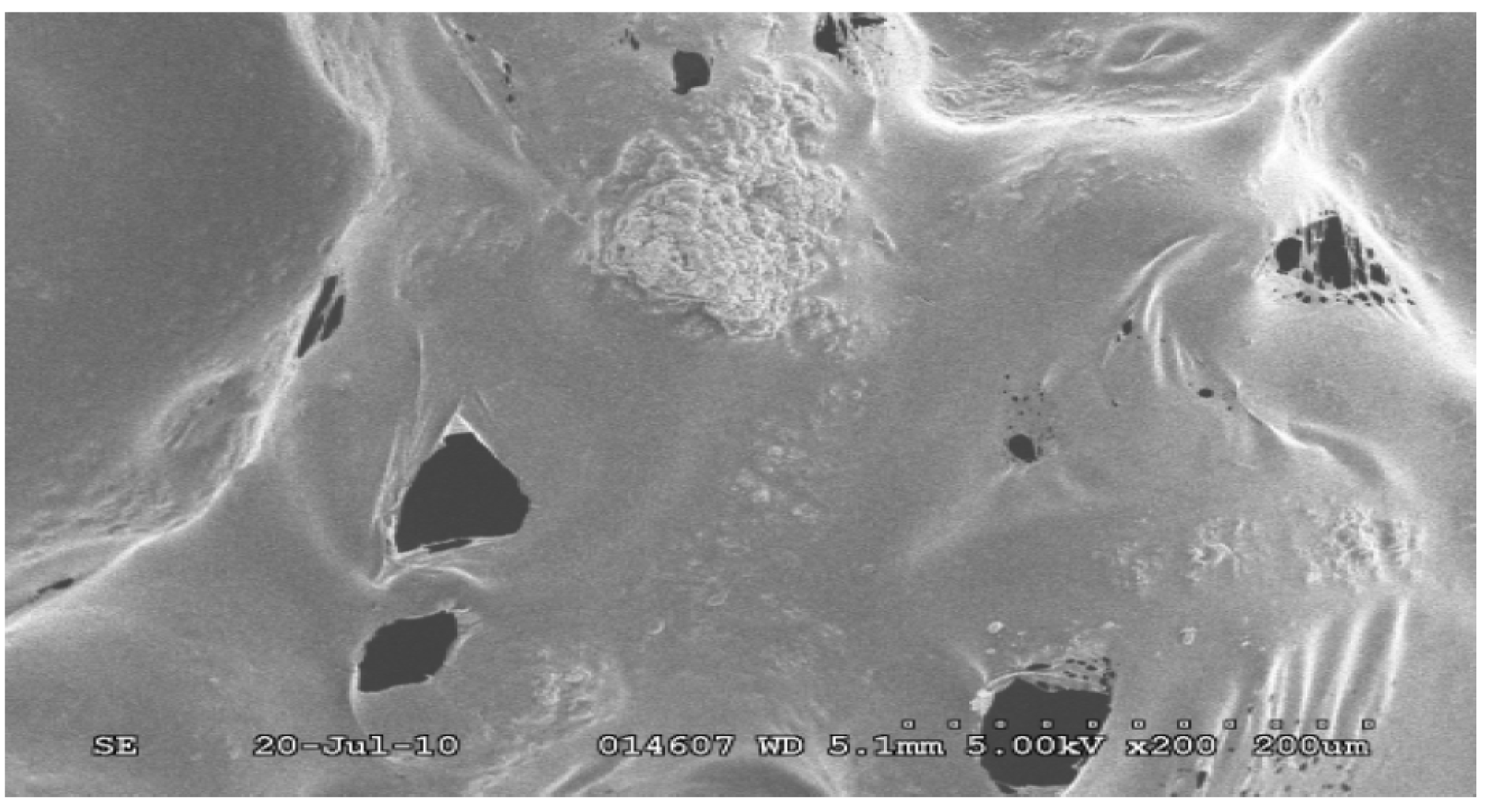

4.3. Degradation of Acetylsalicylic Acid (Aspirin)
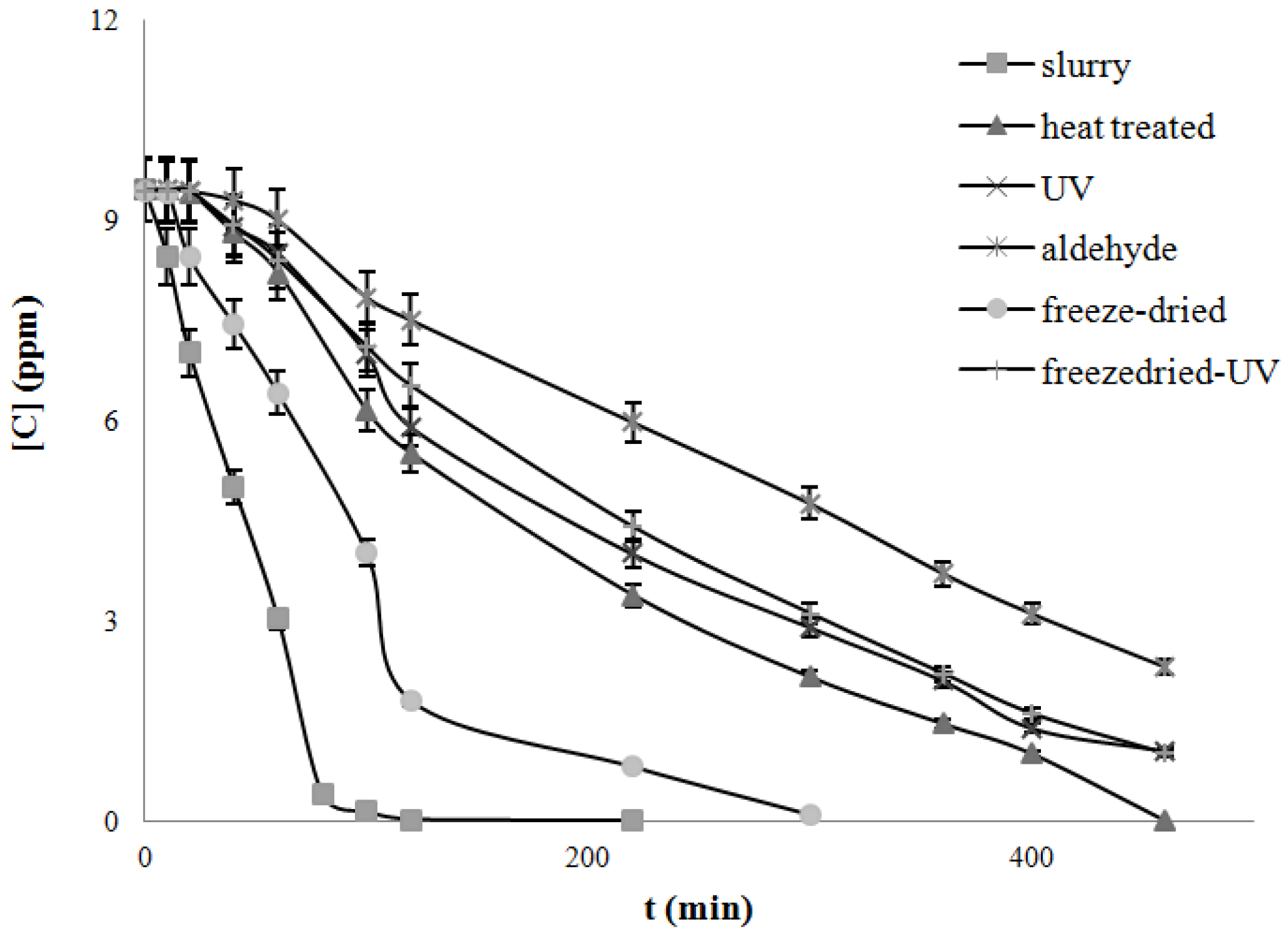
| Catalysts | Freeze dried | Heat treated | UV treated | Acetaldehyde treated | Freeze-dried UV treated |
|---|---|---|---|---|---|
| k, mg/L/min UV light | 0.050 | 0.019 | 0.017 | 0.015 | 0.024 |
| k, mg/L/min Solar light | 0.079 | 0.022 | 0.019 | 0.017 | 0.027 |
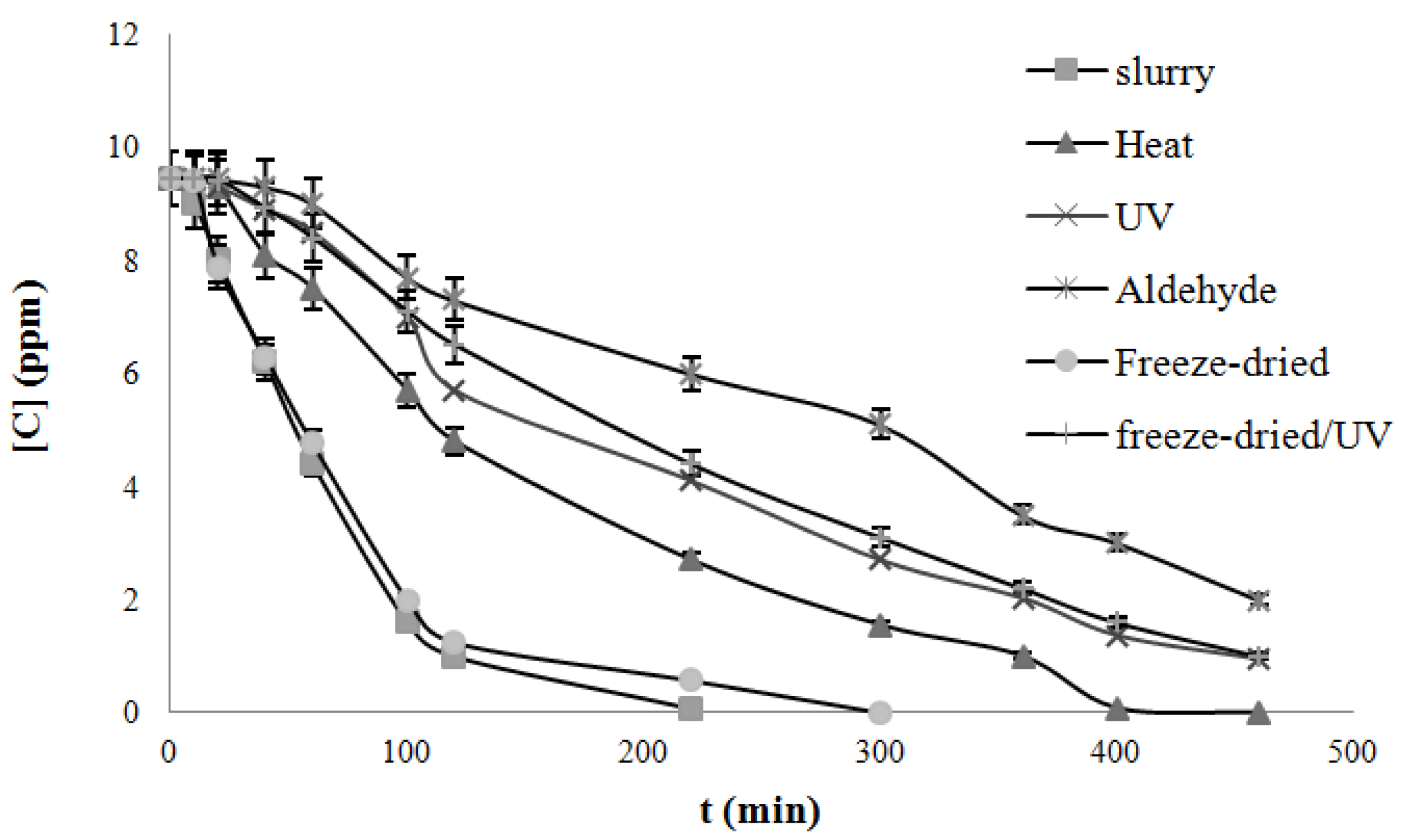
5. Conclusions
Nomenclature
| C and Co | Concentration, ppm |
| K | Langmuir-Hinshelwood rate constant |
| K | Langmuir constant |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinyl pyrrolidone |
| P25 TiO2 | Degussa P25 Titanium dioxide |
| SEM | Scanning Electron Microscopy |
| CL | Cross link |
Subscripts
| Sl | slurry |
| f-d | Freeze dried |
| Ald | Aldehyde |
| UV | Ultraviolet |
| H | Heat treated |
| f-d/UV | Freeze dried UV treated |
Conflicts of Interest
References
- Liu, C.-C.; Hsieh, Y.-H.; Lai, P.-F.; Li, C.-H.; Kao, C.-L. Photodegradation treatment of azo dye waste water by UV/TiO2 process. Dye. Pigment. 2006, 68, 191–195. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Photocatalytic Purification and Treatment of Water and Air; Al-Ekabi, H.A.; Ollis, D. (Eds.) Elsevier: Amsterdam, The Netherlands, 1993.
- Andronic, L.; Duta, A. The influence of TiO2 powder and film on methyl orange degradation. Mater. Chem. Phys. 2008, 112, 1078–1082. [Google Scholar] [CrossRef]
- Vincenzo, A.; Marianna, B.; Vittorio, L.; Giovanni, P.; Leonardo, P.; Sedat, Y. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Akira Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chen, D.; Ray, A.K. Photocatalytic kinetics of phenol and its derivatives over UV irradiated TiO2. Appl. Catal. B 1999, 23, 143–157. [Google Scholar] [CrossRef]
- Rizzo, L.; Koch, J.; Belgiorno, V.; Anderson, M.A. Removal of methylene blue in a photocatalytic reactor using polymethylmethacrylate supported TiO2 nanofilm. Desalination 2007, 211, 1–9. [Google Scholar] [CrossRef]
- Di Paola, A.; García-López, E.; Marcìa, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar]
- Dung, N.T.; van Khoa, N.; Herrmann, J.-M. Photocatalytic degradation of reactive dye RED-3BA in aqueous TiO2 suspension under UV-visible light. Int. J. Photoenergy 2005, 25, 250–255. [Google Scholar]
- Kalfa, O.M.; Alçınkaya, Ö.; Türker, A.R. Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J. Hazard. Mater. 2009, 166, 455–461. [Google Scholar]
- Rashed, M.N.; El-Amin, A.A. Photocatalytic degradation of methyl orange in aqeous TiO2 under different solar irradiation sources. Int. J. Phys. Sci. 2007, 2, 73–81. [Google Scholar]
- Maedaa, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, J.; Wu, P.; Ji, X. Immobilization of TiO2 films on activated carbon fiber and their photocatalytic degradation properties for dye compounds with different molecular size. Catal. Commun. 2008, 9, 1846–1850. [Google Scholar] [CrossRef]
- Ochiai, T.; Hoshi, T.; Slimen, H.; Nakata, K.; Murakami, T.; Tatejima, H.; Koide, Y.; Houas, A.; Horie, T.; Moritobe, Y.; et al. Fabrication of a TiO2 nanoparticles impregnated titanium mesh filter and its application for environmental purification. Catal. Sci. Technol. 2011, 1, 1324–1327. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhao, J.; Zhu, H.; Orthman, J. Photodegradation of dye pollutants on TiO2 particles dispersed in silicate under UV-Vis irradiation. Appl. Catal. B 2002, 37, 331–338. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Nakataa, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Kontos, P.F.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; Entezari, M.H.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Tanii, A.; Misaka, J.; Nakao, H.; Anpo, M. Design of TiO2 activated carbon filter systems by an Ionized Cluster Beam Method and their application for photocatalytic water purification. Mol. Cryst. Liq. Cryst. 2002, 388, 453–458. [Google Scholar]
- Lin, L.-H.; Lee, H.-T. A new modified silicone–TiO2 polymer composite film and its photocatalytic degradation. J Appl. Polym. Sci. 2006, 102, 3341–3344. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mukherjee, D.; Barghi, S.; Ray, A.K. Preparation and Characterization of the TiO2 Immobilized Polymeric Photocatalyst for Degradation of Aspirin under UV and Solar Light. Processes 2014, 2, 12-23. https://doi.org/10.3390/pr2010012
Mukherjee D, Barghi S, Ray AK. Preparation and Characterization of the TiO2 Immobilized Polymeric Photocatalyst for Degradation of Aspirin under UV and Solar Light. Processes. 2014; 2(1):12-23. https://doi.org/10.3390/pr2010012
Chicago/Turabian StyleMukherjee, Debjani, Shahzad Barghi, and Ajay K. Ray. 2014. "Preparation and Characterization of the TiO2 Immobilized Polymeric Photocatalyst for Degradation of Aspirin under UV and Solar Light" Processes 2, no. 1: 12-23. https://doi.org/10.3390/pr2010012





