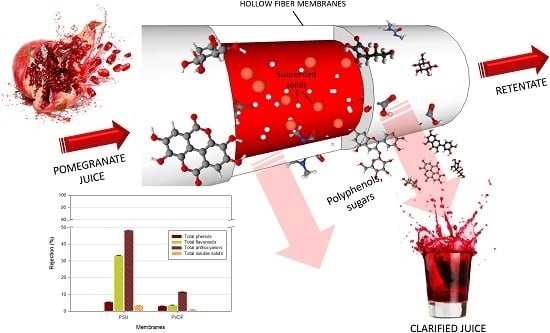
Functional Properties of Punica granatum L. Juice Clarified by Hollow Fiber Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Juice Extraction
2.3. Membrane Preparation
2.4. Characterization Tests
2.5. Juice Clarification
2.6. pH, Suspended Solids and Soluble Solids Measurements
2.7. Total Phenols Content
2.8. Total Flavonoids Content
2.9. Total Anthocyanins Content
2.10. Ascorbic Acid Content
2.11. In Vitro Antioxidant Activities
2.11.1. DPPH (2,2-diphenil-1-picrylhydrazyl) Test
2.11.2. ABTS (2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid)) Assay
2.11.3. FRAP Assay
2.11.4. β-Carotene Bleaching Test
2.12. Carbohydrate-Hydrolysing Enzymes Inhibitory Activity
2.12.1. α-Amylase Inhibitory Activity
2.12.2. α-Glucosidase Inhibition Assay
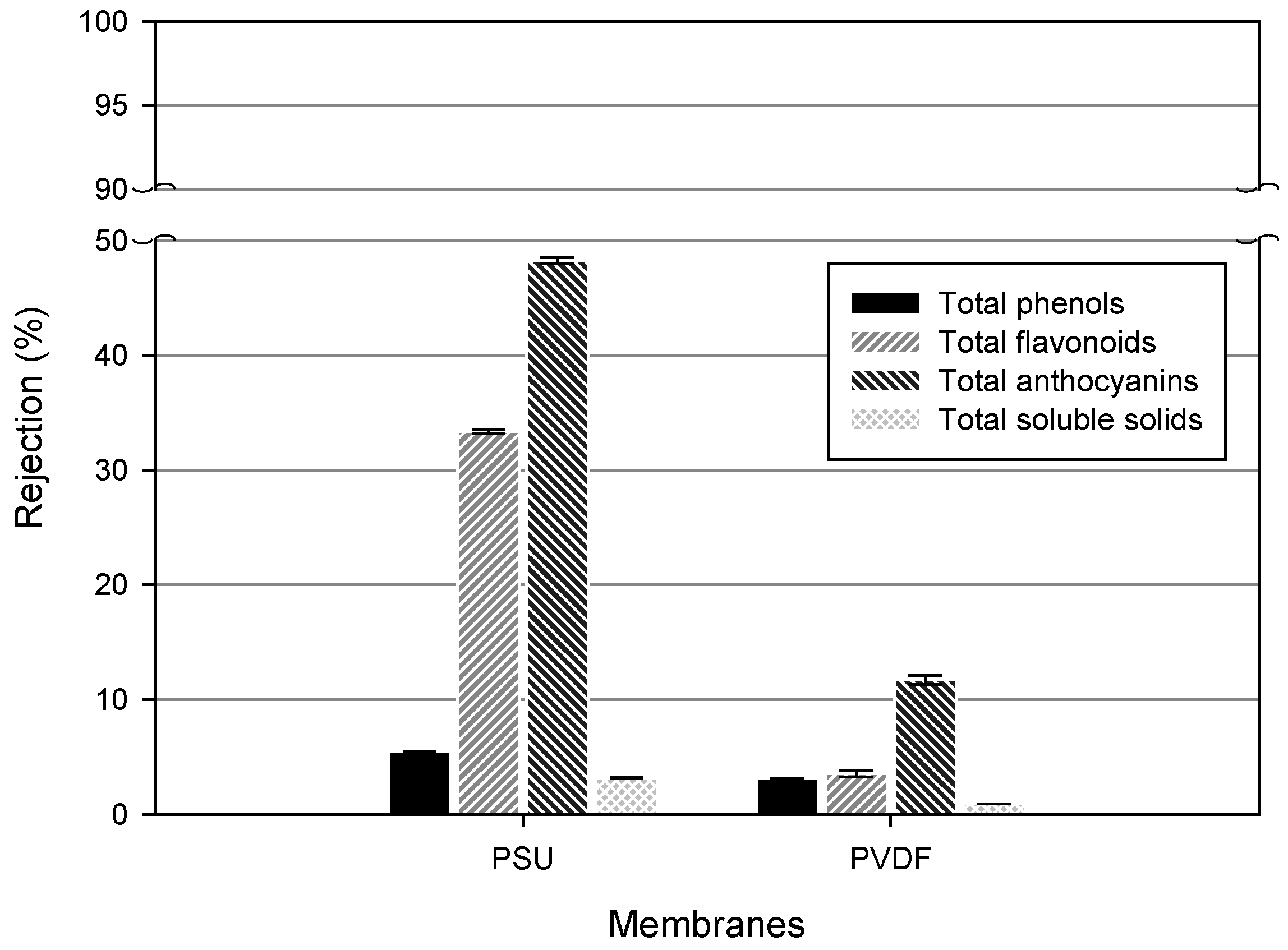
2.13. Rejection
2.14. Statistical Analysis
3. Results and Discussion
3.1. Membrane Characterization
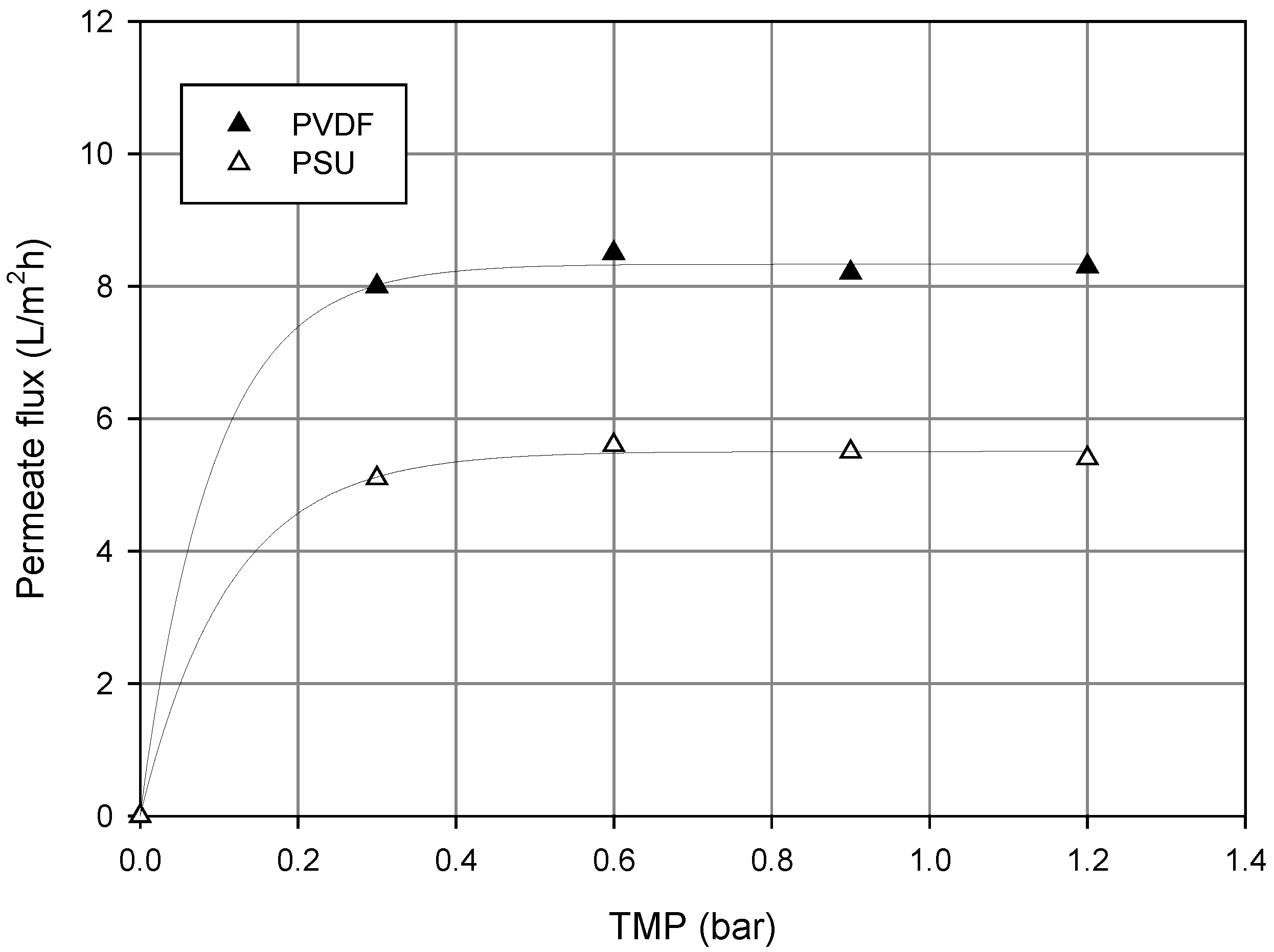
3.2. Pomegranate Juice Filtration
3.3. Phytochemicals Content
3.4. Bioactivity of the Treated Juice
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hyson, D.A. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Bell, C.; Hawthorne, S. Ellagic acid, pomegranate and prostate cancer-a mini review. J. Pharm. Pharmacol. 2008, 60, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Developmental changes in maturity indices of pomegranate fruit: A descriptive review. Sci. Hortic. 2013, 159, 152–161. [Google Scholar] [CrossRef]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wlesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food. Chem. 2009, 57, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Al-Maiman, S.A.; Ahmad, D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chem. 2002, 76, 437–441. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and type 2 diabetes. Pomegranate and type 2 diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Urala, N.; Lähteenmäki, L. Reasons behind consumers’ functional food choices. Nutr. Food Sci. 2003, 33, 148–158. [Google Scholar] [CrossRef]
- Tastan, O.; Baysal, T. Clarification of pomegranate juice with chitosan: Changes on quality characteristics during storage. Food Chem. 2015, 180, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Delaquis, P.; Girard, B. Microfiltration and ultrafiltration ceramic membranes for apple juice clarification. J. Food Sci. 1998, 63, 845–850. [Google Scholar] [CrossRef]
- Bagci, P.O. Effective clarification of pomegranate juice: A comparative study of pretreatment methods and their influence on ultrafiltration flux. J. Food Eng. 2014, 141, 58–64. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Tasselli, F. Clarification of pomegranate juice (Punica granatum L.) by hollow fiber membranes: Analyses of membrane fouling and performance. J. Chem. Technol. Biotechnol. 2015, 90, 859–866. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Aroujalian, A.; Navidbakhsh, M. Clarification of pomegranate juice by microfiltration with PVDF membranes. Desalination 2010, 264, 243–248. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fiber membranes from PVDF/PVP blends and their application in VMD. J. Membr. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of Sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.K.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry, Unit F1.2; John Wiley and Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Klein, B.P.; Perry, A.K. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 1982, 47, 941–945. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Rashed, K.; Said, A.; Bonesi, M.; Menichini, F.; Tundis, R. Antiproliferative and antioxidant properties of Alhagi maurorum Boiss (Leguminosae) aerial parts. Ind. Crops Prod. 2014, 53, 289–295. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; de Luca, D.; O’Brien, N.; Menichini, F.; Tundis, R. Influence of drying and cooking process on the phytochemical content, antioxidant and hypoglycaemic properties of two bell Capsicum annum L. cultivars. Food Chem. Toxicol. 2013, 53, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Sukitpaneenit, P.; Chung, T.S. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–20. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phaseinversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; Macedonio, F.; AL-Jlil, S.A.; Al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Lee, K.W.; Seo, B.K.; Nam, S.T.; Han, M.T. Trade-Off between thermodynamic enhancement and kinetic hindrance during phase inversion in the preparation of polysulfone membranes. Desalination 2003, 159, 289–296. [Google Scholar] [CrossRef]
- Alsalhy, Q.; Algebory, S.; Alwan, G.M.; Simone, S.; Figoli, A.; Drioli, E. Hollow fiber ultrafiltration membranes from poly(vinyl chloride): Preparation, morphologies, and properties. Sep. Sci. Technol. 2011, 46, 2199–2210. [Google Scholar] [CrossRef]
- Lafrenière, L.Y.; Talbot, F.D.F.; Matsuura, T.; Sourirajan, S. Effect of poly(vinyl pyrrolidone) additive on the performance of polyethersulfone ultrafiltration membranes. Ind. Eng. Chem. Res. 1987, 26, 2385–2389. [Google Scholar]
- Pellegrin, B.; Mezzari, F.; Hanafi, Y.; Szymczyk, A.; Remigy, J.C.; Causserand, C. Filtration performance and pore size distribution of hypochlorite aged PES/PVP ultrafiltration membranes. J. Membr. Sci. 2015, 474, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.L.; Chung, T.S.; Huang, Y. Effect of polyvinylpyrrolidone molecular weights on morphology, oil/water separation, mechanical and thermal properties of polyetherimide/polyvinylpyrrolidone hollow fiber membranes. J. Appl. Polym. Sci. 1999, 74, 2220–2233. [Google Scholar] [CrossRef]
- Lutz, H. Ultrafiltration: Fundamentals and Engineering. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier B.V.: Kidlington, UK, 2010; Volume 2, pp. 115–139. [Google Scholar]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 317S–325S. [Google Scholar] [PubMed]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Heijnen, C.G.M.; Haenen, G.R.M.M.; Oostveen, R.M.; Stalpers, E.M.; Bas, A. Protection of flavonoids against lipid peroxidation: Structure activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Ahmadkhaniha, R.; Shafiee, A. Effect of membrane clarification on the physicochemical properties of pomegranate juice. Int. J. Food Sci. Technol. 2010, 45, 1457–1463. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Mirsaeedghazi, H.; Aboonajmi, M.; Kianmehr, M.H. The effect of ultrasound waves on the efficiency of membrane clarification of pomegranate juice. Int. J. Food Sci. Technol. 2015, 50, 892–898. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, MS, USA, 1986; pp. 183–189. [Google Scholar]
- Valero, M.; Vegara, S.; Martí, N.; Saura, D. Clarification of pomegranate juice at industrial scale. J. Food Process. Technol. 2014, 5, 324–330. [Google Scholar] [CrossRef]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, C570–C576. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Drioli, E. Clarification and concentration of pomegranate juice (Punica granatum L.) using membrane processes. J. Food Eng. 2011, 107, 366–373. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Shi, J.; Chan, K.; Li, G.Q. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on α-amylase and α-glucosidase. Phytother. Res. 2013, 27, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2008, 34, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Intern. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banihani, S.A.; Makahleh, S.M.; El-Akawi, Z.; Al-Fashtaki, R.A.; Khaboura, O.F.; Gharibeh, M.Y.; Saadah, N.A.; Al-Hashimi, F.H.; Al-Khasieb, N.J. Fresh pomegranate juice ameliorates insulin resistance, enhances β-cell function, and decreases fasting serum glucose in type 2 diabetic patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef] [PubMed]

| HF | PVDF | PSU |
|---|---|---|
| Polymer | PVDF 6010 (25 wt%) | PSU (20 wt%) |
| Additive | PVP K 17 (35 wt%) | PVP K 17 (15 wt %) |
| Solvent | DMF (40 wt%) | DMA (65 wt%) |
| Bore fluid | DMF (45 wt%) | DMA (30 wt%) |
| Coagulation bath | Water | Water |
| T of the polymer solution (°C) | 115 | 50 |
| Air gap (cm) | 23 | 25.5 |
| Humidity | Yes | No |
| Polymer spinning rate (g/min) | 9.5 | 9.2 |
| Nitrogen pressure (bar) | 4 | 0.5 |
| Parameter | Fiber | |
|---|---|---|
| PVDF | PSU | |
| O.D. (mm) | 2.49 ± 0.44 | 1.30 ± 0.41 |
| I.D. (mm) | 1.44 ± 0.56 | 0.94 ± 0.32 |
| Thickness (mm) | 0.53 ± 0.8 | 0.18 ± 0.54 |
| Emod (N/mm2) | 40 ± 2.5 | 126.42 ± 3.19 |
| εbreak (%) | 79.5 ± 3.3 | 42.91 ± 6.30 |
| Bubble point (bar) | 1.10 | 1.175 |
| Pore diameter (μm) | 0.13 | 0.13 |
| Porosity (%) | 88 ± 0.51 | 78 ± 0.22 |
| Contact angle (°) | 72 ± 3 | 63 ± 3 |
| Water permeability (L/m2 h bar) | 343 ± 40 | 139 ± 23 |
| Parameter | Initial Juice | PVDF-P | PSU-P |
|---|---|---|---|
| pH | 3.99 ± 0.08 | 3.64 ± 0.07 | 4.06 ± 0.08 |
| Suspended solids (% w/w) | 4.16 ± 0.08 | - | - |
| Total soluble solids (Brix) | 22.1 ± 0.44 | 21.9 ± 0.43 | 21.4 ± 0.43 |
| Total phenols a | 1996.7 ± 38.9 | 1934.3 ± 41.9 | 1888.1 ± 22.7 |
| Total flavonoids b | 295.4 ± 3.6 | 285.0 ± 2.4 | 196.9 ± 3.9 |
| Total anthocyanins c | 124.7 ± 2.9 | 110.1 ± 2.3 | 64.5 ± 1.8 |
| Ascorbic acid d | 13.8 ± 0.5 | 8.6 ± 0.1 | 10.5 ± 0.3 |
| Sample | DPPH Test (IC50 μg/mL) | ABTS Test (IC50 μg/mL) | FRAP Test (μM Fe(II)/g) a | β-Carotene Bleaching Test (IC50 μg/mL) | ||
|---|---|---|---|---|---|---|
| Clarified Juice | 30 min | 60 min | ||||
| PVDF-P | 733.08 ± 2.8 | 300.86 ± 2.7 | 13.83 ± 0.6 | 20.79 ± 1.4 | 43.22 ± 1.0 | |
| PSU-P | 42.41% # | 467.80 ± 3.9 | 6.56 ± 0.4 | 69.03 ± 1.7 | 63.05 ± 2.6 | |
| Positive Control | Ascorbic acid | 5.0 ± 0.8 | 1.0 ± 0.03 | |||
| BHT | 63.2 ± 4.5 | |||||
| Propyl gallate | 1.0 ± 0.04 | 1.0 ± 0.03 | ||||
| Sample | IC50 (μg/mL) | ||
|---|---|---|---|
| α-Amylase | α-Glucosidase | ||
| Clarified Juice | PVDF-P | 75.86 ± 3.9 a | 69.07 ± 2.1 a |
| PSU-P | 221.31 ± 4.9 a | 89.71 ± 2.5 a | |
| Positive Control | Acarbose | 50.0 ± 0.9 | 35.5 ± 1.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiano, F.; Figoli, A.; Conidi, C.; Menichini, F.; Bonesi, M.; Loizzo, M.R.; Cassano, A.; Tundis, R. Functional Properties of Punica granatum L. Juice Clarified by Hollow Fiber Membranes. Processes 2016, 4, 21. https://doi.org/10.3390/pr4030021
Galiano F, Figoli A, Conidi C, Menichini F, Bonesi M, Loizzo MR, Cassano A, Tundis R. Functional Properties of Punica granatum L. Juice Clarified by Hollow Fiber Membranes. Processes. 2016; 4(3):21. https://doi.org/10.3390/pr4030021
Chicago/Turabian StyleGaliano, Francesco, Alberto Figoli, Carmela Conidi, Francesco Menichini, Marco Bonesi, Monica R. Loizzo, Alfredo Cassano, and Rosa Tundis. 2016. "Functional Properties of Punica granatum L. Juice Clarified by Hollow Fiber Membranes" Processes 4, no. 3: 21. https://doi.org/10.3390/pr4030021