Origins and Evolution of Inorganic-Based and MOF-Based Mixed-Matrix Membranes for Gas Separations
Abstract
:1. Introduction
2. Origins of Inorganic-Based and MOF-Based MMMs
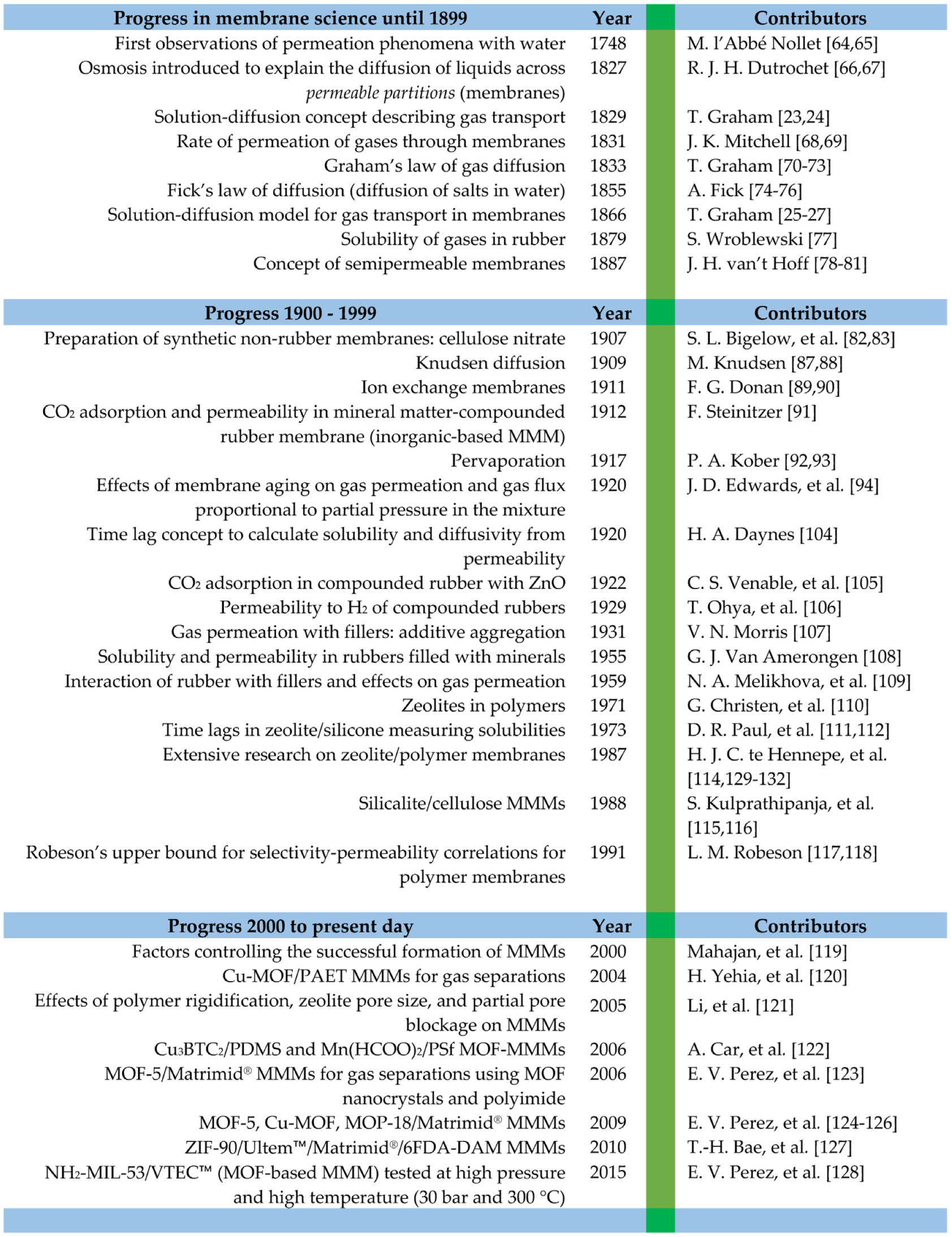
2.1. Membrane History
2.2. Mixed-Matrix Membrane Origins
3. Evolution of Inorganic-Based MMMs
3.1. Zeolites Silicates and Metal Oxides in MMMs
3.2. Carbon Materials in MMMs
4. Evolution of MOF-Based MMMs
4.1. Metal-Organic Frameworks
4.2. MOFs in MMMs
5. Future Directions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| [emim][NTf2] | [1-ethyl-3-methyl imidazolium] (cation) [bis(trifluoromethanesulfonyl) imide] (anion) |
| [emim][BF4] | [1-ethyl-3-methyl imidazolium] (cation) [tetrafluoroborate] (anion) |
| [emim][B(CN)4] | [1-ethyl-3-methyl imidazolium] (cation) [tetracyanoborate] (anion) |
| [vbim][NTF2] | Polymerizable RTIL (1-vinyl-3-butylimidazolium-bis(trifluoromethylsulfonyl)imidate) |
| θ | Membrane stage cut |
| Barrer | Non-international system unit for gas permeation (1 Barrer = 1 × 10−10 cm3(STP)·cm·cm−2·s−1·cmHg−1) |
| BSE | Backscattered electron |
| CA | Cellulose acetate |
| CMS | Carbon molecular sieve |
| CNT | Carbon nanotube |
| COF | Covalent organic framework |
| CP | Coordination polymer |
| DEA | Diethanolamine |
| Df | Filler diffusion coefficient |
| Dm | Matrix diffusion coefficient |
| DFT | Density functional theory |
| FCNT | Functionalized carbon nanotube |
| FIB-SEM | Focused ion beam scanning electron microscopy |
| FMWCNT | Functionalized multi-walled carbon nanotube |
| GO | Graphene oxide |
| GPU | Gas permeation unit (1 GPU = 1 × 10−6 cm3(STP)·cm−2·s−1·cmHg−1) |
| IRMOF | Isoreticular metal-organic framework |
| IUPAC | International union of pure and applied chemistry |
| K | Interfacial adsorption equilibrium constant |
| LCMS | Liquid chromatography mass spectrometry |
| MIL | Materials Institute Lavoisier |
| MMM | Mixed-matrix membrane |
| MOC | Metal-organic cube |
| MOF | Metal-organic framework |
| MOP | Metal-organic polyhedra |
| MWCNT | Multi-walled carbon nanotube |
| NMP | N-methyl-2-pyrrolidone |
| ODA | 4,4-Oxydianiline |
| ODPA | 4,4′-Oxydiphthalic anhydride |
| PAET | Poly(3-acetoxyethylthiophene) |
| PAF-1 | Porous aromatic framework 1 |
| PALS | Positron annihilation lifetime spectroscopy |
| PBI | Polybenzimidazole |
| PDMS | Polydimethylsiloxane |
| PES | Polyethersulfone |
| Pf | Filler permeability coefficient |
| PFG NMR | Pulsed field gradient nuclear magnetic resonance |
| PI | Polyimide |
| PIM-1 | Polymer of intrinsic microporosity |
| Pm | Matrix permeability coefficient |
| PMDA | Pyromellitic dianhydride |
| PMP | Poly(4-methyl-1-pentyne) |
| PMP-2 | Poly(4-methyl-2-pentyne) |
| PMPS | Polymethylphenylsiloxane |
| PolyRTILs | Polymerizable room temperature ionic liquids |
| PPEES | Poly(1,4-phenylene ether-ether-sulfone) |
| PPZ | Polyphosphazene |
| PS | Polystyrene |
| PSf | Polysulfone |
| PTMSP | Poly(1-trimethylsilil-1-propyne) |
| PVAc | Poly(vinyl acetate) |
| PVAm | Poly(vinyl amine) |
| PVPy | Polyvinylpyrrolidone |
| RTILs | Room temperature ionic liquids |
| SAPO-34 | Silicoaluminophosphate |
| SBS | Poly(styrene-b-butadiene-b-styrene) |
| SBU | Secondary building unit |
| SEM | Scanning electron microscopy |
| SPEEK | Sulfonated poly(ether ether ketone) |
| SPES | Sulfonated polyethersufone |
| SWCNT | Single-walled carbon nanotube |
| TEM | Transmission electron microscopy |
| Tg | Glass transition temperature |
| TGA | Thermogravimetric analysis |
| TMPDA | 2,4,6-Trimethyl-1,3-phenylenediamine |
| XRD | Powder X-ray diffraction |
| ZIF | Zeolitic imidazolate framework |
| XPS | X-ray photoelectron spectroscopy |
References
- Henis, J.M.S.; Tripodi, M.K. The developing technology of gas separating membranes. Science 1983, 220, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.; Ushio, S.; Lewald, R. Membranes widen roles in gas separations. Chem. Eng. 1984, 16, 14–19. [Google Scholar]
- Babita, K.; Sridhar, S.; Raghavan, K.V. Membrane reactors for fuel cell quality hydrogen through WGSR—Review of their status, challenges and opportunities. Int. J. Hydrog. Energy 2011, 36, 6671–6688. [Google Scholar] [CrossRef]
- Diniz da Costa, J.C.; Reed, G.P.; Thambimuthu, K. High temperature gas separation membranes in coal gasification. Energy Procedia 2009, 1, 295–302. [Google Scholar] [CrossRef]
- Fang, M.; Wu, C.; Yang, Z.; Wang, T.; Xia, Y.; Li, J. ZIF-8/PDMS mixed matrix membranes for propane/nitrogen mixture separation: Experimental result and permeation model validation. J. Membr. Sci. 2015, 474, 103–113. [Google Scholar] [CrossRef]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Marano, J.J.; Ciferino, J.P. Integration of Gas Separation Membranes with IGCC Identifying the Right Membrane for the Right Job. Energy Procedia 2009, 1, 361–368. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation-Part B: Effect of Cu3BTC2 on membrane transport properties. J. Membr. Sci. 2013, 428, 331–340. [Google Scholar] [CrossRef]
- Ploegmakers, J.; Japip, S.; Nijmeijer, K. Mixed matrix membranes containing MOFs for ethylene/ethane separation Part A: Membrane preparation and characterization. J. Membr. Sci. 2013, 428, 445–453. [Google Scholar] [CrossRef]
- Shoko, E.; McLellan, B.; Dicks, A.L.; da Costa, J.C.D. Hydrogen from coal: Production and utilisation technologies. Int. J. Coal Geol. 2006, 65, 213–222. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Han, S.-S.; Park, J.-H.; Kim, J.-N.; Cho, S.-H. Propylene recovery from propylene/propane/nitrogen mixture by PSA process. Adsorption 2005, 11, 621–624. [Google Scholar] [CrossRef]
- Silva, F.A.D.; Rodrigues, A.E. Propylene/propane separation by vacuum swing adsorption using 13X zeolite. AlChE J. 2001, 47, 341–357. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. Propane/propylene separation by pressure swing adsorption: Sorbent comparison and multiplicity of cyclic steady states. Chem. Eng. Sci. 2002, 57, 1139–1149. [Google Scholar] [CrossRef]
- Habgood, D.C.C.; Hoadley, A.F.A.; Zhang, L. Techno-economic analysis of gasification routes for ammonia production from Victorian brown coal. Chem. Eng. Res. Des. 2015, 102, 57–68. [Google Scholar] [CrossRef]
- Hellman, A.; Honkala, K.; Dahl, S.; Christensen, C.H.; Nørskov, J.K. Ammonia synthesis: State of the bellwether reaction. In Comprehensive Inorganic Chemistry II (Second Edition); Poeppelmeier, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 459–474. [Google Scholar]
- Driscoll, D.; Morreale, B.; Headley, L. NETL Test Protocol: Testing of Hydrogen Separation Membranes; U.S. Department of Energy (DOE): Washington, DC, USA; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2008. [Google Scholar]
- Stiegel, G.J.; Ramezan, M. Hydrogen from coal gasification: An economical pathway to a sustainable energy future. Int. J. Coal Geol. 2006, 65, 173–190. [Google Scholar] [CrossRef]
- Miller, L.C.; Cicero, D.C.; Ackiewicz, M. Hydrogen from Coal Program: Research, Development, and Demonstration Plan for the Period 2009 through 2016; U.S. Department of Energy (DOE): Washington, DC, USA; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2009. [Google Scholar]
- Angelini, P.; Armstrong, T.; Counce, R.; Griffith, W.; Klasson, T.; Muralidharan, G.; Narula, C.; Sikka, V.; Closset, G.; Keller, G.; et al. Materials for Separation Technologies: Energy and Emission Reduction Opportunities; Oak Ridge National Laboratory: Washington, WA, USA, 2005; p. 118. [Google Scholar]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Graham, T. Notice of the singular inflation of a bladder. Q. J. Sci. Lit. Art 1829, 2, 88–89. [Google Scholar]
- Graham, T. Notice of the singular inflation of a bladder. J. Membr. Sci. 1995, 100. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. Philos. Trans. R. Soc. Lond. 1866, 156, 399–439. [Google Scholar] [CrossRef]
- Graham, T. LV. On the absorption and dialytic separation of gases by colloid septa. Philos. Mag. Ser. 4 1866, 32, 401–420. [Google Scholar]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa Part I.—Action of a septum of caoutchouc. J. Membr. Sci. 1995, 100, 27–31. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Yasuda, H. Units of gas permeability constants. J. Appl. Polym. Sci. 1975, 19, 2529–2536. [Google Scholar] [CrossRef]
- Su, Z.; Chen, J.H.; Sun, X.; Huang, Y.; Dong, X. Amine-functionalized metal organic framework (NH2-MIL-125(Ti)) incorporated sodium alginate mixed matrix membranes for dehydration of acetic acid by pervaporation. RSC Adv. 2015, 5, 99008–99017. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Ban, Y.; Peng, Y.; Jiao, W.; Wang, P.; Guo, A.; Li, Y.; Yang, W. Conversion of xylose into furfural in a MOF-based mixed matrix membrane reactor. Chem. Eng. J. 2015. [Google Scholar] [CrossRef]
- Han, G.L.; Zhou, K.; Lai, A.N.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. [Cu2(bdc)2(bpy)]n/SPES-C mixed matrix membranes for separation of methanol/methyl tert-butyl ether mixtures. J. Membr. Sci. 2014, 454, 36–43. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Shen, J.; Jin, W. Fabrication of MOFs/PEBA mixed matrix membranes and their application in bio-butanol production. Sep. Purif. Technol. 2014, 133, 40–47. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, N.; Wang, L.; Huang, H.; Zhang, R.; Yang, F.; Xie, Y.; Ji, S.; Li, J.-R. Hybrid membranes of metal-organic molecule nanocages for aromatic/aliphatic hydrocarbon separation by pervaporation. Chem. Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, G.; Zhao, X.; Jin, W. Hydrophobic-ZIF-71 filled PEBA mixed matrix membranes for recovery of biobutanol via pervaporation. J. Membr. Sci. 2013, 446, 181–188. [Google Scholar] [CrossRef]
- De la Iglesia, O.; Sorribas, S.; Almendro, E.; Zornoza, B.; Tellez, C.; Coronas, J. Metal-organic framework MIL-101(Cr) based mixed matrix membranes for esterification of ethanol and acetic acid in a membrane reactor. Renew. Energy 2016, 88, 12–19. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, H.; Zhang, H.; Shen, J.; Xue, L.; Gao, C.; van der Bruggen, B. Mixed matrix membranes containing MIL-53(Al) for potential application in organic solvent nanofiltration. RSC Adv. 2015, 5, 73068–73076. [Google Scholar] [CrossRef]
- Liu, X.-L.; Li, Y.-S.; Zhu, G.-Q.; Ban, Y.-J.; Xu, L.-Y.; Yang, W.-S. An organophilic pervaporation membrane derived from metal-organic framework nanoparticles for efficient recovery of bio-alcohols. Angew. Chem. Int. Ed. 2011, 50, 10636–10639. [Google Scholar] [CrossRef] [PubMed]
- Sorribas, S.; Kudasheva, A.; Almendro, E.; Zornoza, B.; de la Iglesia, O.; Tellez, C.; Coronas, J. Pervaporation and membrane reactor performance of polyimide based mixed matrix membranes containing MOF HKUST-1. Chem. Eng. Sci. 2015, 124, 37–44. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Tellez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Ying, Y.; Xiao, Y.; Ma, J.; Guo, X.; Huang, H.; Yang, Q.; Liu, D.; Zhong, C. Recovery of acetone from aqueous solution by ZIF-7/PDMS mixed matrix membranes. RSC Adv. 2015, 5, 28394–28400. [Google Scholar] [CrossRef]
- Fan, H.; Shi, Q.; Yan, H.; Ji, S.; Dong, J.; Zhang, G. Simultaneous spray self-assembly of highly loaded ZIF-8-PDMS nanohybrid membranes exhibiting exceptionally high biobutanol-permselective pervaporation. Angew. Chem. Int. Ed. 2014, 53, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, J.; Fan, H.; Ji, S.; Zhang, G.; Zhang, Z. Sonication-enhanced in situ assembly of organic/inorganic hybrid membranes: Evolution of nanoparticle distribution and pervaporation performance. J. Membr. Sci. 2015, 481, 94–105. [Google Scholar] [CrossRef]
- Wee, L.H.; Li, Y.; Zhang, K.; Davit, P.; Bordiga, S.; Jiang, J.; Vankelecom, I.F.J.; Martens, J.A. Submicrometer-sized ZIF-71 filled organophilic membranes for improved bioethanol recovery: Mechanistic in-sights by Monte Carlo simulation and FTIR spectroscopy. Adv. Funct. Mater. 2015, 25, 516–525. [Google Scholar] [CrossRef]
- Sue, Y.-C.; Wu, J.-W.; Chung, S.-E.; Kang, C.-H.; Tung, K.-L.; Wu, K.C.W.; Shieh, F.-K. Synthesis of hierarchical micro/mesoporous structures via solid-aqueous interface growth: Zeolitic imidazolate framework-8 on siliceous mesocellular foams for enhanced pervaporation of water/ethanol mixtures. ACS Appl. Mater. Interfaces 2014, 6, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Lin, Y.-F.; Huang, Y.-S.; Tung, K.-L.; Chang, K.-S.; Chen, J.-T.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Synthesis of ZIF-7/chitosan mixed-matrix membranes with improved separation performance of water/ethanol mixtures. J. Membr. Sci. 2013, 438, 105–111. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a potential filler to prepare pervaporation membranes for bio-alcohol recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, Y.; Yang, T.; Chung, T.-S. ZIF-90/P84 mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 453, 155–167. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Gas sorption in polymers, molecular sieves, and mixed matrix membranes. J. Appl. Polym. Sci. 2007, 104, 4053–4059. [Google Scholar] [CrossRef]
- Husain, S.; Koros, W.J. Mixed matrix hollow fiber membranes made with modified HSSZ-13 zeolite in polyetherimide polymer matrix for gas separation. J. Membr. Sci. 2007, 288, 195–207. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material advancements in fabrication of mixed-matrix membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kinzer, K.E.; Tseng, H.S. Microporous membrane formation via thermally induced phase separation. I. Solid-liquid phase separation. J. Membr. Sci. 1990, 52, 239–261. [Google Scholar] [CrossRef]
- Van de Witte, P.; Dijkstra, P.J.; van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef]
- Kim, S.S.; Lloyd, D.R. Microporous membrane formation via thermally-induced phase separation. III. Effect of thermodynamic interactions on the structure of isotactic polypropylene membranes. J. Membr. Sci. 1991, 64, 13–29. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Kim, S.S.; Kinzer, K.E. Microporous membrane formation via thermally-induced phase separation. II. Liquid—liquid phase separation. J. Membr. Sci. 1991, 64, 1–11. [Google Scholar] [CrossRef]
- Koros, W.J.; Böddeker, K.W.; Fane, A.G.; Lonsdale, H.K. The early history of membrane science: Selected papers celebrating volume 100. J. Membr. Sci. 1995, 100, 1–68. [Google Scholar]
- Böddeker, K.W. Commentary: Tracing membrane science. J. Membr. Sci. 1995, 100, 65–68. [Google Scholar] [CrossRef]
- Böddeker, K.W. Tracing membrane science, an historical account. In Liquid Separations with Membranes: An Introduction to Barrier Interference; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2008; pp. 113–119. [Google Scholar]
- Nollet, M.l.A. Sur les causes du bouillonnement des liquides. Hist. Acad. Sci. 1748, 57–104. [Google Scholar]
- Nollet, J.A. Investigations on the causes for the ebullition of liquids. J. Membr. Sci. 1995, 100, 1–3. [Google Scholar] [CrossRef]
- Dutrochet, M. Nouvelles observations sur l’endosmose et l’exosmose, et sur la cause de ce double phenomene. Ann. Chim. Phys. 1827, 35, 393–400. [Google Scholar]
- Dutrochet, R.H. New observations on endosmosis and exosmosis, and on the cause of this dual phenomenon. J. Membr. Sci. 1995, 100, 5–7. [Google Scholar] [CrossRef]
- Mitchell, J.K. On the penetrativeness of fluids. Am. J. Med. Sci. 1830, 7, 36–67. [Google Scholar] [CrossRef]
- Mitchell, J.K. On the penetrativeness of fluids. J. Membr. Sci. 1995, 100, 11–16. [Google Scholar] [CrossRef]
- Graham, T. XXVII. On the law of the diffusion of gases. Philos. Mag. Ser. 3 1833, 2. [Google Scholar] [CrossRef]
- Graham, T. On the law of the diffusion of gases. J. Membr. Sci. 1995, 100, 17–21. [Google Scholar] [CrossRef]
- Graham, T. LVIII. On the law of the diffusion of gases. Philos. Mag. Ser. 3 1833, 2, 351–358. [Google Scholar]
- Graham, T. XLIV. On the law of the diffusion of gases. Philos. Mag. Ser. 3 1833, 2, 269–276. [Google Scholar]
- Fick, A. Uber diffusion. Pogg. Ann. Phys. Chem. 1855, 94, 59–86. [Google Scholar] [CrossRef]
- Fick, A. On liquid diffusion. Philos. Mag. Ser. 4 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Fick, A. On liquid diffusion. J. Membr. Sci. 1995, 100, 33–38. [Google Scholar] [CrossRef]
- Wroblewski, S.V. Ueber die natur der absorption der gase. Ann. Phys. 1879, 244, 29–52. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H. The role of osmotic pressure in the analogy between solutions and gases. J. Membr. Sci. 1995, 100, 39–44. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H. Die rolle des osmotischen druckes in der analogie zwischen losungen und gasen. Z. Phys. Chem. 1887, 1, 481–508. [Google Scholar]
- Van’t Hoff, J.H. The role of osmotic pressure in the analogy between solutions and gases. In The Modern Theory of Solution: Memoirs by Pfeffer, van’t Hoff, Arrhenius, and Raoult; Jones, H.C., Ed.; Harper & Brothers Publishers: New York, NY, USA; London, UK, 1899; pp. 11–42. [Google Scholar]
- Van’t Hoff, J.H. The function of osmotic pressure in the analogy between solutions and gases. Philos. Mag. Ser. 5 1888, 26, 81–105. [Google Scholar] [CrossRef]
- Bigelow, S.L.; Gemberling, A. Collodion membranes. J. Am. Chem. Soc. 1907, 29, 1576–1589. [Google Scholar] [CrossRef]
- Bigelow, S.L.; Gemberling, A. Collodion membranes. J. Membr. Sci. 1995, 100, 57–59. [Google Scholar] [CrossRef]
- Kundt, A.; Warburg, E. Ueber reibung und wärmeleitung verdünnter gase. Ann. Phys. 1875, 231, 337–365. [Google Scholar] [CrossRef]
- Warburg, E. Ueber die gleitung der gase an glaswänden. Ann. Phys. 1876, 235, 399–415. [Google Scholar] [CrossRef]
- Christiansen, C. Die atmolytische strömung der gase. Ann. Phys. 1890, 277, 565–587. [Google Scholar] [CrossRef]
- Knudsen, M. Die gesetze der molekularströmung und der inneren reibungsströmung der gase durch röhren. Ann. Phys. 1909, 333, 75–130. [Google Scholar] [CrossRef]
- Knudsen, M. The laws of molecular flow and of inner friction flow of gases through tubes. J. Membr. Sci. 1995, 100, 23–25. [Google Scholar] [CrossRef]
- Donnan, F.G. Theorie der membrangleichgewichte und membranpotentiale bei vorhandensein von nicht dialysierenden elektrolyten. Ein beitrag zur physikalisch-chemischen physiologie. Z. Elektrochem. Angew. Phys. Chem. 1911, 17, 572–581. [Google Scholar]
- Donnan, F.G. Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J. Membr. Sci. 1995, 100, 45–55. [Google Scholar] [CrossRef]
- Steinitzer, F. Das verhalten von kautschuk zu kohlensaure. Gummi Ztg. 1912, 26, 1626–1628. [Google Scholar]
- Kober, P.A. Pervaporation, perstillation and percrystallization. J. Membr. Sci. 1995, 100, 61–64. [Google Scholar] [CrossRef]
- Kober, P.A. Pervaporation, perstillation and percrystallization. J. Am. Chem. Soc. 1917, 39, 944–948. [Google Scholar] [CrossRef]
- Edwards, J.D.; Pickering, S.F. Permeability of rubber to gases. Sci. Pap. Bur. Stand. 1920, 387, 327–362. [Google Scholar] [CrossRef]
- Kovacs, A.J. Transition vitreuse dans les polymères amorphes. Etude phénoménologique. Adv. Polym. Sci. 1963, 3, 394–507. [Google Scholar]
- Illers, V.K.H. Einfluß der thermischen vorgeschichte auf die eigenschaften von polyvinylchlorid. Makromol. Chem. 1969, 127, 1–33. [Google Scholar] [CrossRef]
- Petrie, S.E.B. Thermal behavior of annealed organic glasses. J. Polym. Sci. B Polym. Phys. 1972, 10, 1255–1272. [Google Scholar] [CrossRef]
- Petrie, S.E.B. The effect of excess thermodynamic properties versus structure formation on the physical properties of glassy polymers. J. Macromol. Sci. Phys. B 1976, 12, 225–247. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical aging in amorphous polymers and other materials. Elsevier: Amsterdam, The Netherlands, 1978; p. 229. [Google Scholar]
- Tant, M.R.; Wilkes, G.L. An overview of the nonequilibrium behavior of polymer glasses. Polym. Eng. Sci. 1981, 21, 874–895. [Google Scholar] [CrossRef]
- Tant, M.R.; Wilkes, G.L. Physical aging studies of semicrystalline poly(ethylene terephthalate). J. Appl. Polym. Sci. 1981, 26, 2813–2825. [Google Scholar] [CrossRef]
- Toi, K.; Ito, T.; Ikemoto, I. Effect of aging and conditioning on the gas transport of poly(vinyl acetate). J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 525–529. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Daynes, H.A. The process of diffusion through a rubber membrane. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Venable, C.S.; Fuwa, T. The solubility of gases in rubber and rubber stock and effect of solubility on penetrability. J. Ind. Eng. Chem. 1922, 14, 139–142. [Google Scholar] [CrossRef]
- Ohya, T.; Davey, W.C. Permeability of rubber mixings. Inst. Rubber Ind. Trans. 1929, 5, 27–30. [Google Scholar]
- Morris, V.N. Permeability of rubber to air II—Effect of stretch, thickness, milling, compounding ingredients, kind of crude rubber, and temperature of vulcanization. Ind. Eng. Chem. 1931, 23, 837–843. [Google Scholar] [CrossRef]
- Amerongen, G.J.V. The effect of fillers on the permeability of rubber to gases. Rubber Chem. Technol. 1955, 28, 821–832. [Google Scholar] [CrossRef]
- Melikhova, N.A.; Reitlinger, S.A.; Kuzina, E.N. Effect of fillers on the gas-permeability of synthetic rubbers. Soviet Rubber Technol. 1959, 18, 34–38. [Google Scholar]
- Christen, G.; Fabre, A.; Faure, A. Membrane Hétérogène Pour le Fractionnement de Mélanges Fluides, et Son Emploi. France Patent 2,079,460 (A5), 1971. [Google Scholar]
- Paul, D.R.; Kemp, D.R. The diffusion time lag in polymer membranes containing adsorptive fillers. J. Polym. Sci. Polym. Symp. 1973, 41, 79–93. [Google Scholar] [CrossRef]
- Kemp, D.R.; Paul, D.R. Gas sorption in polymer membranes containing adsorptive fillers. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 485–500. [Google Scholar] [CrossRef]
- Paul, D.R. Effect of immobilizing adsorption on the diffusion time lag. J. Polym. Sci. B Polym. Phys. 1969, 7, 1811–1818. [Google Scholar] [CrossRef]
- Te Hennepe, H.J.C.; Bargeman, D.; Mulder, M.H.V.; Smolders, C.A. Zeolite-filled silicone rubber membranes. Part 1: Membrane preparation and pervaporation results. J. Membr. Sci. 1987, 35, 39–55. [Google Scholar] [CrossRef]
- Kulprathipanja, S.; Neuzil, R.W.; Li, N.N. Separation of Fluids by Means of Mixed Matrix Membranes. U.S. Patent 4,740,219, 26 April 1988. [Google Scholar]
- Kulprathipanja, S.; Funk, E.W.; Kulkarni, S.S.; Chang, Y.A. Separation of a Monosaccharide with Mixed Matrix Membranes. U.S. Patent 4,735,193A, 5 April 1988. [Google Scholar]
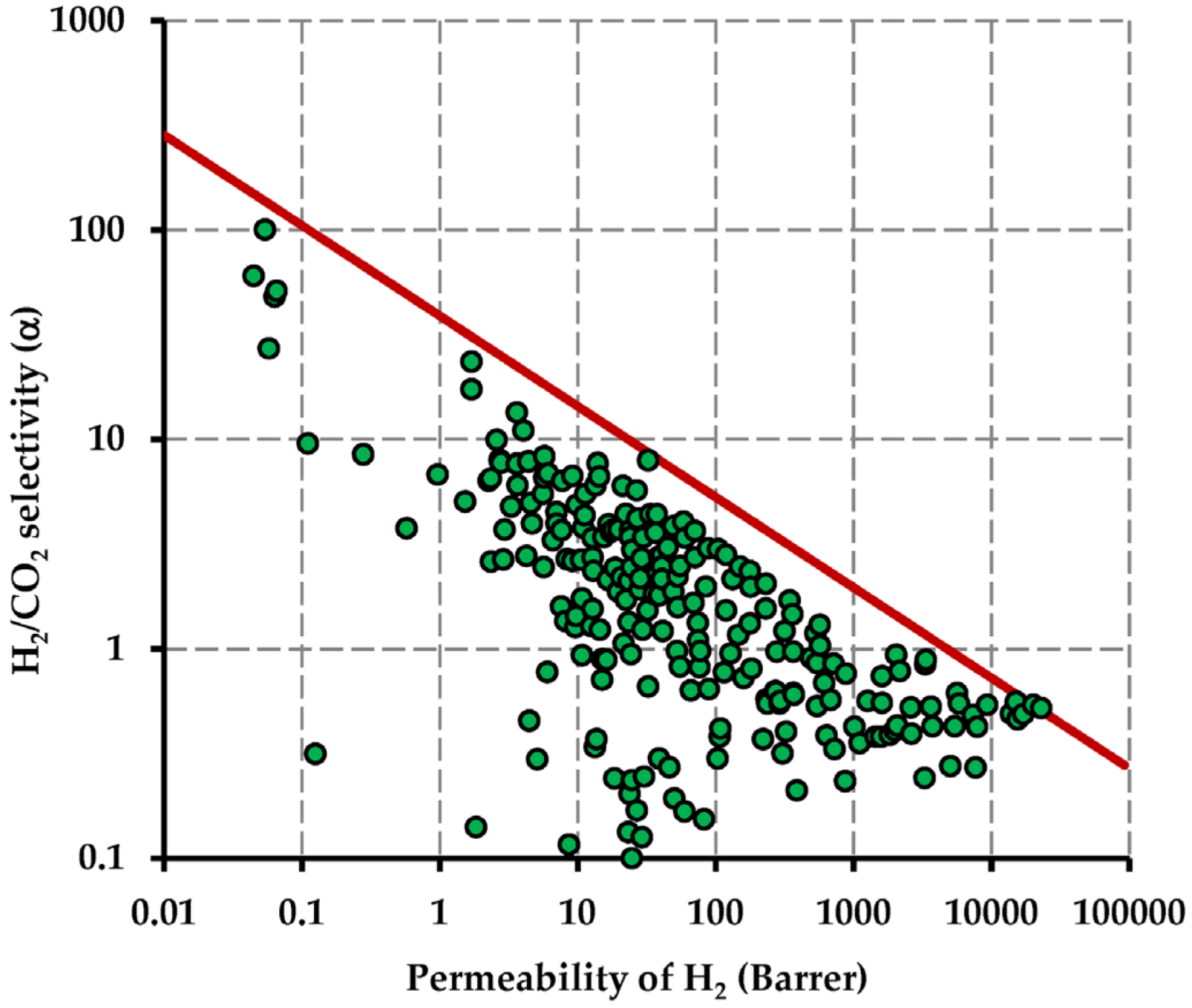
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Mahajan, R.; Koros, W.J. Factors controlling successful formation of mixed-matrix gas separation materials. Ind. Eng. Chem. Res. 2000, 39, 2692–2696. [Google Scholar] [CrossRef]
- Yehia, H.; Pisklak, T.J.; Ferraris, J.P.; Balkus, K.J., Jr.; Musselman, I.H. Methane facilitated transport using copper (II) biphenyl dicarboxylate-triethylenediamine poly(3-acetoxyethylthiophene) mixed matrix membranes. Polym. Prepr. (Am. Chem. Soc. Div. Polym. Chem.) 2004, 45, 35–36. [Google Scholar]
- Li, Y.; Chung, T.-S.; Cao, C.; Kulprathipanja, S. The effects of polymer chain rigidification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes. J. Membr. Sci. 2005, 260, 45–55. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Peinemann, K.-V. Hybrid membrane materials with different metal-organic frameworks (MOFs) for gas separation. Desalination 2006, 200, 424–426. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes for gas separation using metal-organic frameworks. PMSE Prepr. 2006, 95, 815–816. [Google Scholar]
- Perez, E.V.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Mixed-Matrix Membranes Containing MOF-5 for Gas Separations. J. Membr. Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Perez, E.V. Mixed-Matrix Membranes for Gas Separation Using Metal-Organic Frameworks. Ph.D. Thesis, The University of Texas at Dallas, Richardson, TX, USA, 2009. [Google Scholar]
- Perez, E.V.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Metal-organic polyhedra 18 mixed-matrix membranes for gas separation. J. Membr. Sci. 2014, 463, 82–93. [Google Scholar] [CrossRef]
- Bae, T.-H.; Lee, J.S.; Qiu, W.; Koros, W.J.; Jones, C.W.; Nair, S. A high-performance gas-separation membrane containing submicrometer-sized metal-organic framework crystals. Angew. Chem. Int. Ed. 2010, 49, 9863–9866. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.V.; Kalaw, G.J.D.; Ferraris, J.P.; Balkus, K.J., Jr.; Musselman, I.H. High temperature and high pressure H2/CO2 separations with NH2-MIL-53/VTEC™ mixed-matrix membranes. In Proceedings of the 25th North American Membrane Society Meeting—NAMS 2015, Boston, MA, USA, 30 May–3 June 2015.
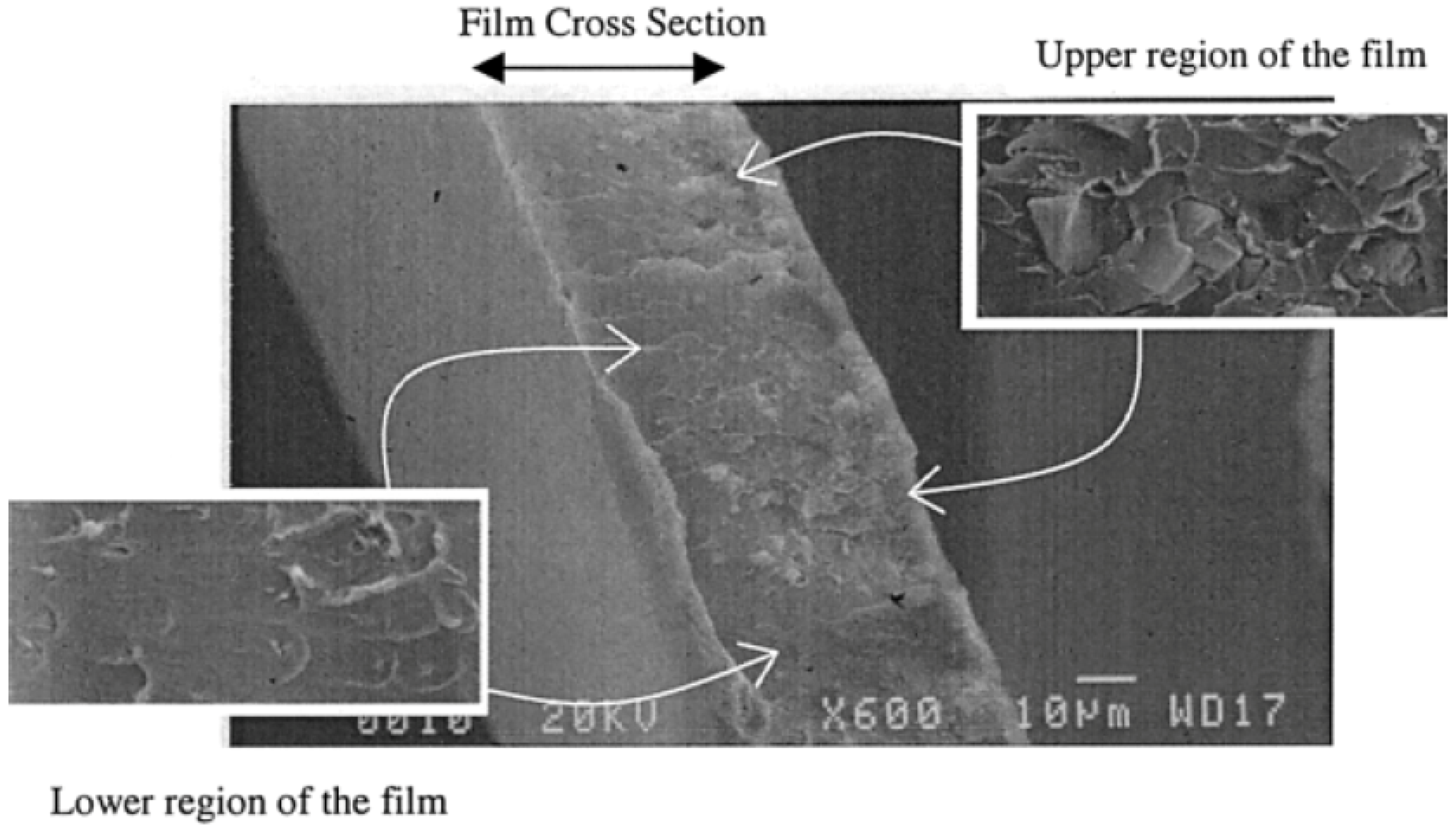
- Te Hennepe, H.J.C. Zeolite Filled Polymeric Membranes: A New Concept in Separation Science. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 1988. [Google Scholar]
- Te Hennepe, H.J.C.; Bargeman, D.; Mulder, M.H.V.; Smolders, C.A. Permeation through zeolite filled silicone rubber membranes. Stud. Surf. Sci. Catal. 1988, 39, 411–420. [Google Scholar]
- Te Hennepe, H.J.C.; Mulder, M.H.V.; Smolders, C.A.; Bargeman, D.; Schröder, G.A.T. Pervaporation Process and Membrane. U.S. Patent 4,925,562, 15 May 1990. [Google Scholar]
- Te Hennepe, H.J.C.; Boswerger, W.B.F.; Bargeman, D.; Mulder, M.H.V.; Smolders, C.A. Zeolite-filled silicone rubber membranes Experimental determination of concentration profiles. J. Membr. Sci. 1994, 89, 185–196. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and opportunities for mixed-matrix membranes for gas separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Sterzel, H.-J.; Sanner, A. Membranes of Organic Polymers Which Contain Crystalline Carrier Compounds, and Their Preparation. Patent 4973606A, 1990. [Google Scholar]
- Drago, R.S.; Balkus, K.J. Cobalt(II)-facilitated transport of dioxygen in a polystyrene membrane. Inorg. Chem. 1986, 25, 716–718. [Google Scholar] [CrossRef]
- Süer, M.G.; Baç, N.; Yilmaz, L. Gas permeation characteristics of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Battal, T.; Bac, N.; Yilmaz, L. Effect of feed composition on the performance of polymer-zeolite mixed matrix gas separation membranes. Sep. Sci. Technol. 1995, 30, 2365–2384. [Google Scholar] [CrossRef]
- Tantekin-Ersolmaz, S.B.; Atalay-Oral, C.; Tatlier, M.; Erdem-Senatalar, A.; Schoeman, B.; Sterte, J. Effect of zeolite particle size on the performance of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 2000, 175, 285–288. [Google Scholar] [CrossRef]
- Mahajan, R.; Burns, R.; Schaeffer, M.; Koros, W.J. Challenges in forming successful mixed matrix membranes with rigid polymeric materials. J. Appl. Polym. Sci. 2002, 86, 881–890. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, reverse-selective nanocomposite membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J. Diffusive permeability and selectivity of nanocomposite membranes. Ind. Eng. Chem. Res. 2006, 45, 6890–6898. [Google Scholar] [CrossRef]
- Scholz, H.P.; Tassler, W.; Wiesegard, H.; Schoenmeier, A.; Wrabetz, G.; Plogsties, H.D.; Gross, M.; Opitz, H.; Theer, W.; Finger, G.D. Verfahren und Vorrichtung zur Abtrennung, Anreicherung und Reinigung von Bestandteilen Eines Gasgemisches Durch Permeation. Patent DE2,248,801 A1, 12 April 1973. [Google Scholar]
- Li, Y.; Chung, T.-S.; Huang, Z.; Kulprathipanja, S. Dual-layer polyethersulfone (PES)/BTDA-TDI/MDI co-polyimide (P84) hollow fiber membranes with a submicron PES-zeolite beta mixed matrix dense-selective layer for gas separation. J. Membr. Sci. 2006, 277, 28–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Mixed matrix membranes composed of Matrimid and carbon aerogel and carbon aerogel+zeolite composite nanoparticles. PMSE Prepr. 2006, 95, 812–814. [Google Scholar]
- Zhang, Y.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas permeability properties of mixed-matrix Matrimid membranes containing a carbon aerogel: A material with both micropores and mesopores. Ind. Eng. Chem. Res. 2008, 47, 2794–2802. [Google Scholar] [CrossRef]
- Zhang, Y.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Mixed-matrix membranes composed of Matrimid and mesoporous ZSM-5 nanoparticles. J. Membr. Sci. 2008, 325, 28–39. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Wen, R.; Teoh, M.M.; Kulprathipanja, S. Enhanced gas separation properties by using nanostructured PES-Zeolite 4A mixed matrix membranes. J. Appl. Polym. Sci. 2006, 101, 3800–3805. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S.; Kulprathipanja, S. Novel Ag+-zeolite/polymer mixed matrix membranes with a high CO2/CH4 selectivity. AlChE J. 2007, 53, 610–616. [Google Scholar] [CrossRef]
- Yave, W.; Shishatskiy, S.; Abetz, V.; Matson, S.; Litvinova, E.; Khotimskiy, V.; Peinemann, K.-V. A novel poly(4-methyl-2-pentyne)/TiO2 hybrid nanocomposite membrane for natural gas conditioning: Butane/methane separation. Macromol. Chem. Phys. 2007, 208, 2412–2418. [Google Scholar] [CrossRef]
- Matteucci, S.; Kusuma, V.A.; Sanders, D.; Swinnea, S.; Freeman, B.D. Gas transport in TiO2 nanoparticle-filled poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2008, 307, 196–217. [Google Scholar] [CrossRef]
- Ward, J.K.; Koros, W.J. Crosslinkable mixed matrix membranes with surface modified molecular sieves for natural gas purification: II. Performance characterization under contaminated feed conditions. J. Membr. Sci. 2011, 377, 82–88. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Amine-functionalized zeolite FAU/EMT-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2011, 379, 468–478. [Google Scholar] [CrossRef]
- Joly, C.; Goizet, S.; Schrotter, J.C.; Sanchez, J.; Escoubes, M. Sol-gel polyimide-silica composite membrane: Gas transport properties. J. Membr. Sci. 1997, 130, 63–74. [Google Scholar] [CrossRef]
- Gomes, D.; Nunes, S.P.; Peinemann, K.-V. Membranes for gas separation based on poly(1-trimethylsilyl-1-propyne)–silica nanocomposites. J. Membr. Sci. 2005, 246, 13–25. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.J.; Pinnau, I.; Song, J.; Du, N.; Robertson, G.P.; Guiver, M.D. Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2010, 346, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Paul, D.R. Gas permeation in poly(ether imide) nanocomposite membranes based on surface-treated silica. Part 1: Without chemical coupling to matrix. Polymer 2006, 47, 7519–7534. [Google Scholar] [CrossRef]
- Takahashi, S.; Paul, D.R. Gas permeation in poly(ether imide) nanocomposite membranes based on surface-treated silica. Part 2: With chemical coupling to matrix. Polymer 2006, 47, 7535–7547. [Google Scholar] [CrossRef]
- Rubio, C.; Zornoza, B.; Gorgojo, P.; Tellez, C.; Coronas, J. Separation of H2 and CO2 containing mixtures with mixed matrix membranes based on layered materials. Curr. Org. Chem. 2014, 18, 2351–2363. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Krych, W.; Ramanan, H.; Nair, S.; Marand, E.; Tsapatsis, M. Fabrication of polymer/selective-flake nanocomposite membranes and their use in gas separation. Chem. Mater. 2004, 16, 3838–3845. [Google Scholar] [CrossRef]
- Defontaine, G.; Barichard, A.; Letaief, S.; Feng, C.; Matsuura, T.; Detellier, C. Nanoporous polymer—Clay hybrid membranes for gas separation. J. Colloid Interface Sci. 2010, 343, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Coronas, J.; Jordan, E.; Oh, W.; Nair, S.; Onorato, F.; Shantz, D.F.; Tsapatsis, M. Layered silicates by swelling of AMH-3 and nanocomposite membranes. Angew. Chem. Int. Ed. 2008, 47, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Krantz, W.B.; Chung, T.-S. A novel primer to prevent nanoparticle agglomeration in mixed matrix membranes. AlChE J. 2007, 53, 2470–2475. [Google Scholar] [CrossRef]
- Jha, P.; Way, J.D. Carbon dioxide selective mixed-matrix membranes formulation and characterization using rubbery substituted polyphosphazene. J. Membr. Sci. 2008, 324, 151–161. [Google Scholar] [CrossRef]
- Khan, A.L.; Cano-Odena, A.; Gutierrez, B.; Minguillon, C.; Vankelecom, I.F.J. Hydrogen separation and purification using polysulfone acrylate-zeolite mixed matrix membranes. J. Membr. Sci. 2010, 350, 340–346. [Google Scholar] [CrossRef]
- Marsh, N.; Khan, A.L.; Klaysom, C.; Gahlaut, A.; Li, X.; Vankelecom, I.F.J. Euromembrane Conference 2012: SPEEK and functionalized mesoporous MCM–41 mixed–matrix membranes for gas separation. Procedia Eng. 2012, 44, 1902–1905. [Google Scholar]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Li, X.; Vankelecom, I.F.J. SPEEK and functionalized mesoporous MCM–41 mixed matrix membranes for CO2 separations. J. Mater. Chem. 2012, 22, 20057–20064. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Yave, W.; Golemme, G. Some approaches for high performance polymer based membranes for gas separation: Block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Corbin, D.R.; Foley, H.C.; Shiflett, M.B. Mixed Matrix Nanoporous Carbon Membranes. U.S. Patent 6,740,143, 25 May 2004. [Google Scholar]
- Koros, W.J.; Vu, D.Q.; Mahajan, R.; Miller, S.J. Carbon Molecular Sieves and Methods for Making the Same. U.S. Patent 6,562,110, 13 May 2002. [Google Scholar]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves. I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.-S.; Jiang, L.; Kulprathipanja, S. Carbon-zeolite composite membranes for gas separation. Carbon 2005, 43, 2025–2027. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Shaharun, M.S.; Abu Bakar, M.Z. Effect of fixed carbon molecular sieve (CMS) loading and various di-ethanolamine (DEA) concentrations on the performance of a mixed matrix membrane for CO2/CH4 separation. RSC Adv. 2015, 5, 60814–60822. [Google Scholar] [CrossRef]
- Chung, T.-S.; Chan, S.S.; Wang, R.; Lu, Z.; He, C. Characterization of permeability and sorption in Matrimid®/C60 mixed matrix membranes. J. Membr. Sci. 2003, 211, 91–99. [Google Scholar] [CrossRef]
- Sun, H.; Ma, C.; Wang, T.; Xu, Y.; Yuan, B.; Li, P.; Kong, Y. Preparation and characterization of C60-filled ethyl cellulose mixed-matrix membranes for gas separation of propylene/propane. Chem. Eng. Technol. 2014, 37, 611–619. [Google Scholar] [CrossRef]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent developments in carbon nanotube membranes for water purification and gas separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Pechar, T.W.; Marand, E. Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 19, 330–339. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, S.; Vijay, Y.K. Study of gas transport properties of multi-walled carbon nanotubes/polystyrene composite membranes. Int. J. Hydrog. Energy 2012, 37, 3914–3921. [Google Scholar] [CrossRef]
- Ismail, A.F.; Rahim, N.H.; Mustafa, A.; Matsuura, T.; Ng, B.C.; Abdullah, S.; Hashemifard, S.A. Gas separation performance of polyethersulfone/multi-walled carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 80, 20–31. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S.H.S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Bhole, Y.S.; Wanjale, S.D.; Kharul, U.K.; Jog, J.P. Assessing feasibility of polyarylate–clay nanocomposites towards improvement of gas selectivity. J. Membr. Sci. 2007, 306, 277–286. [Google Scholar] [CrossRef]
- Zulhairun, A.K.; Ismail, A.F. The role of layered silicate loadings and their dispersion states on the gas separation performance of mixed matrix membrane. J. Membr. Sci. 2014, 468, 20–30. [Google Scholar] [CrossRef]
- Tantekin-Ersolmaz, S.B.; Senorkyan, L.; Kalaonra, N.; Tatlier, M.; Erdem-Senatalar, A. n-Pentane/i-pentane separation by using zeolite-PDMS mixed matrix membranes. J. Membr. Sci. 2001, 189, 59–67. [Google Scholar] [CrossRef]
- Pechar, T.W.; Tsapatsis, M.; Marand, E.; Davis, R. Preparation and characterization of a glassy fluorinated polyimide zeolite-mixed matrix membrane. Desalination 2002, 146, 3–9. [Google Scholar] [CrossRef]
- Qi, R.; Wang, Y.; Chen, J.; Li, J.; Zhu, S. Removing thiophenes from n-octane using PDMS-AgY zeolite mixed matrix membranes. J. Membr. Sci. 2007, 295, 114–120. [Google Scholar] [CrossRef]
- Kanezashi, M.; Sano, M.; Yoshioka, T.; Tsuru, T. Extremely thin Pd-silica mixed-matrix membranes with nano-dispersion for improved hydrogen permeability. Chem. Commun. 2010, 46, 6171–6173. [Google Scholar] [CrossRef] [PubMed]
- Hudiono, Y.C.; Carlisle, T.K.; LaFrate, A.L.; Gin, D.L.; Noble, R.D. Novel mixed matrix membranes based on polymerizable room-temperature ionic liquids and SAPO-34 particles to improve CO2 separation. J. Membr. Sci. 2011, 370, 141–148. [Google Scholar] [CrossRef]
- Xing, R.; Ho, W.S.W. Crosslinked polyvinylalcohol-polysiloxane/fumed silica mixed matrix membranes containing amines for CO2/H2 separation. J. Membr. Sci. 2011, 367, 91–102. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Golemme, G.; Tone, C.M.; de Santo, M.P.; Ciuchi, F.; Perrotta, E. Amine-functionalized SBA-15 in poly(styrene-b-butadiene-b-styrene) (SBS) yields permeable and selective nanostructured membranes for gas separation. J. Mater. Chem. A 2013, 1, 11853–11866. [Google Scholar] [CrossRef]
- Suhaimi, H.S.M.; Khir, M.N.I.M.; Leo, C.P.; Ahmad, A.L. Preparation and characterization of polysulfone mixed-matrix membrane incorporated with palladium nanoparticles dispersed in polyvinylpyrrolidone for hydrogen separation. J. Polym. Res. 2014, 21, 1–8. [Google Scholar] [CrossRef]
- Biradha, K.; Ramanan, A.; Vittal, J.J. Coordination polymers versus metal–organic frameworks. Cryst. Growth Des. 2009, 9, 2969–2970. [Google Scholar] [CrossRef]
- Batten Stuart, R.; Champness Neil, R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar]
- Yaghi, O.M.; Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar]
- Furukawa, H.; Kim, J.; Plass, K.E.; Yaghi, O.M. Crystal structure, dissolution, and deposition of a 5 nm functionalized metal-organic great rhombicuboctahedron. J. Am. Chem. Soc. 2006, 128, 8398–8399. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, J.; Chen, X. [Zn(bim)2]·(H2O)1.67: A metal-organic open-framework with sodalite topology. Chin. Sci. Bull. 2003, 48, 1531–1534. [Google Scholar]
- Huang, X.C.; Lin, Y.Y.; Zhang, J.P.; Chen, X.M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) Imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Doonan, C.J.; Furukawa, H.; Banerjee, R.; Yaghi, O.M. Crystals as molecules: Postsynthesis covalent functionalization of zeolitic imidazolate frameworks. J. Am. Chem. Soc. 2008, 130, 12626–12627. [Google Scholar] [CrossRef] [PubMed]
- Seki, K. Design of an adsorbent with an ideal pore structure for methane adsorption using metal complexes. Chem. Commun. 2001, 1496, 1496–1497. [Google Scholar] [CrossRef]
- Barcena, C. Gas Permeability Properties of Copper (II) Biphenyldicarboxylate-Triethylenediamine in Rubbery Mixed-Matrix Membranes. Master’s Thesis, The University of Texas at Dallas, Richardson, TX, USA, 2006. [Google Scholar]
- Zhang, Y.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas permeability properties of Matrimid® membranes containing the metal-organic framework Cu-BPY-HFS. J. Membr. Sci. 2008, 313, 170–181. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.-S. Poly-/metal-benzimidazole nano-composite membranes for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171–4180. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporus Mesoporus Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.J.D.; Lau, C.H.; Mardel, J.I.; Kitchin, M.; Konstas, K.; Ladewig, B.P.; Hill, M.R. Physical aging in glassy mixed matrix membranes; tuning particle interaction for mechanically robust nanocomposite films. J. Mater. Chem. A 2016, 4, 10627–10634. [Google Scholar] [CrossRef]
- Mahdi, E.M.; Tan, J.-C. Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: Microstructural, thermo-mechanical and viscoelastic effects. Polymer 2016, 97, 31–43. [Google Scholar] [CrossRef]
- Adams, R.; Carson, C.; Ward, J.; Tannenbaum, R.; Koros, W. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater. 2010, 131, 13–20. [Google Scholar] [CrossRef]
- Diaz, K.; Garrido, L.; Lopez-Gonzalez, M.; del Castillo, L.F.; Riande, E. CO2 transport in polysulfone membranes containing zeolitic imidazolate frameworks as determined by permeation and PFG NMR techniques. Macromolecules 2010, 43, 316–325. [Google Scholar] [CrossRef]
- Hu, J.; Cai, H.; Ren, H.; Wei, Y.; Xu, Z.; Liu, H.; Hu, Y. Mixed-matrix membrane hollow fibers of Cu3(BTC)2 MOF and polyimide for gas separation and adsorption. Ind. Eng. Chem. Res. 2010, 49, 12605–12612. [Google Scholar] [CrossRef]
- Keskin, S.; Sholl, D.S. Selecting metal organic frameworks as enabling materials in mixed matrix membranes for high efficiency natural gas purification. Energy Environ. Sci. 2010, 3, 343–351. [Google Scholar] [CrossRef]
- Keskin, S. Molecular simulation study of CH4/H2 mixture separations using metal organic framework membranes and composites. J. Phys. Chem. C 2010, 114, 13047–13054. [Google Scholar] [CrossRef]
- Erucar, I.; Keskin, S. Computational methods for MOF/polymer membranes. Chem. Rec. 2016, 16, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, M.J.C.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Musselman, I.H.; Perez, E.V.; Ordonez, M.J.C.; Balkus, K.J., Jr.; Ferraris, J.P. Incorporation of hybrid crystalline microporous materials in mixed-matrix membranes for gas separations. Microsc. Microanal. 2010, 16, 1668–1669. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Zornoza, B.; Martinez-Joaristi, A.; Serra-Crespo, P.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Functionalized flexible MOFs as fillers in mixed matrix membranes for highly selective separation of CO2 from CH4 at elevated pressures. Chem. Commun. 2011, 47, 9522–9524. [Google Scholar] [CrossRef] [PubMed]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413–414, 48–61. [Google Scholar] [CrossRef]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Tellez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef] [PubMed]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. A method for increasing permeability in O2/N2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone. Chem. Commun. 2012, 48, 2140–2142. [Google Scholar] [CrossRef] [PubMed]
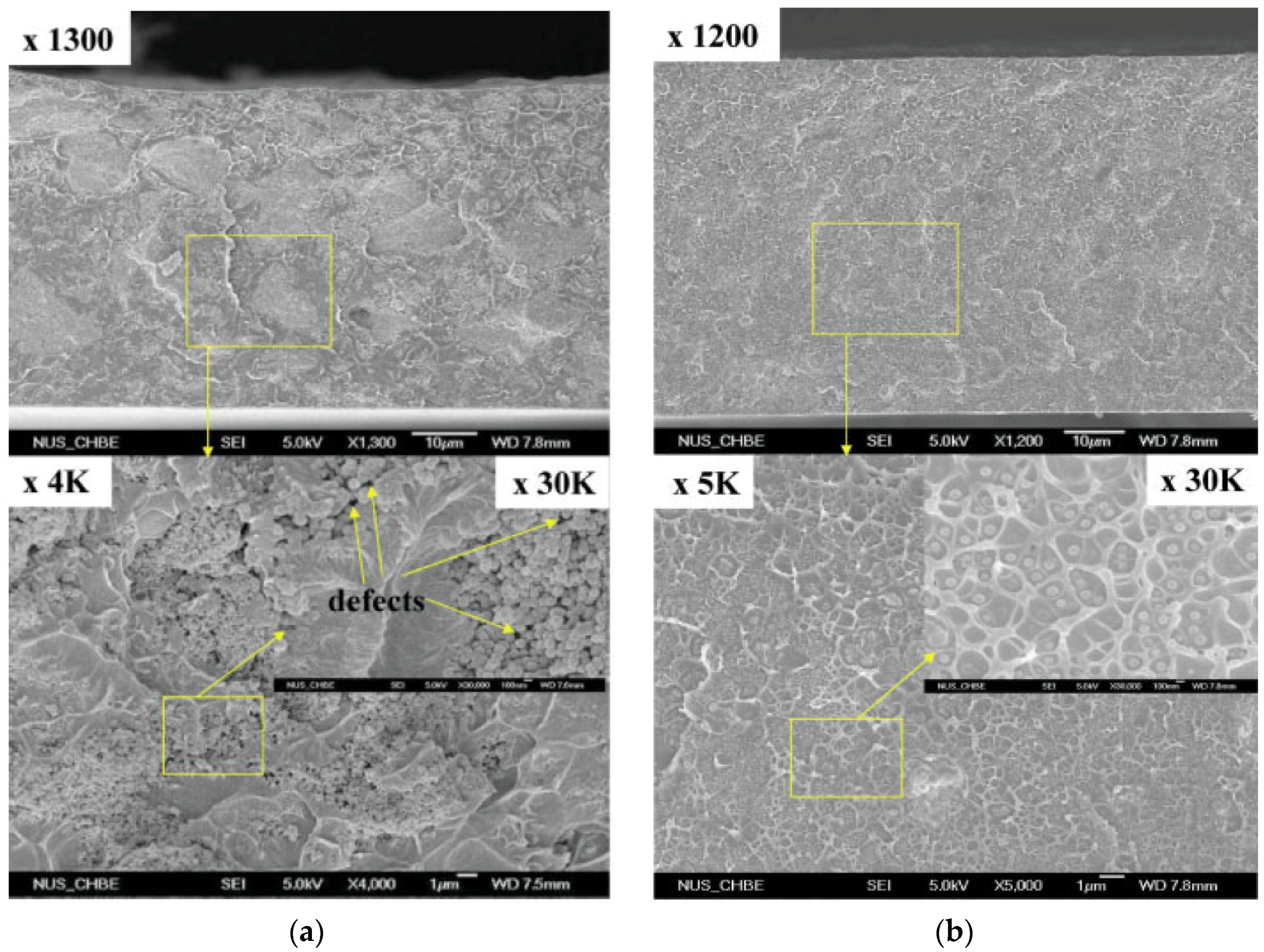
- Dai, Y.; Johnson, J.R.; Karvan, O.; Sholl, D.S.; Koros, W.J. Ultem™/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Membr. Sci. 2012, 401–402, 76–82. [Google Scholar] [CrossRef]
- Yang, T.; Shi, G.M.; Chung, T.-S. Symmetric and asymmetric zeolitic imidazolate frameworks (ZIFs)/polybenzimidazole (PBI) nanocomposite membranes for hydrogen purification at high temperatures. Adv. Energy Mater. 2012, 2, 1358–1367. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Carolus Jansen, J.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Budd, P.; Elabas, E.; Ghanem, B.; Makhseed, S.; McKeown, N.; Msayib, K.; Tattershall, C.; Wang, D. Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Haruyoshi, G.; Yukitaka, K. Production of 5,6,5′,6′-Tetrahydroxy-1,1′-Spirobisindanes. Patent JP3,148,232, 1989. [Google Scholar]
- Jeazet, H.B.T.; Koschine, T.; Staudt, C.; Raetzke, K.; Janiak, C. Correlation of gas permeability in a metal-organic framework MIL-101(Cr)—Polysulfone mixed-matrix membrane with free volume measurements by positron annihilation lifetime spectroscopy (PALS). Membranes 2013, 3, 331–353. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kang, D.-Y.; Nair, S. Rigorous calculations of permeation in mixed-matrix membranes: Evaluation of interfacial equilibrium effects and permeability-based models. J. Membr. Sci. 2013, 448, 160–169. [Google Scholar] [CrossRef]
- Pal, R. Permeation models for mixed matrix membranes. J. Colloid Interface Sci. 2008, 317, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, P.; Yang, T.; Chung, T.-S. Room temperature ionic liquid/ZIF-8 mixed-matrix membranes for natural gas sweetening and post-combustion CO2 capture. J. Membr. Sci. 2013, 436, 221–231. [Google Scholar] [CrossRef]
- Abedini, R.; Omidkhah, M.; Dorosti, F. Hydrogen separation and purification with poly (4-methyl-1-pentyne)/MIL 53 mixed matrix membrane based on reverse selectivity. Int. J. Hydrog. Energy 2014, 39, 7897–7909. [Google Scholar] [CrossRef]
- Diestel, L.; Liu, X.L.; Li, Y.S.; Yang, W.S.; Caro, J. Comparative permeation studies on three supported membranes: Pure ZIF-8, pure polymethylphenylsiloxane, and mixed matrix membranes. Microporous Microporous Mater. 2014, 189, 210–215. [Google Scholar] [CrossRef]
- Diestel, L.; Wang, N.; Schwiedland, B.; Steinbach, F.; Giese, U.; Caro, J. MOF based MMMs with enhanced selectivity due to hindered linker distortion. J. Membr. Sci. 2015, 492, 181–186. [Google Scholar] [CrossRef]
- Duan, C.; Jie, X.; Liu, D.; Cao, Y.; Yuan, Q. Post-treatment effect on gas separation property of mixed matrix membranes containing metal organic frameworks. J. Membr. Sci. 2014, 466, 92–102. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Murali, R.S.; Matsuura, T. The impact of ZIF-8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 2014, 4, 52530–52541. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Performance and plasticization behavior of polymer-MOF membranes for gas separation at elevated pressures. J. Membr. Sci. 2014, 470, 166–177. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. High pressure gas separation performance of mixed-matrix polymer membranes containing mesoporous Fe(BTC). J. Membr. Sci. 2014, 459, 33–44. [Google Scholar] [CrossRef]
- Rodenas, T.; van Dalen, M.; Garcia-Perez, E.; Serra-Crespo, P.; Zornoza, B.; Kapteijn, F.; Gascon, J. Visualizing MOF mixed matrix membranes at the nanoscale: Towards structure-performance relationships in CO2/CH4 separation over NH2-MIL-53(Al)@PI. Adv. Funct. Mater. 2014, 24, 249–256. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Tan, P.Y.; Chai, S.-P.; Zhu, P.W.; Mohamed, A.R. Continuous polycrystalline ZIF-8 membrane supported on CO2-selective mixed matrix supports for CO2/CH4 separation. RSC Adv. 2014, 4, 52461–52466. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending aging in super glassy polymer membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, M.; Teo, J.; Konstas, K.; Lau, C.H.; Sumby, C.J.; Thornton, A.W.; Doonan, C.J.; Hill, M.R. AIMs: A new strategy to control physical aging and gas transport in mixed-matrix membranes. J. Mater. Chem. A 2015, 3, 15241–15247. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Kanehashi, S.; Ozcelik, B.; Kentish, S.E.; Hill, A.J.; Hill, M.R. Tailoring physical aging in super glassy polymers with functionalized porous aromatic frameworks for CO2 Capture. Chem. Mater. 2015, 27, 4756–4762. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Malakhov, A.O.; Knyazeva, E.E.; Novitsky, E.G. Gas transport properties of LiA type zeolite-filled poly(trimethylsilylpropyne) membranes. Pet. Chem. 2015, 55, 708–715. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Denny, J.M.S.; Peterson, G.W.; Mahle, J.J.; Cohen, S.M. Enhanced aging properties of HKUST-1 in hydrophobic mixed-matrix membranes for ammonia adsorption. Chem. Sci. 2016, 6, 2711–2716. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ying, Y.; Yang, Q.; Ban, Y.; Huang, H.; Guo, X.; Xiao, Y.; Liu, D.; Li, Y.; Yang, W.; et al. Mixed-matrix membranes containing functionalized porous metal-organic polyhedrons for the effective separation of CO2–CH4 mixture. Chem. Commun. 2015, 51, 4249–4251. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabres i. Xamena, F.X.; Gascon, J. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Hu, Z.; Qian, Y.; Chi, C.; Yeo, L.Y.; Tee, L.; Zhao, D. Mixed matrix membranes composed of two-dimensional metal-organic framework nanosheets for pre-combustion CO2 capture: A relationship study of filler morphology versus membrane performance. J. Mater. Chem. 2015, 3, 20801–20810. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; Guillouzer, C.L.; Clet, G.; Tellez, C.; et al. Metal organic framework crystals in mixed-matrix membranes: Impact of the filler morphology on the gas separation performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Zhang, Z.; Nguyen, H.T.; Miller, S.A.; Cohen, S.M. polyMOFs: A class of interconvertible polymer-metal-organic-framework hybrid materials. Angew. Chem. Int. Ed. 2015, 54, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Qiao, Z.; Wang, Z.; Zhao, S.; Li, P.; Wang, J.; Wang, S. Enhanced performance of mixed matrix membrane by incorporating a highly compatible covalent organic framework into poly(vinylamine) for hydrogen purification. Int. J. Hydrog. Energy 2016, 41, 9167–9174. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Tanh Jeazet, H.B.; Staudt, C.; Janiak, C. Metal-organic frameworks in mixed-matrix membranes for gas separation. Dalton Trans. 2012, 41, 14003–14027. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, H. Zeolitic imidazolate framework composite membranes and thin films: Synthesis and applications. Chem. Soc. Rev. 2014, 43, 4470–4493. [Google Scholar] [CrossRef] [PubMed]
- Erucar, I.; Keskin, S. Molecular modeling of mOF-based mixed matrix membranes. Curr. Org. Chem. 2014, 18, 2364–2380. [Google Scholar] [CrossRef]
- Erucar, I.; Yilmaz, G.; Keskin, S. Recent advances in metal-organic framework-based mixed matrix membranes. Chem. Asian J. 2013, 8, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Hunger, K.; Schmeling, N.; Tanh Jeazet, H.B.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation. Membranes 2012, 2, 727–763. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal-organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef] [PubMed]
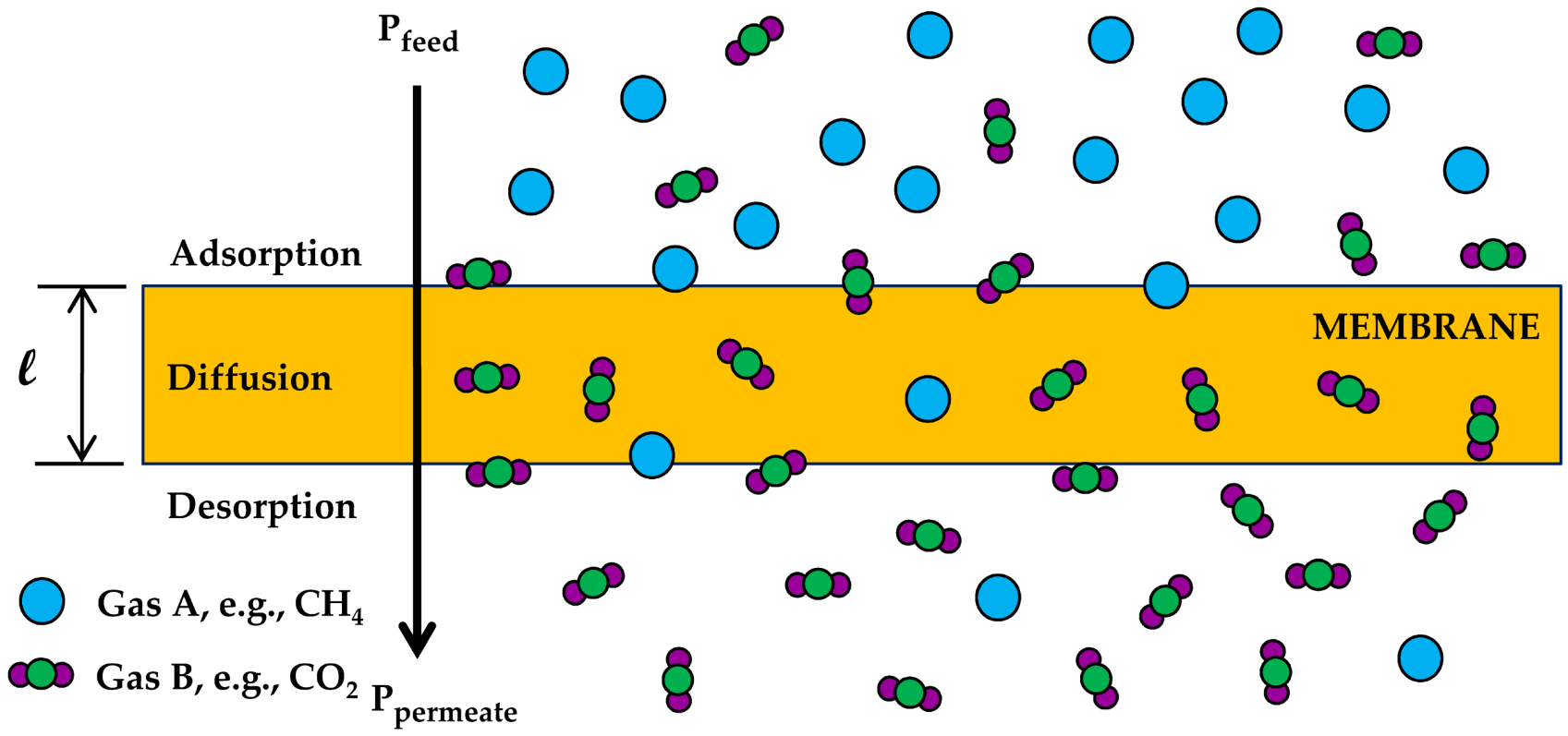
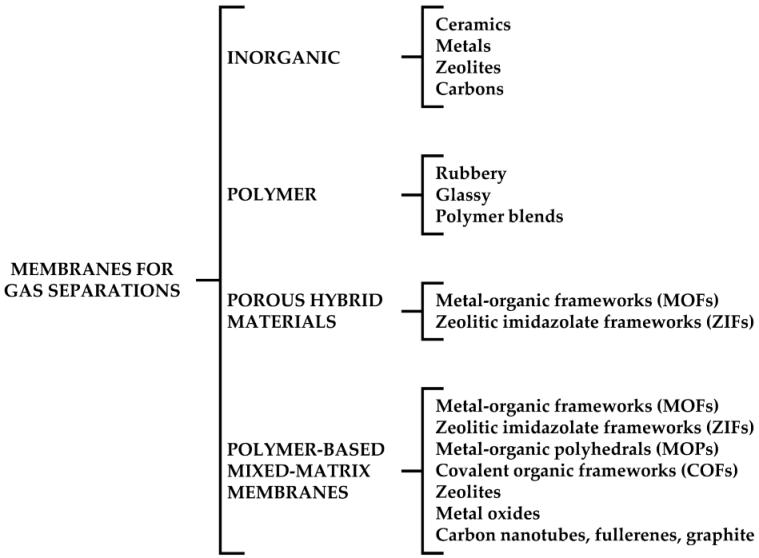
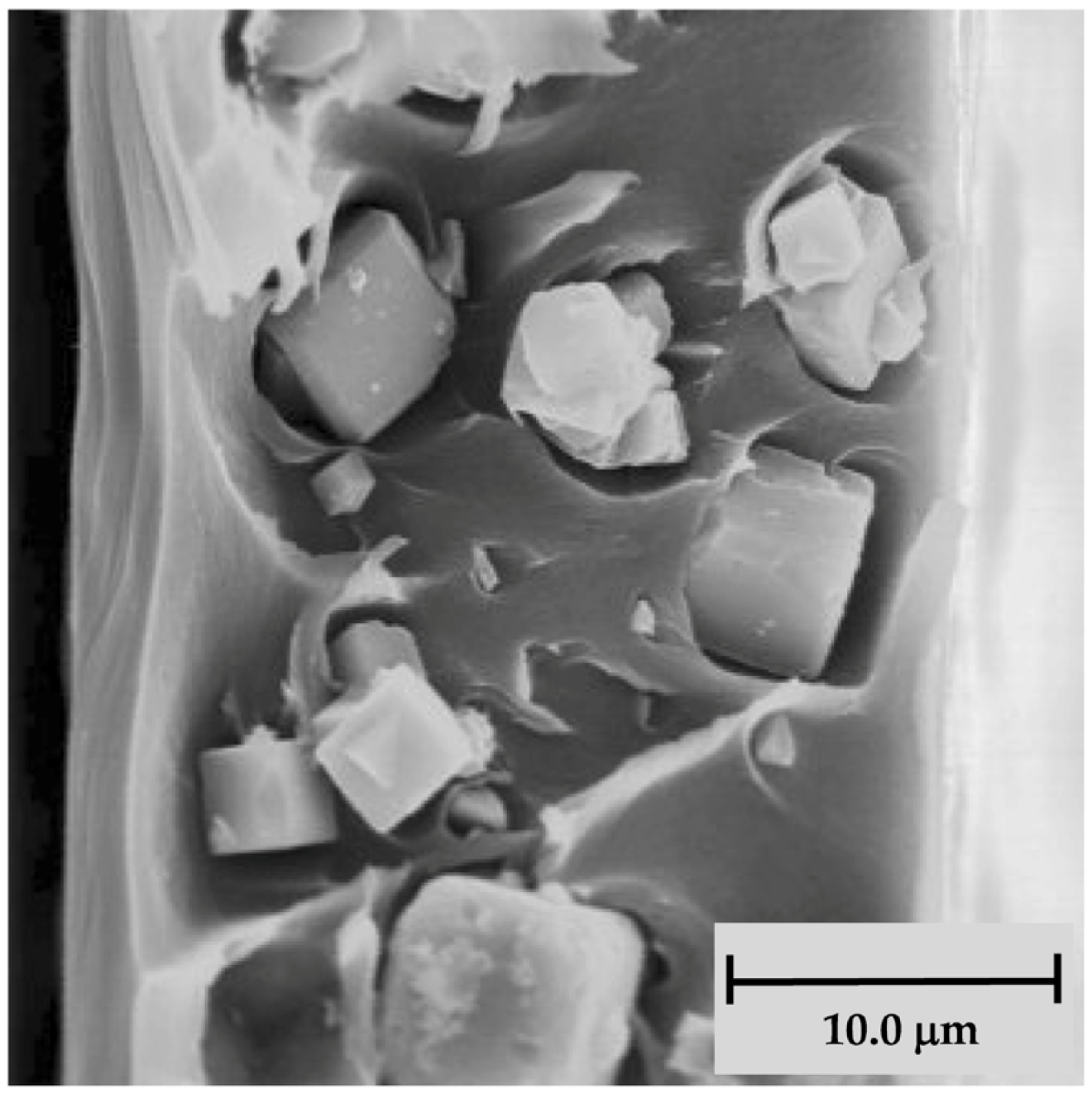
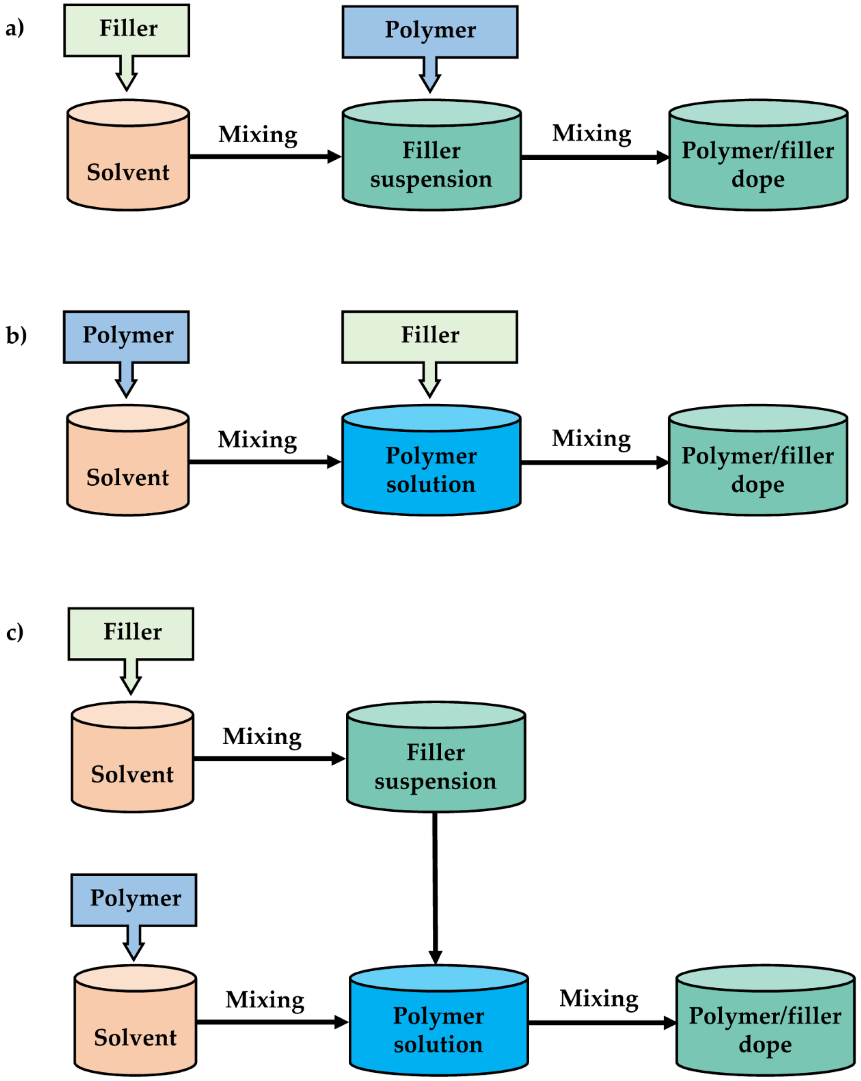
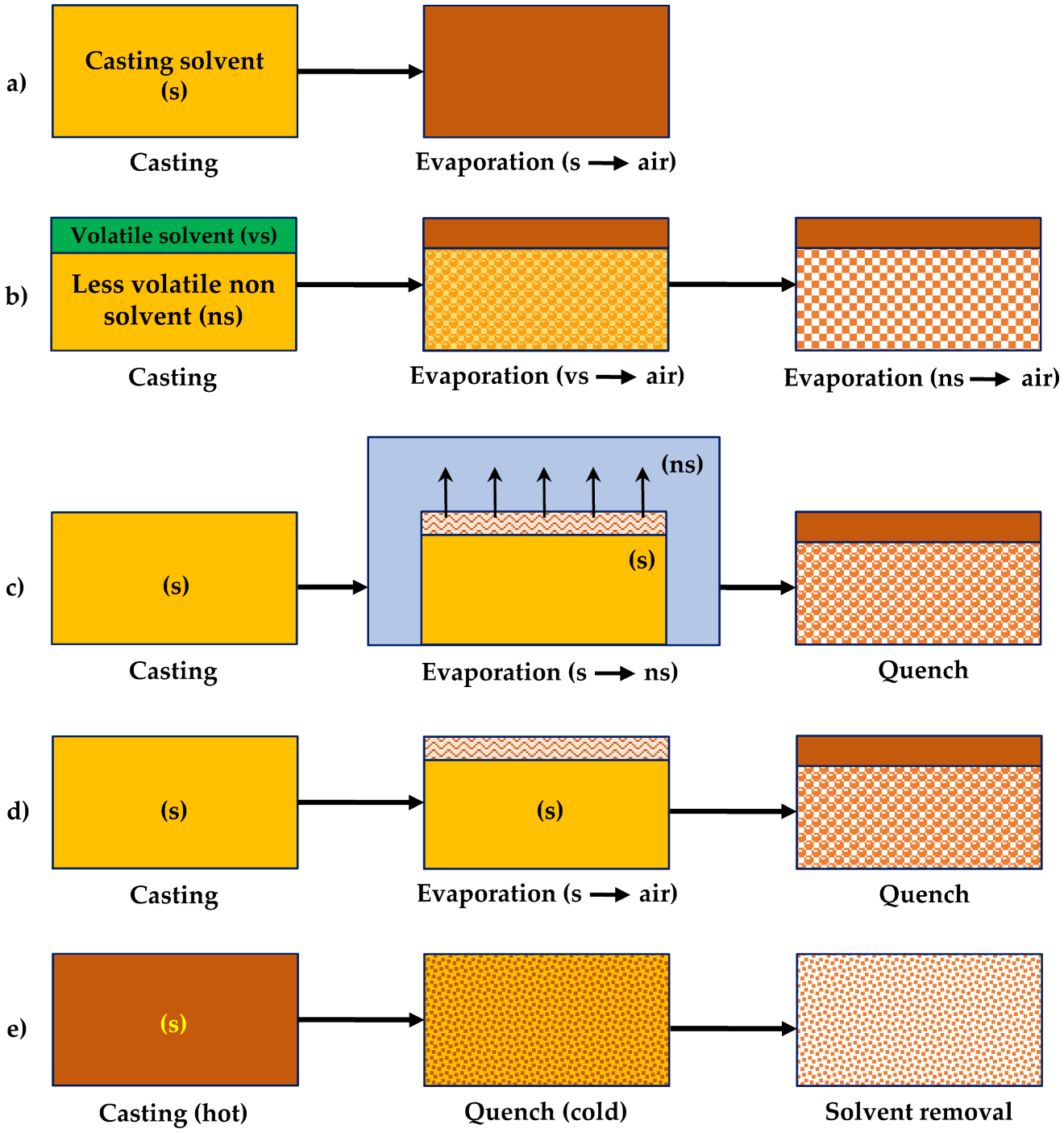

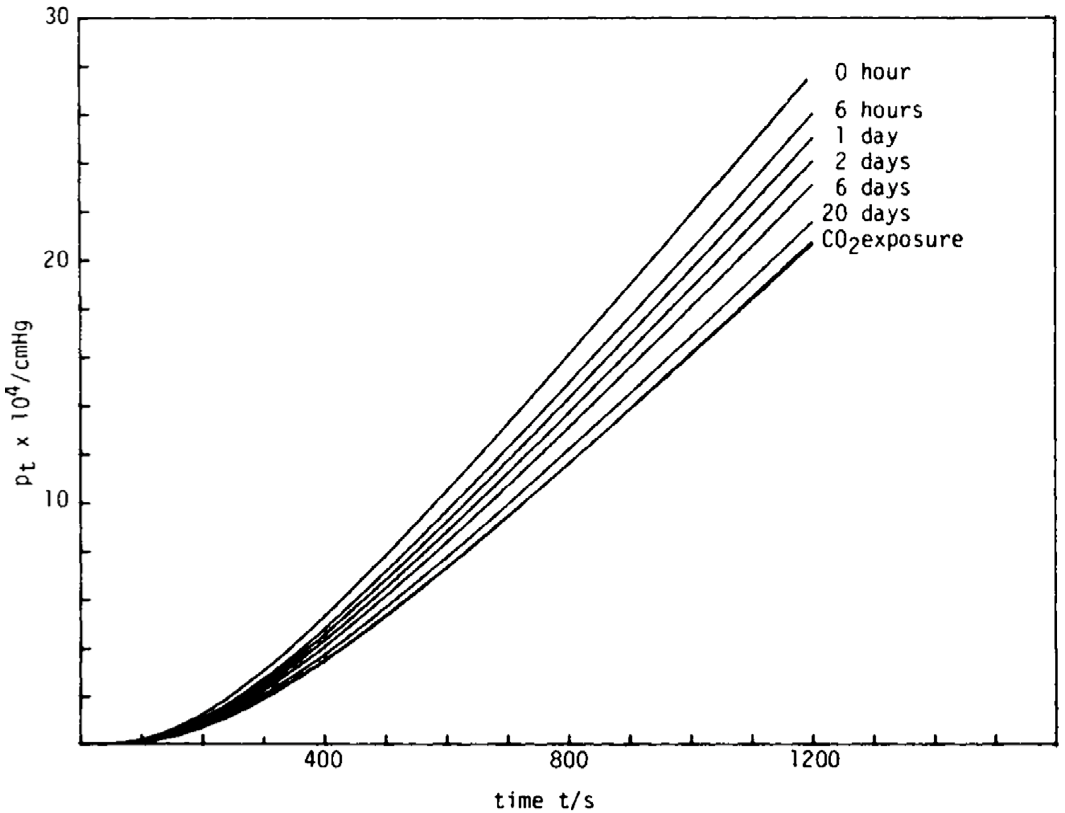
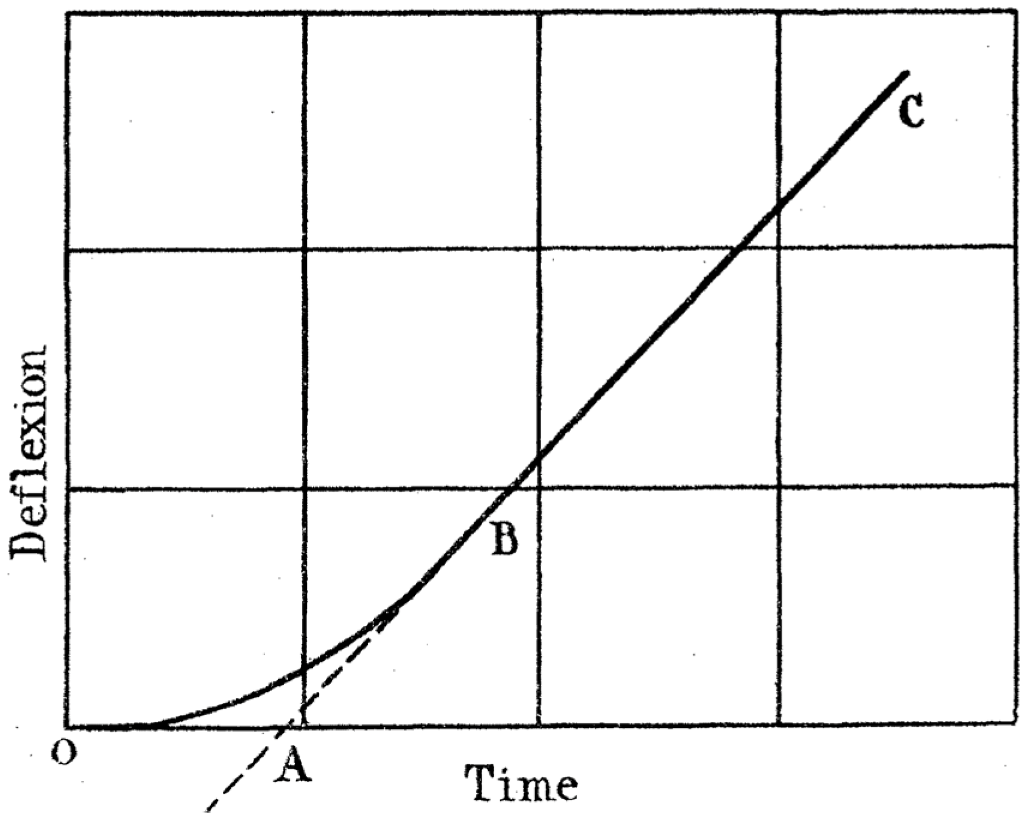
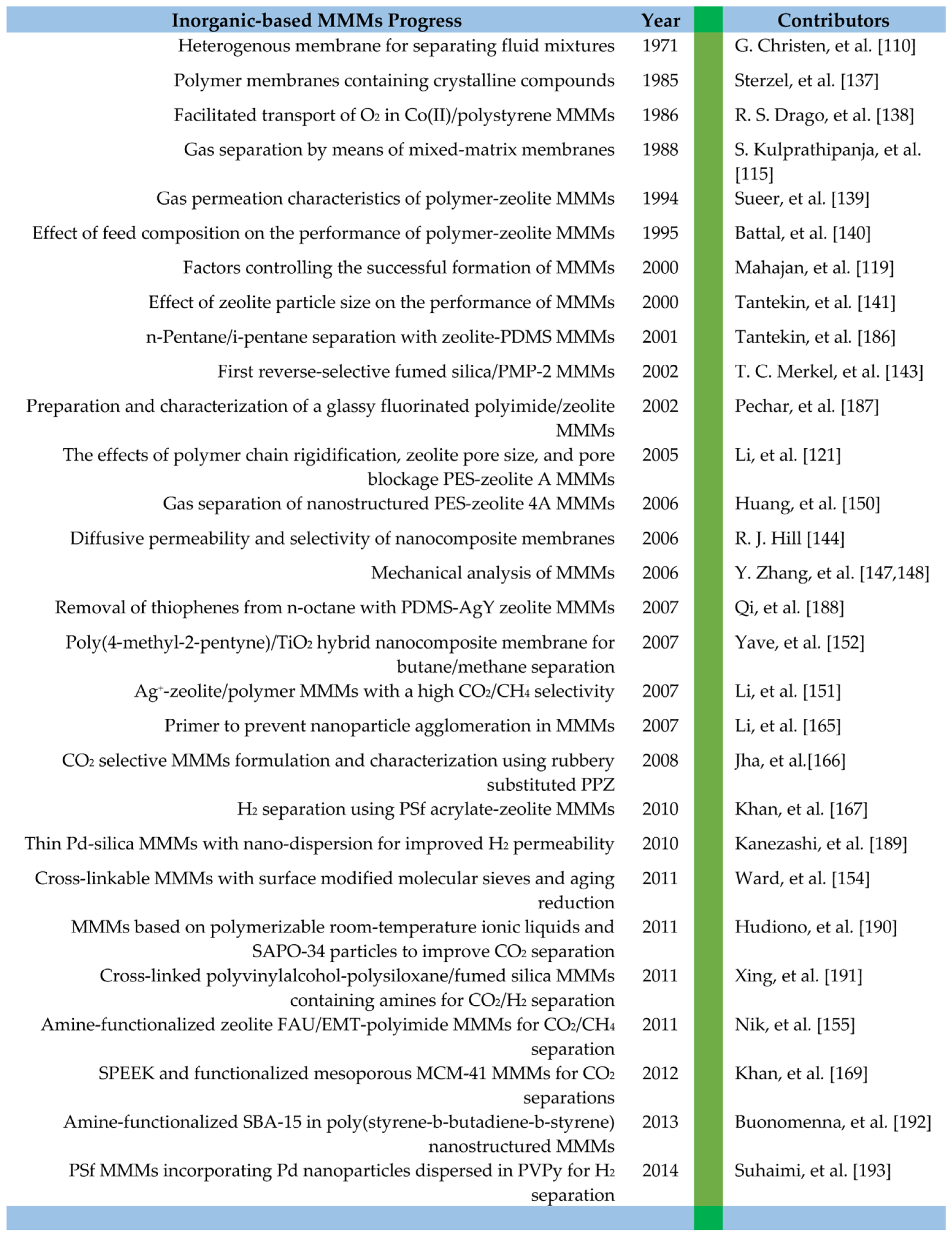


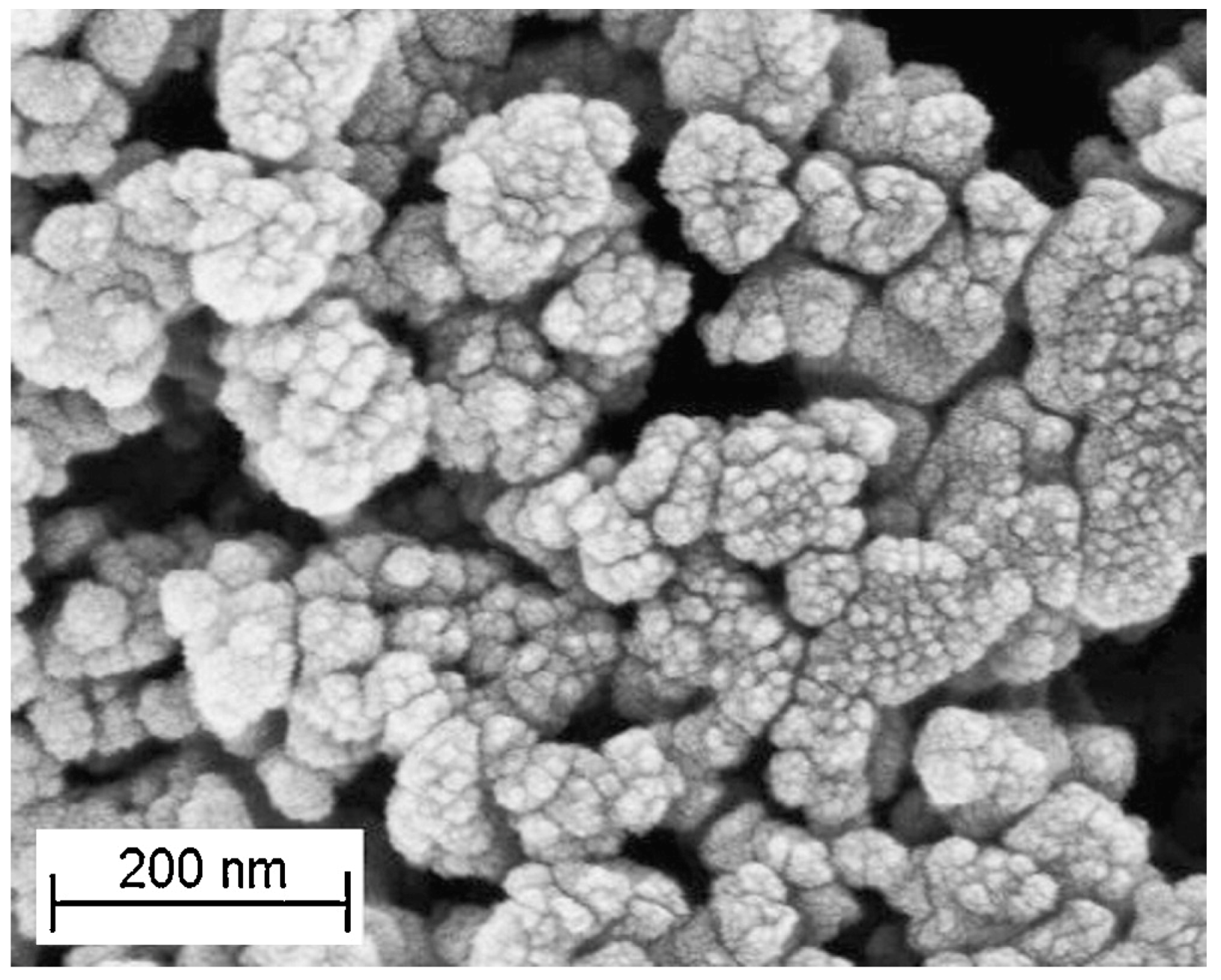

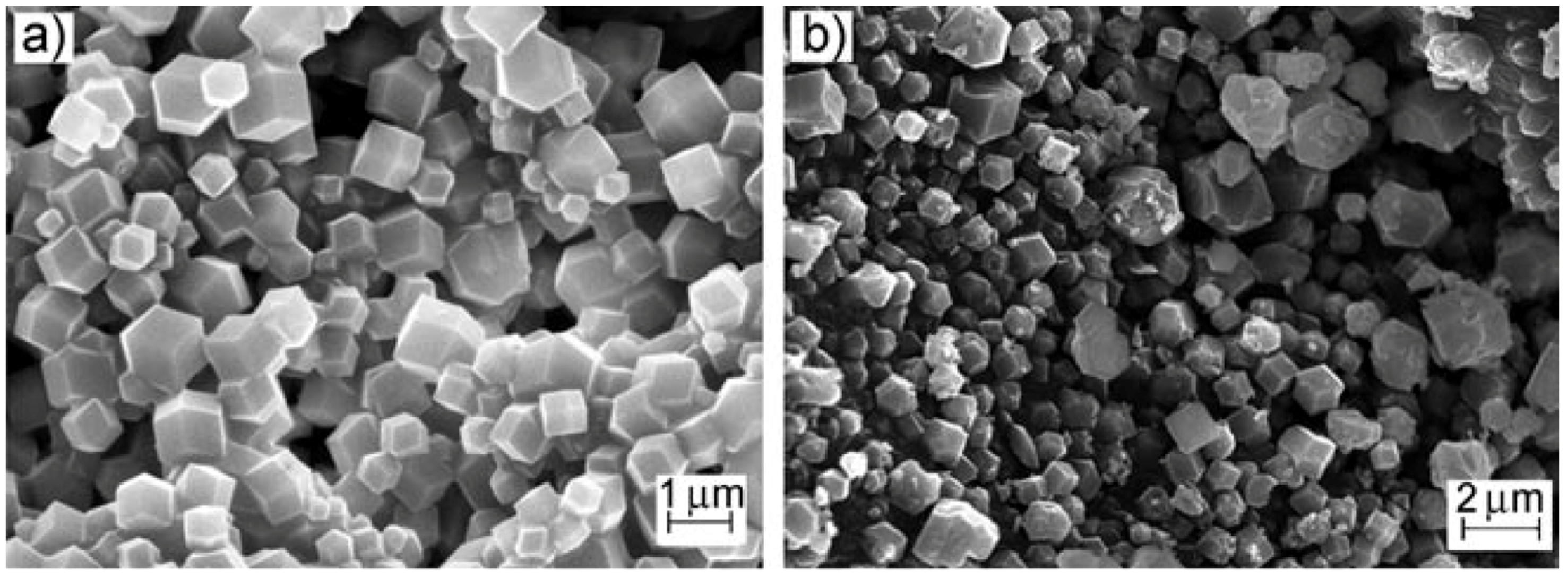
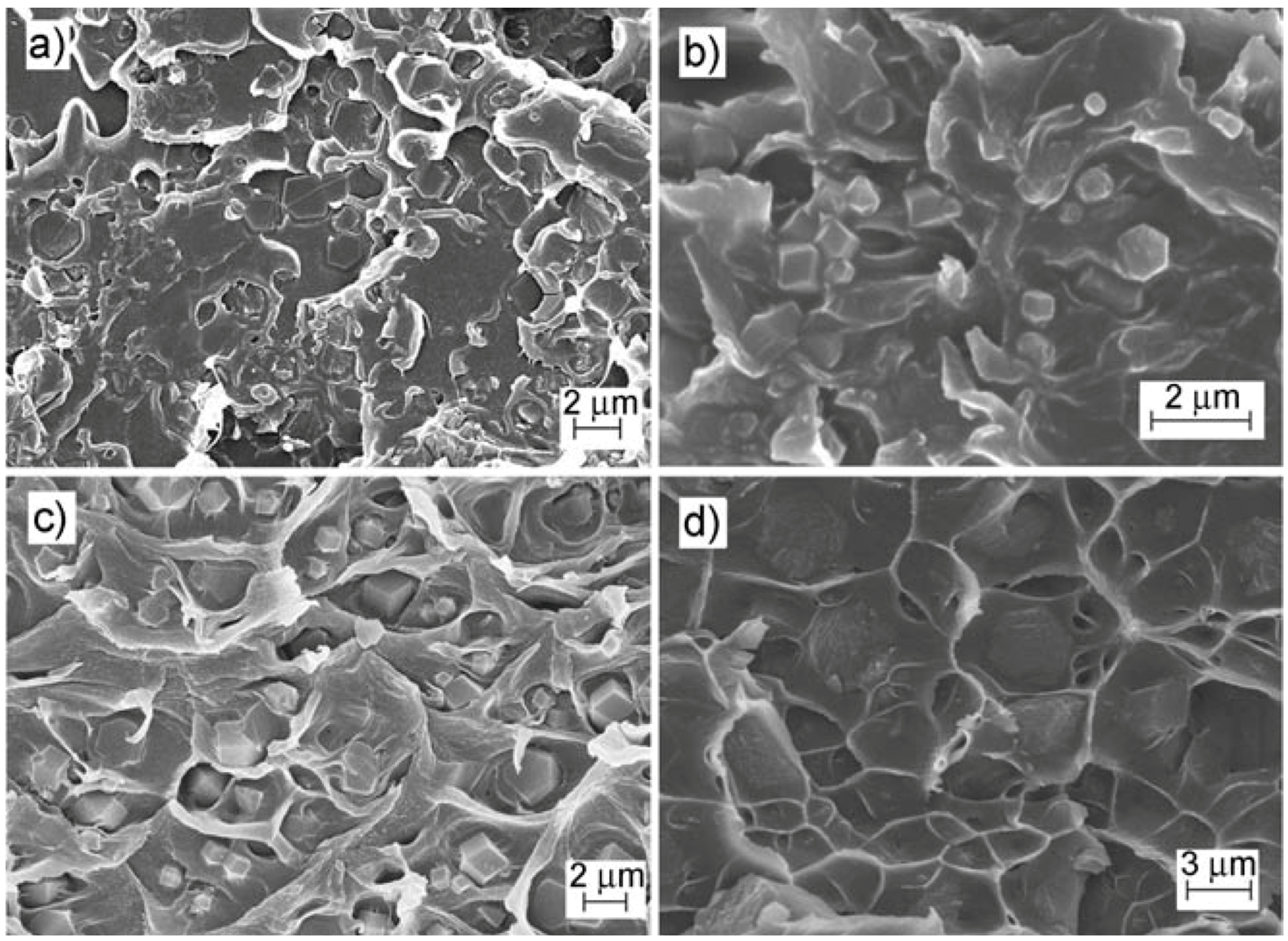

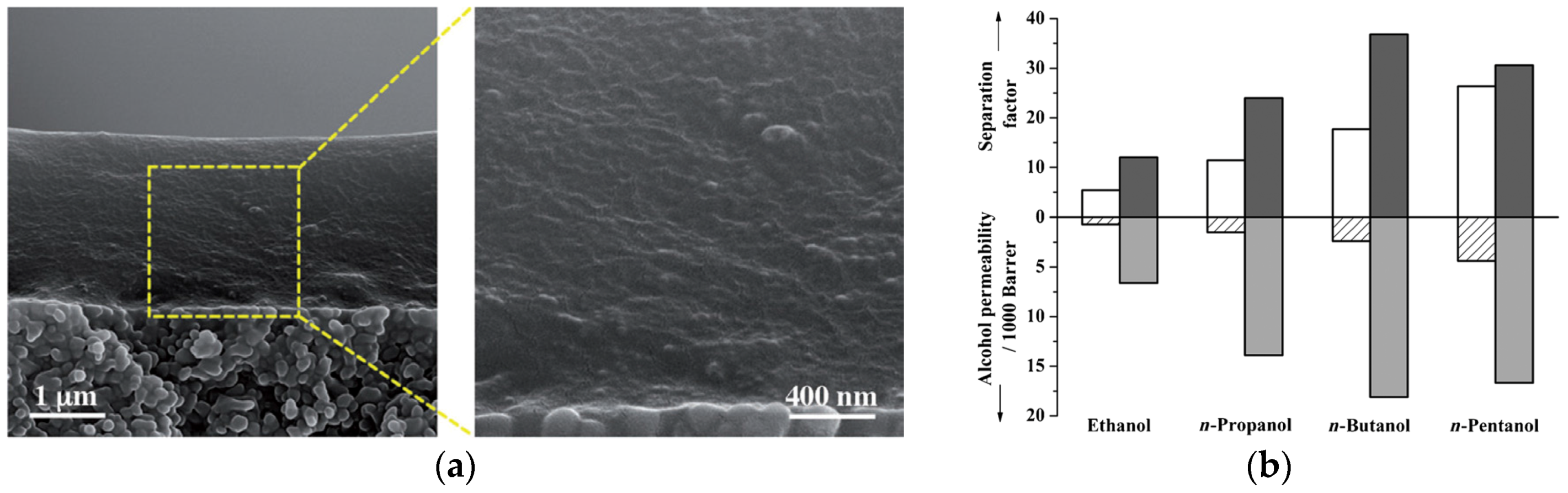
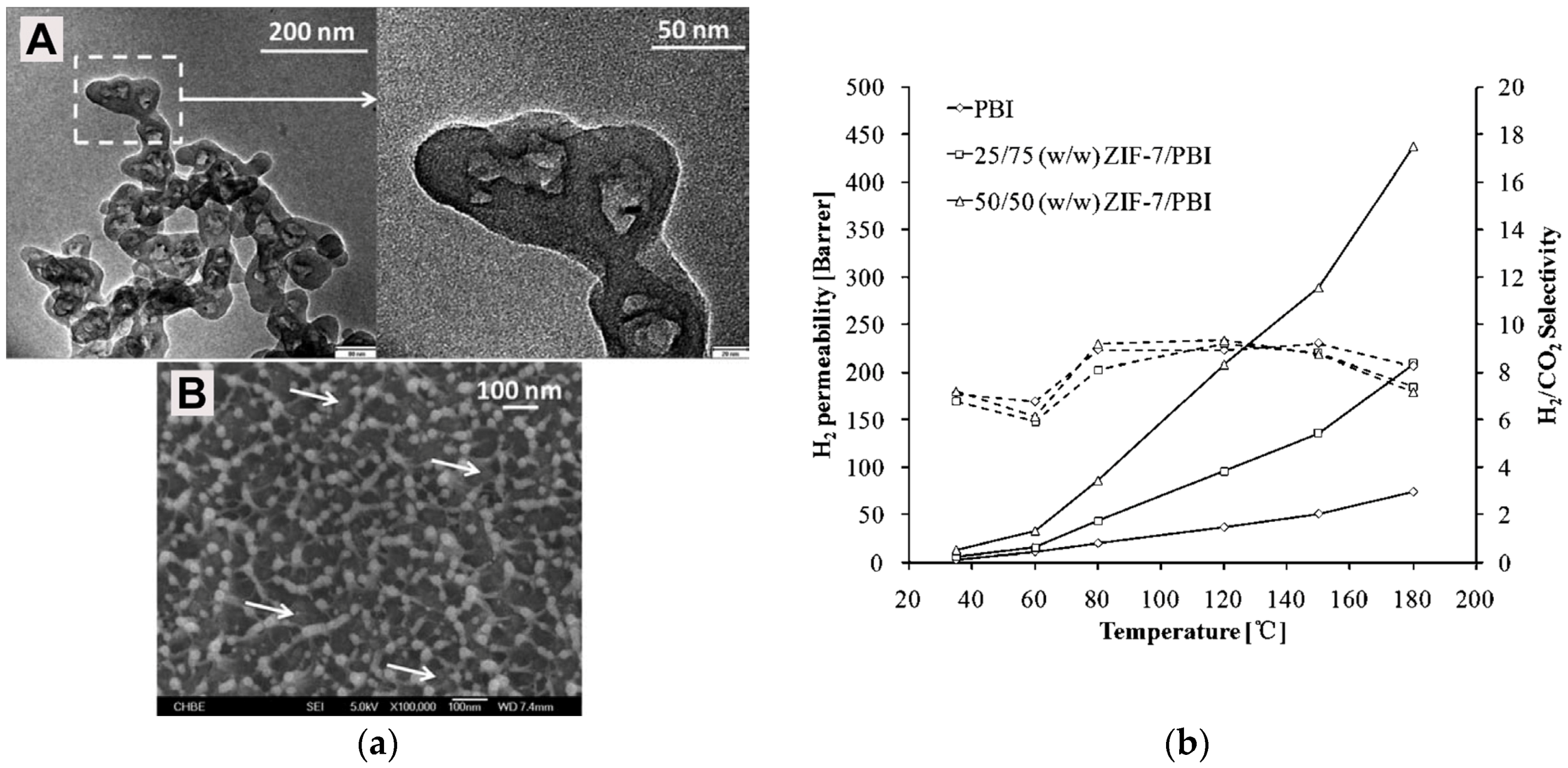
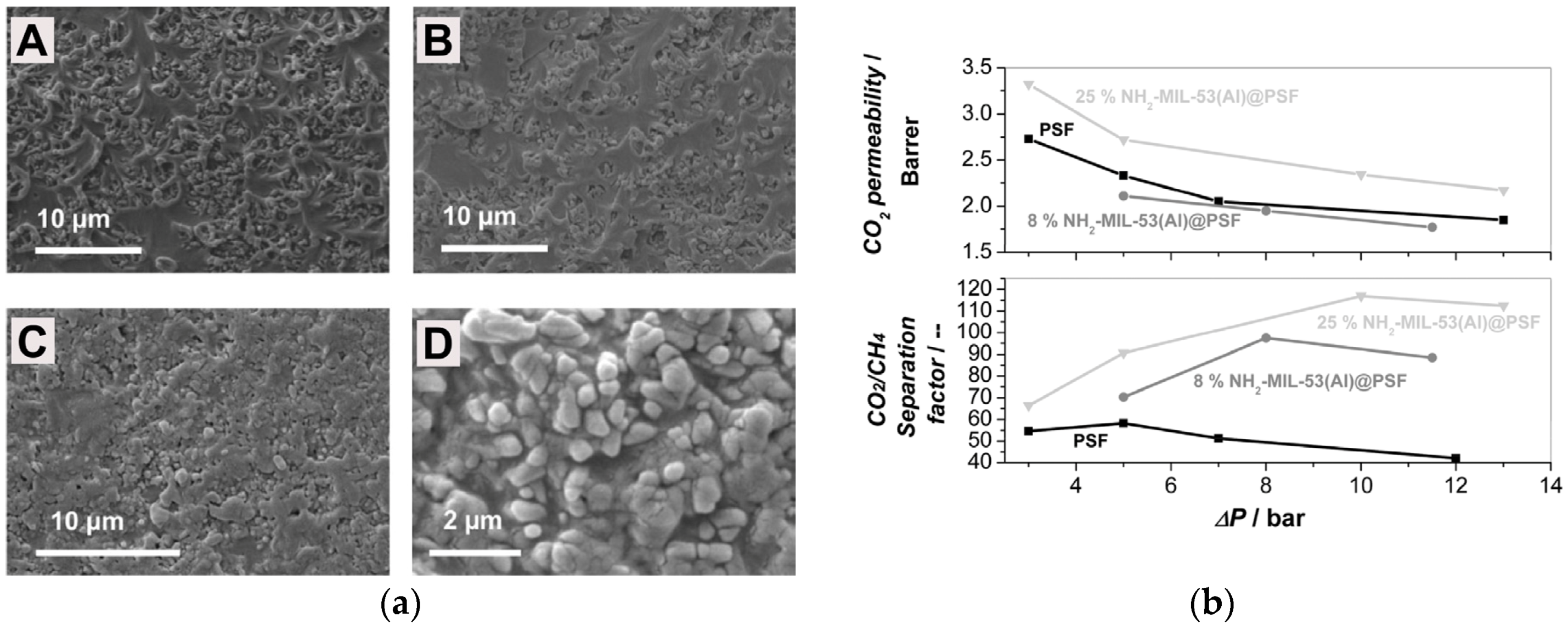
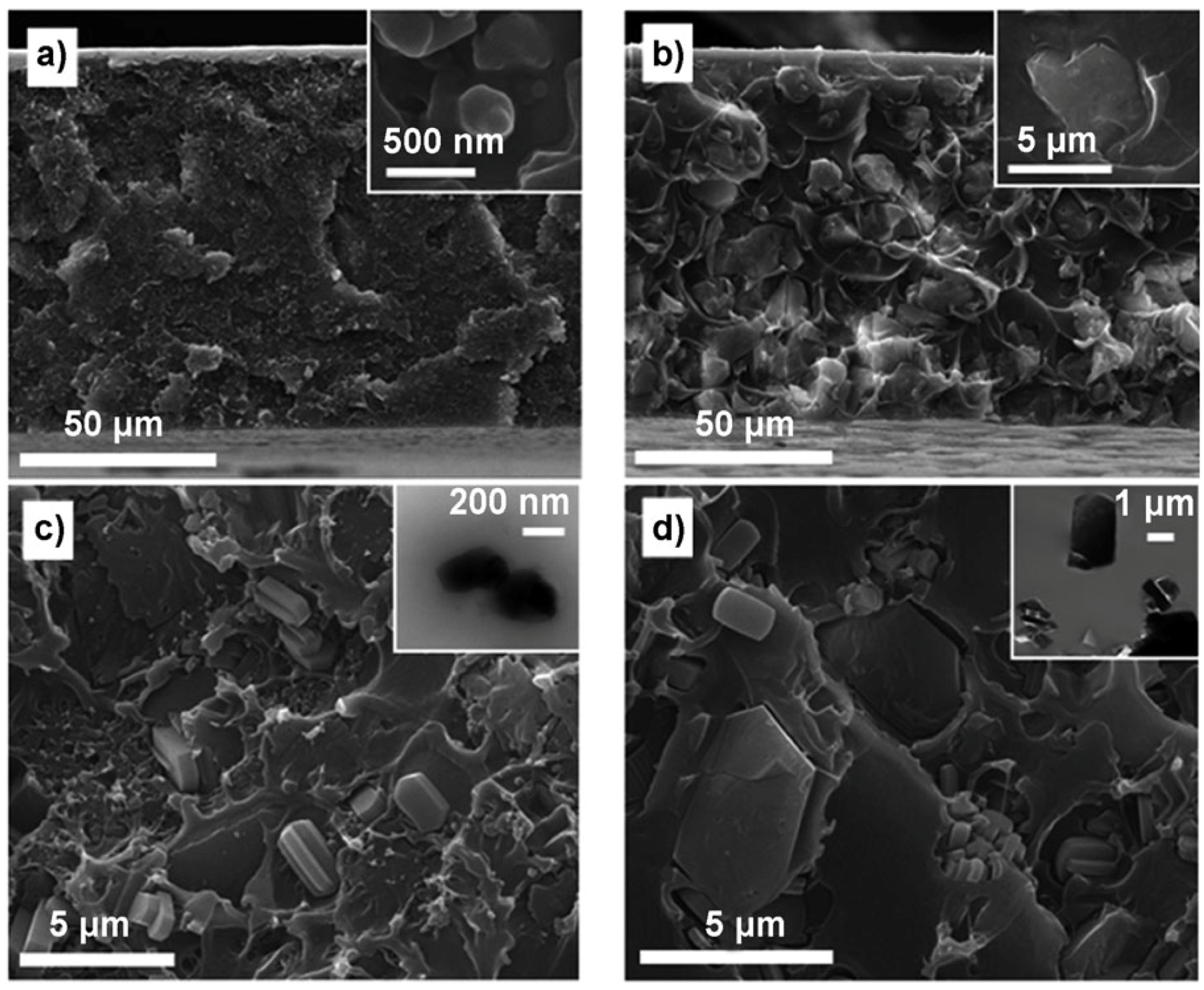
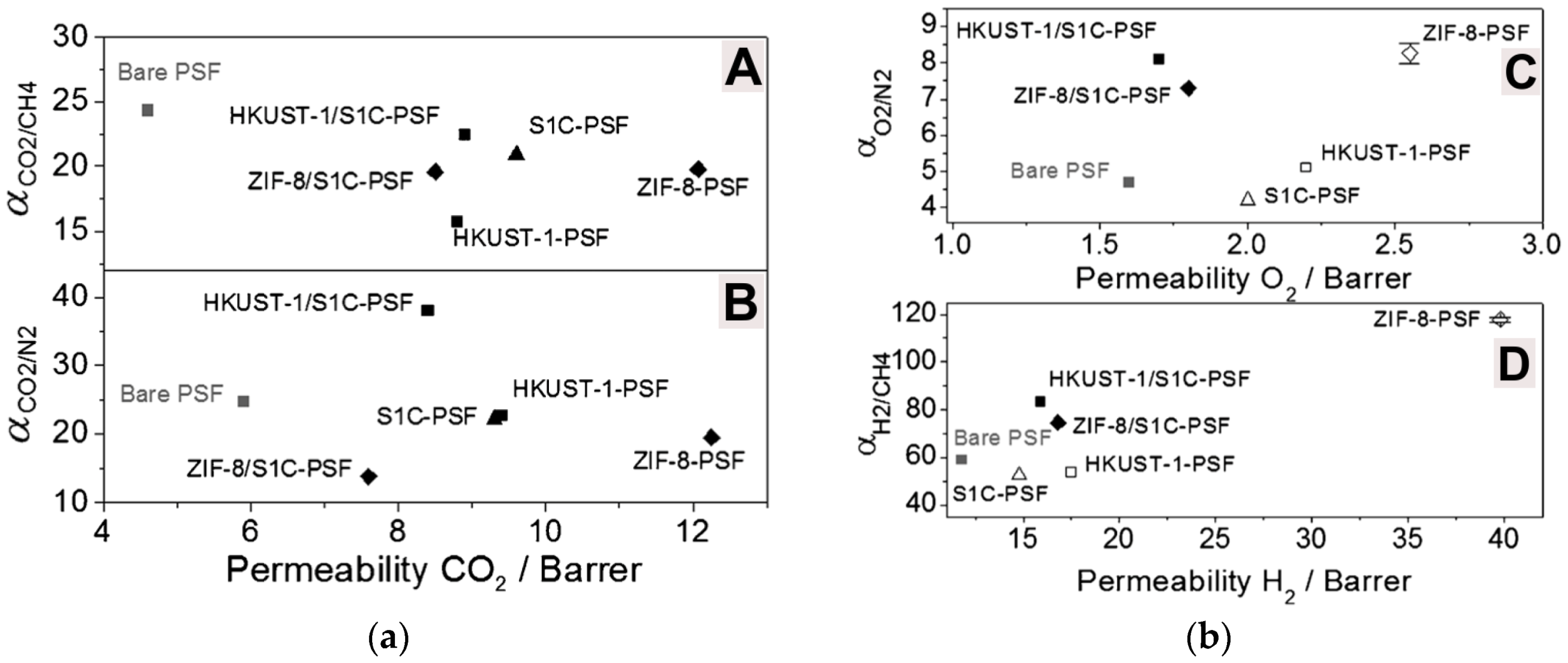
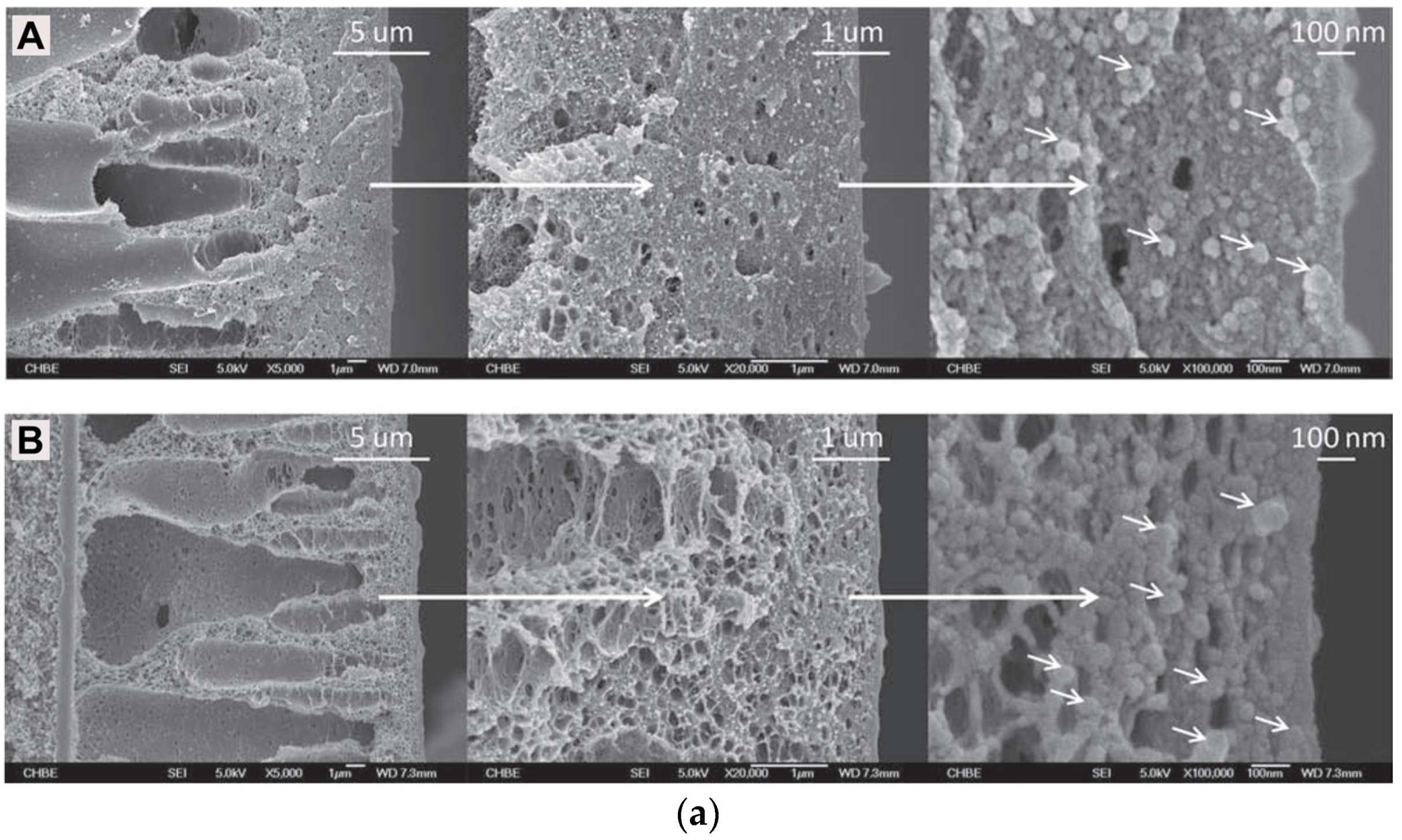
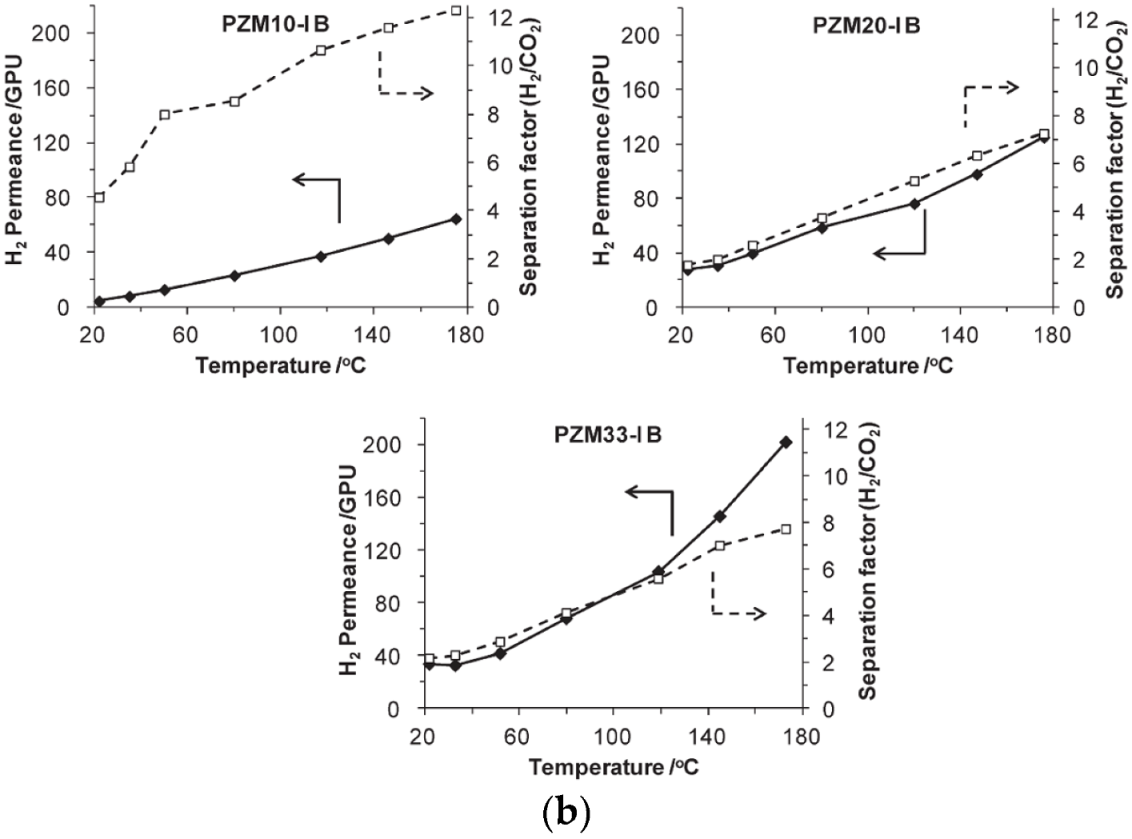
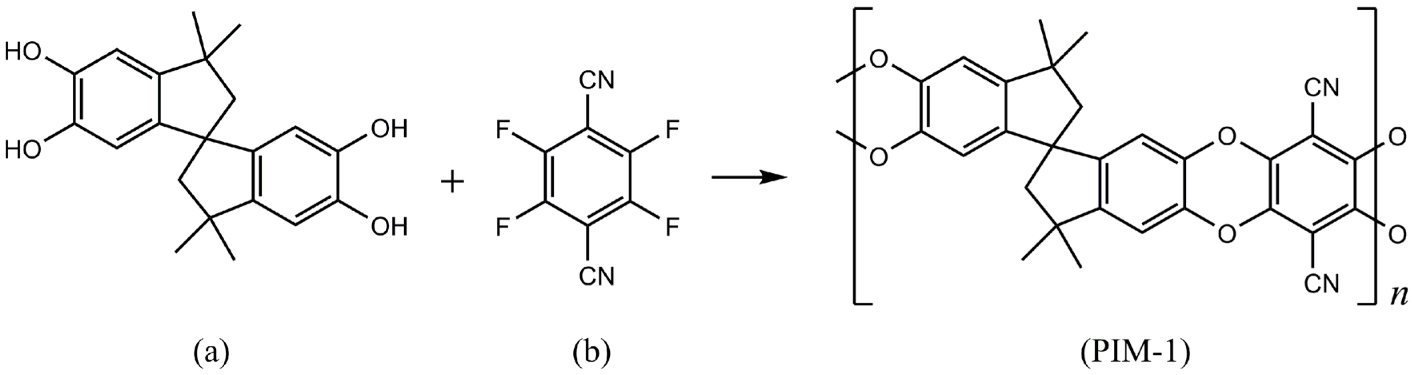
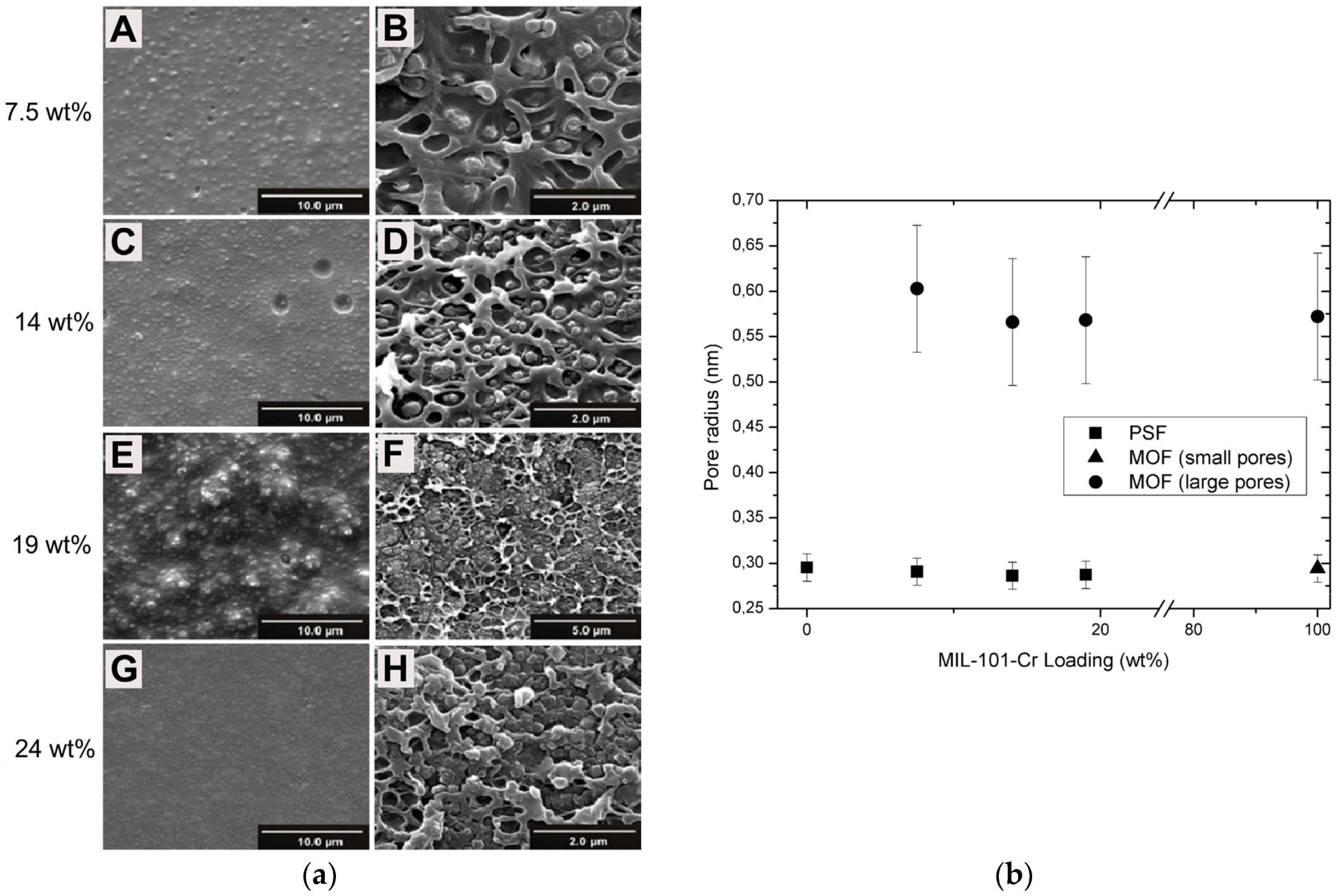
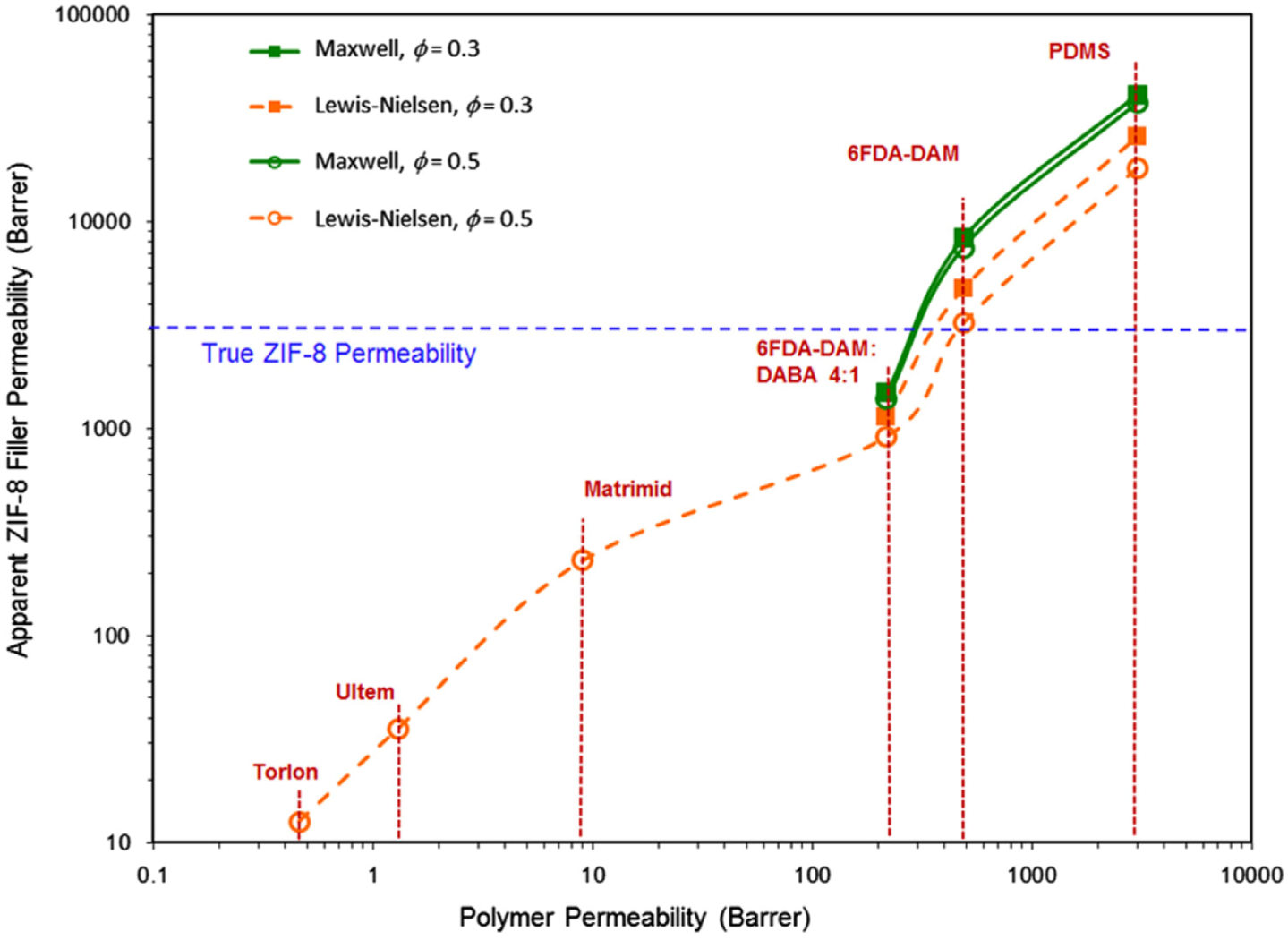
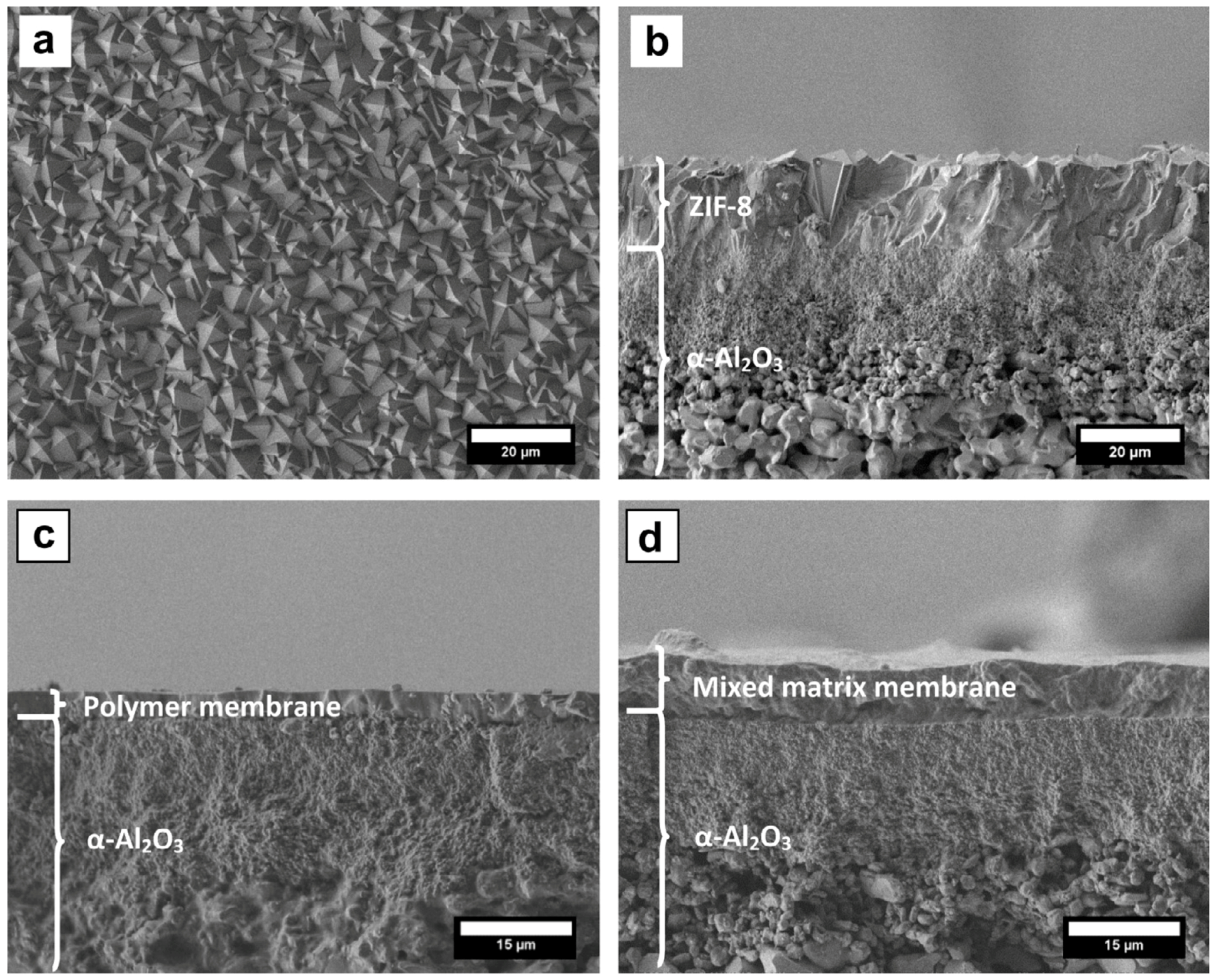
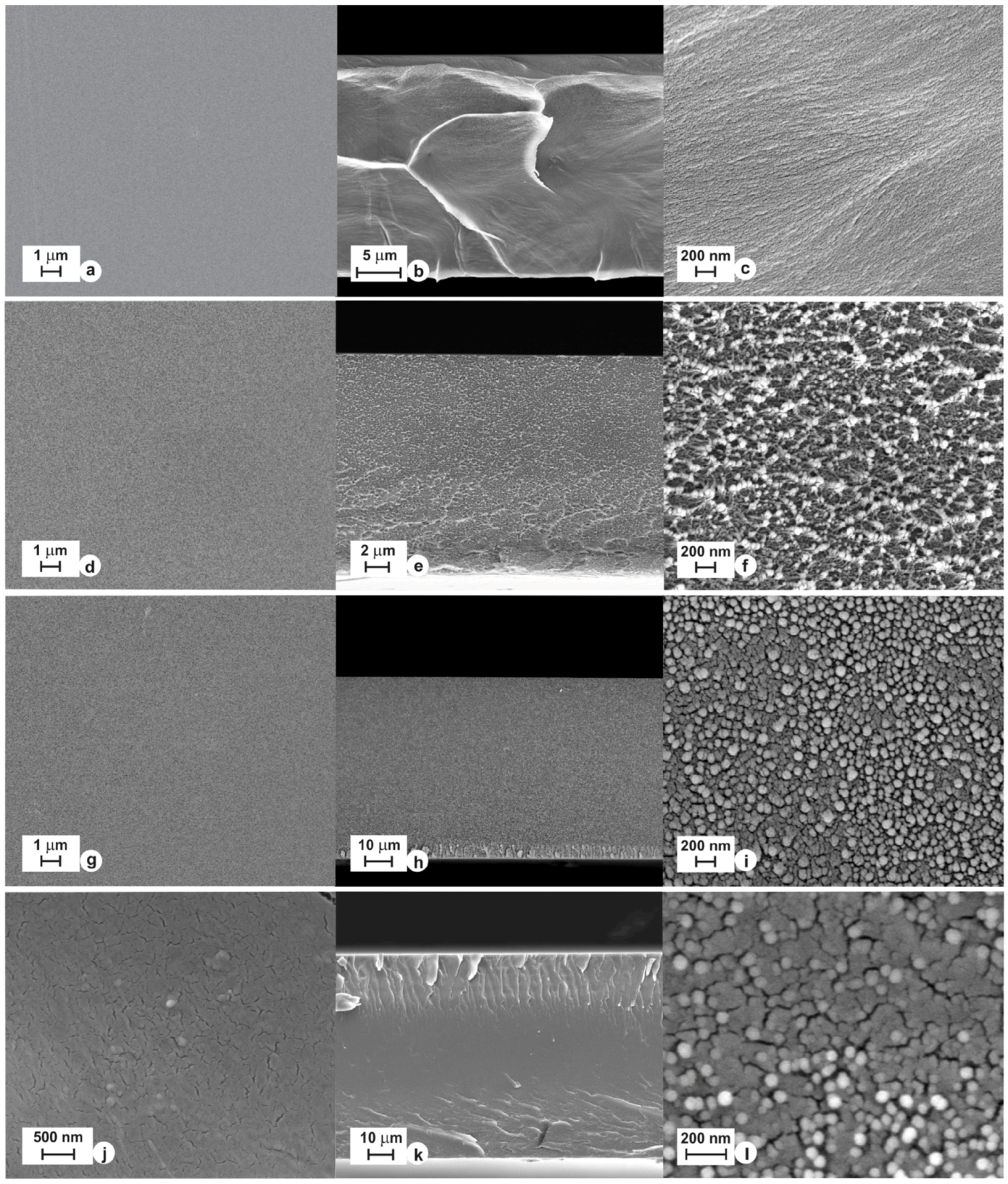
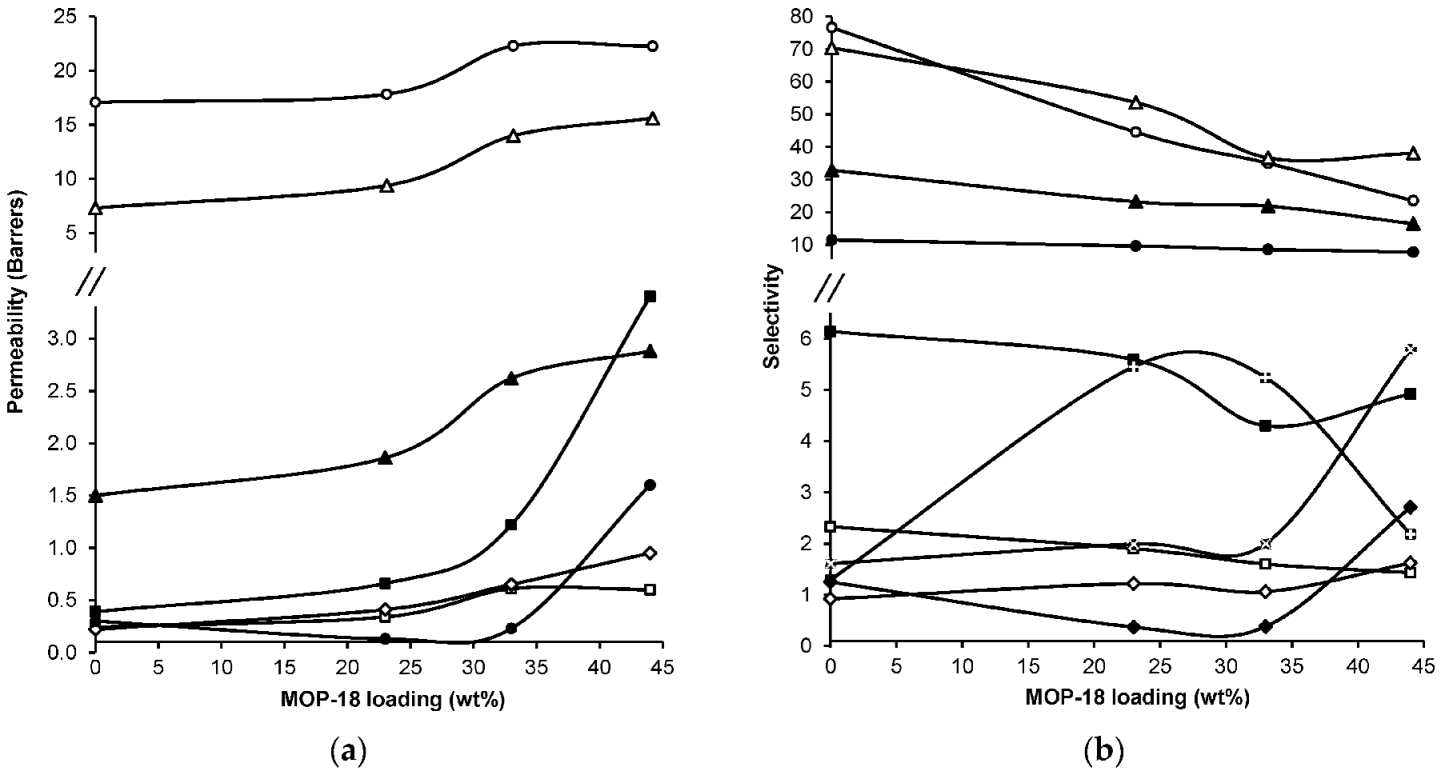
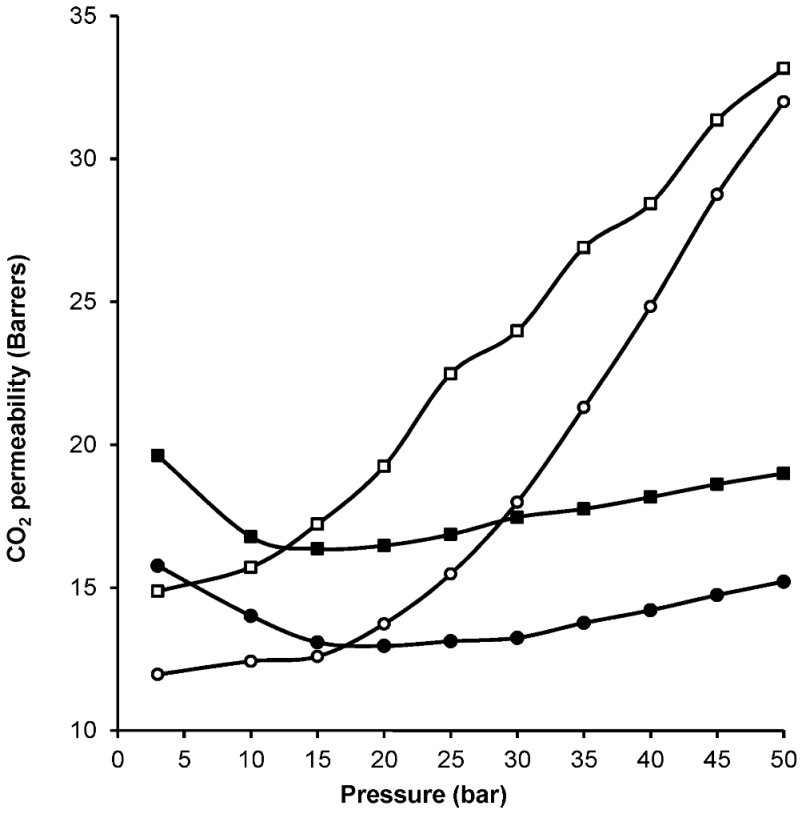
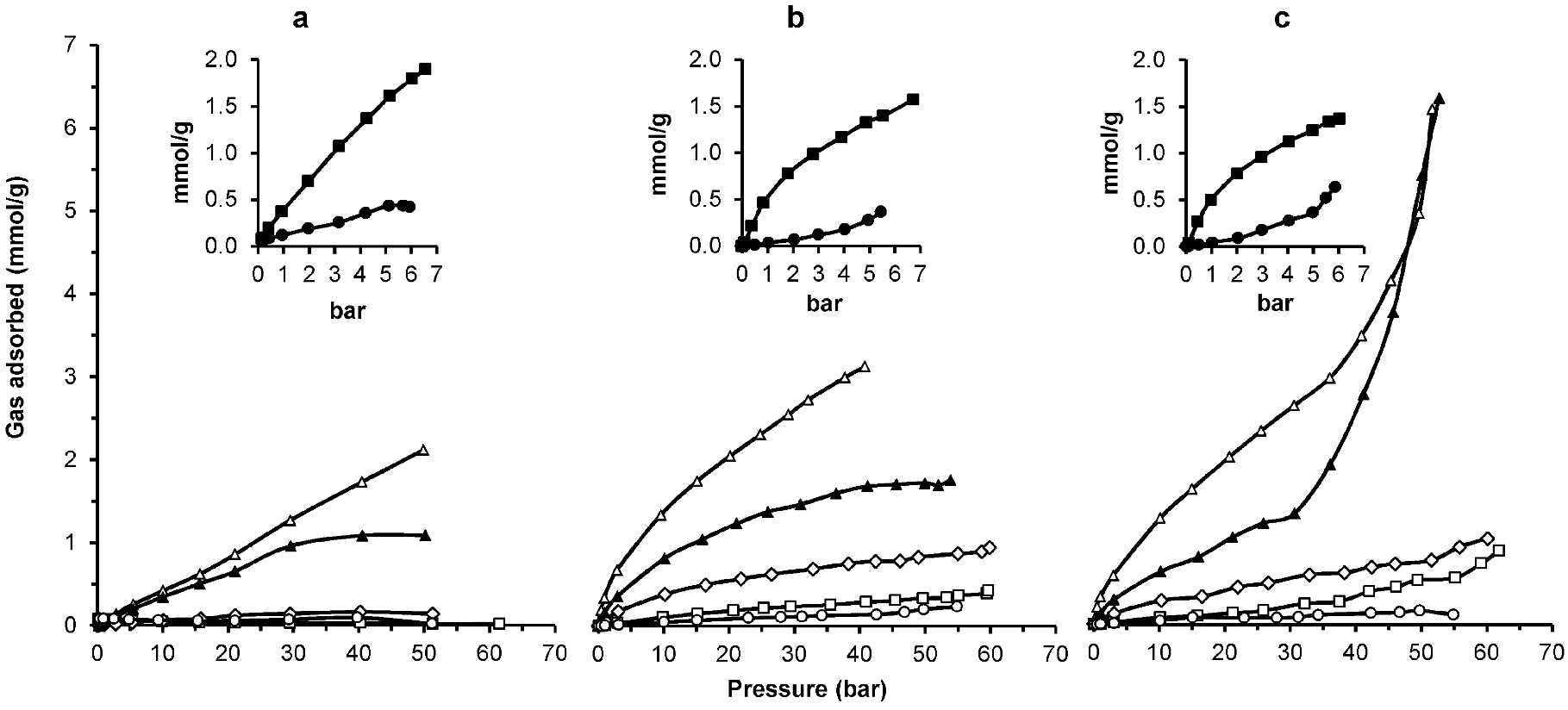
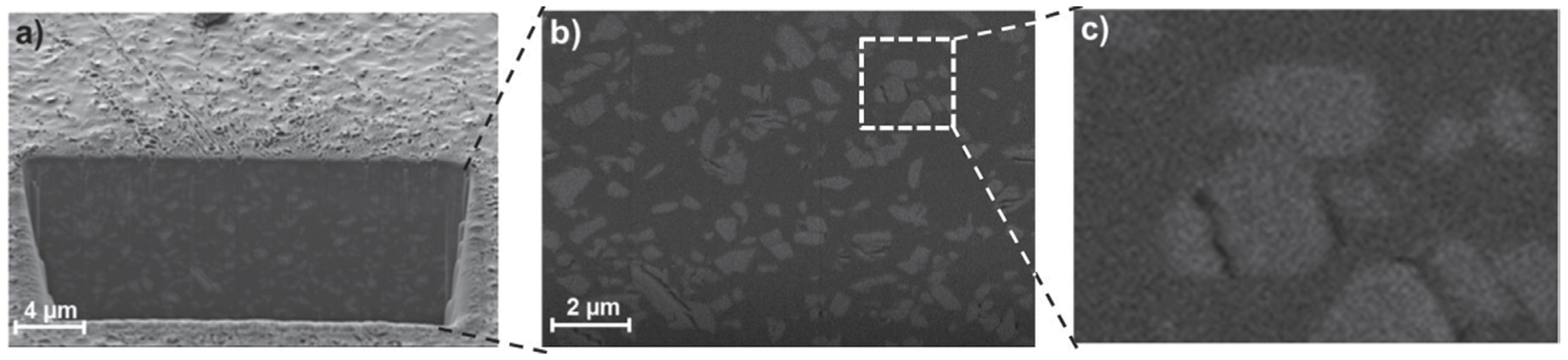

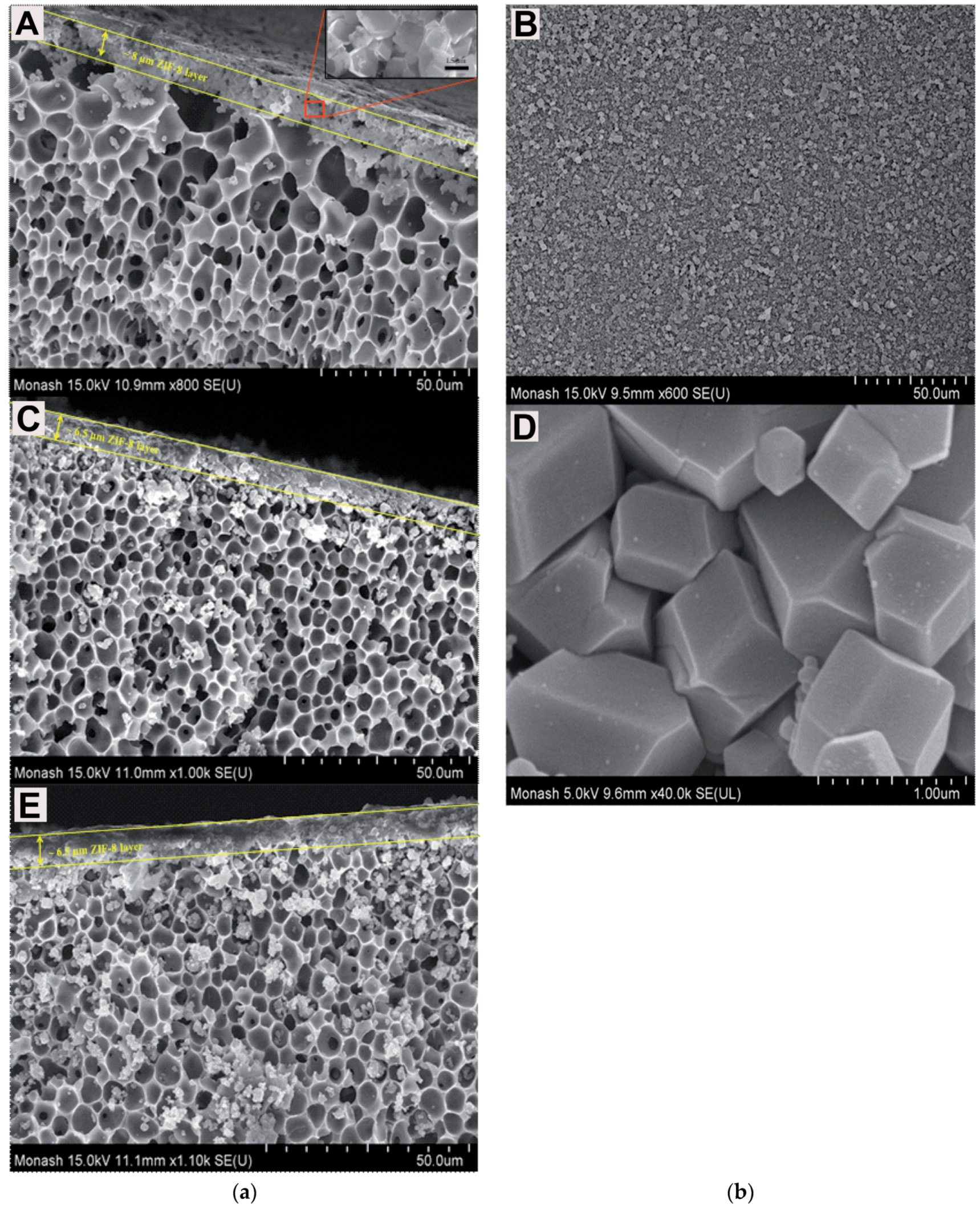
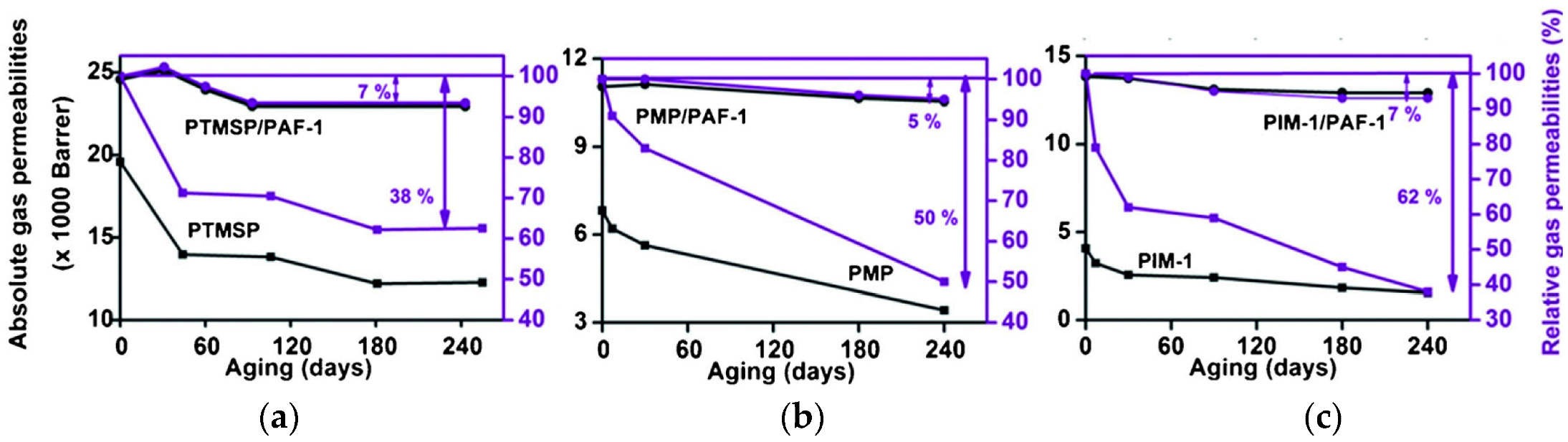
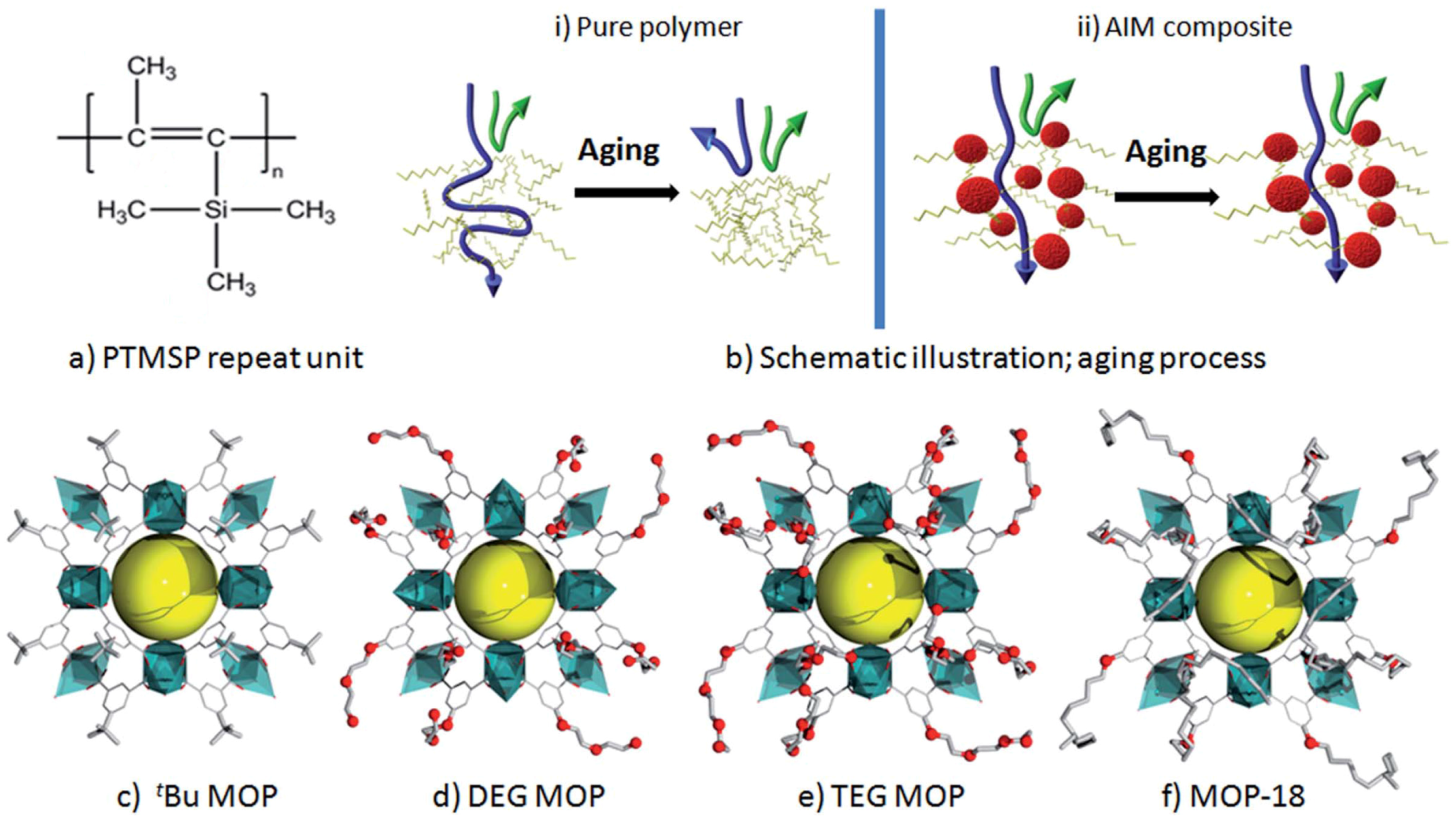
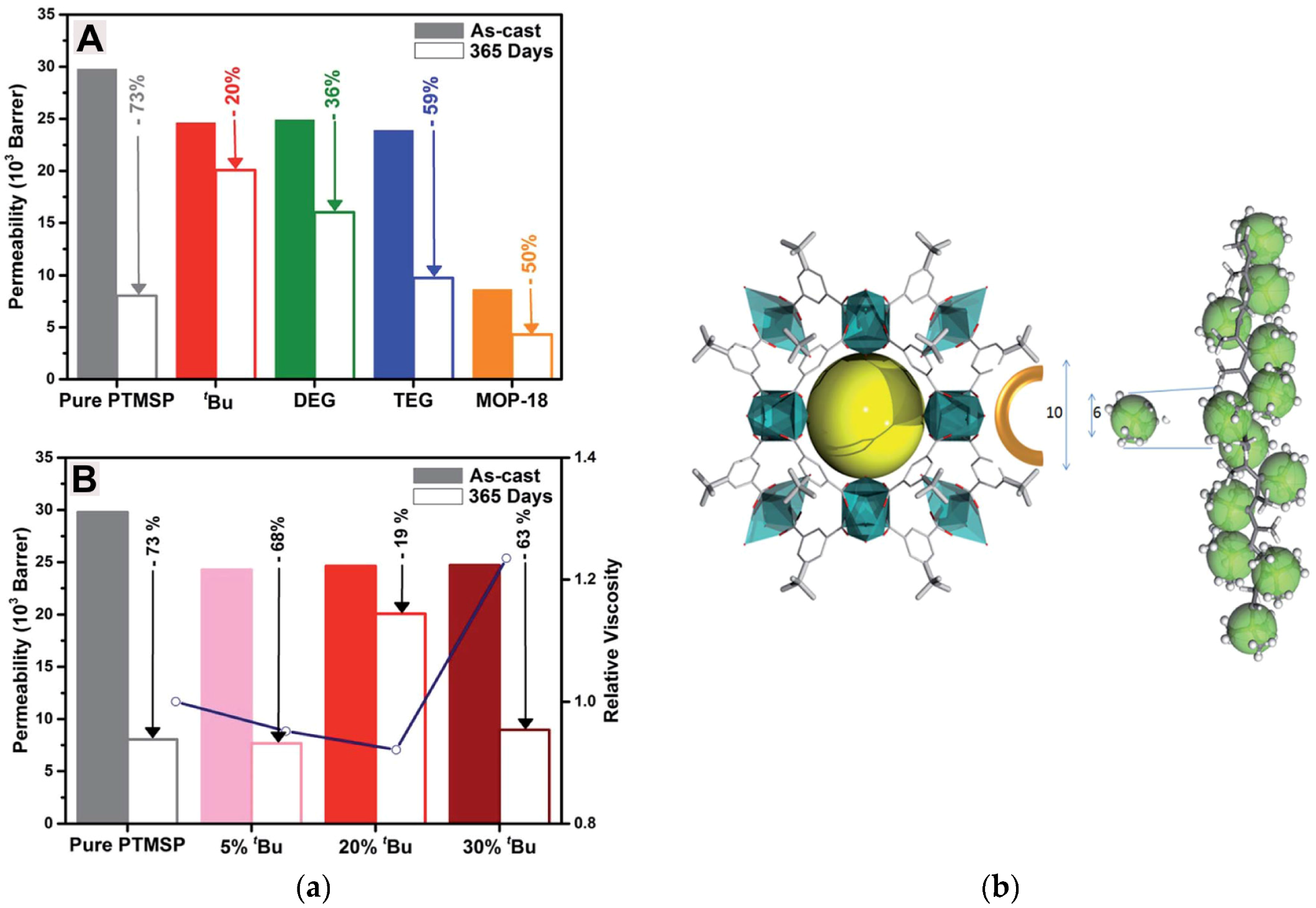
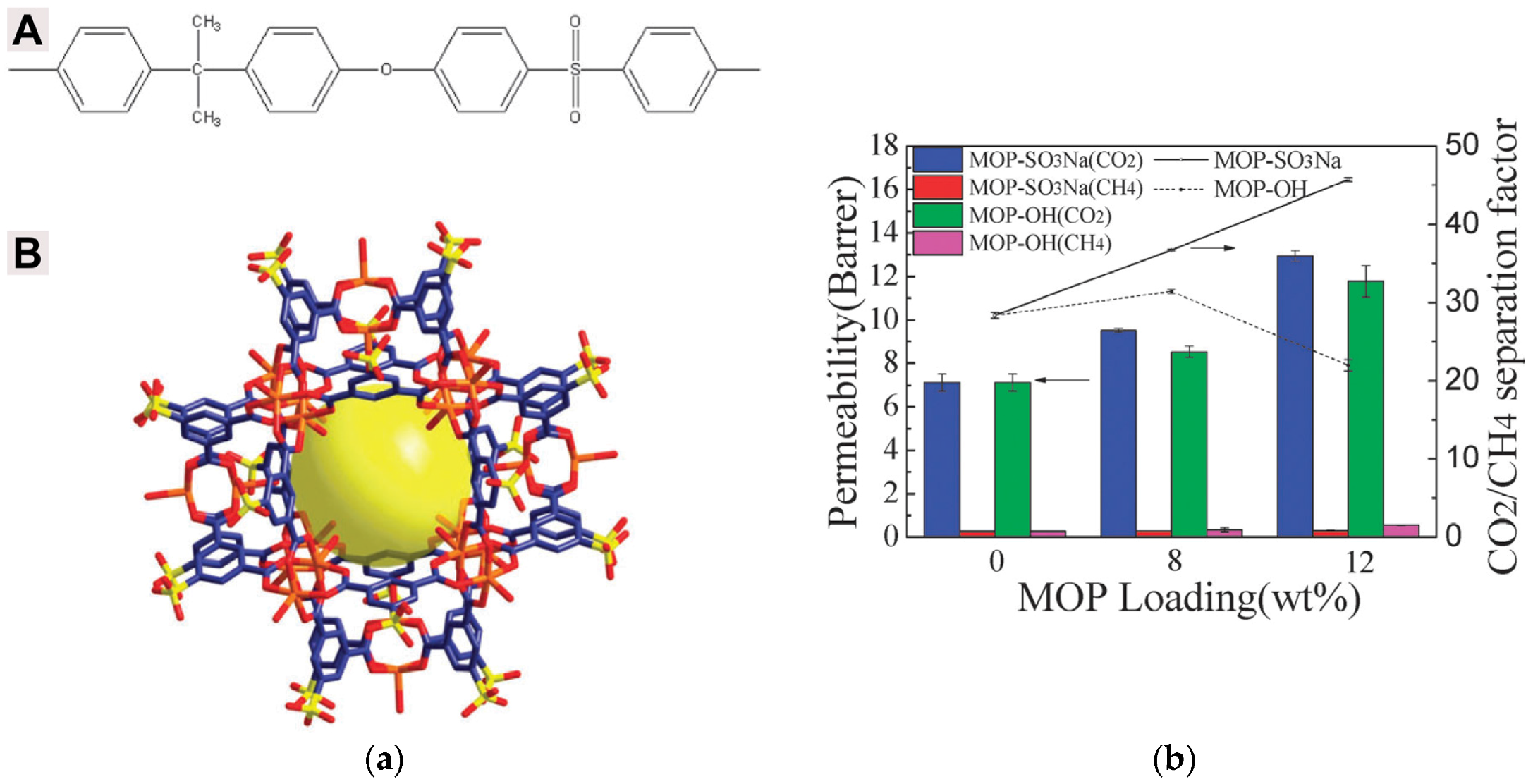
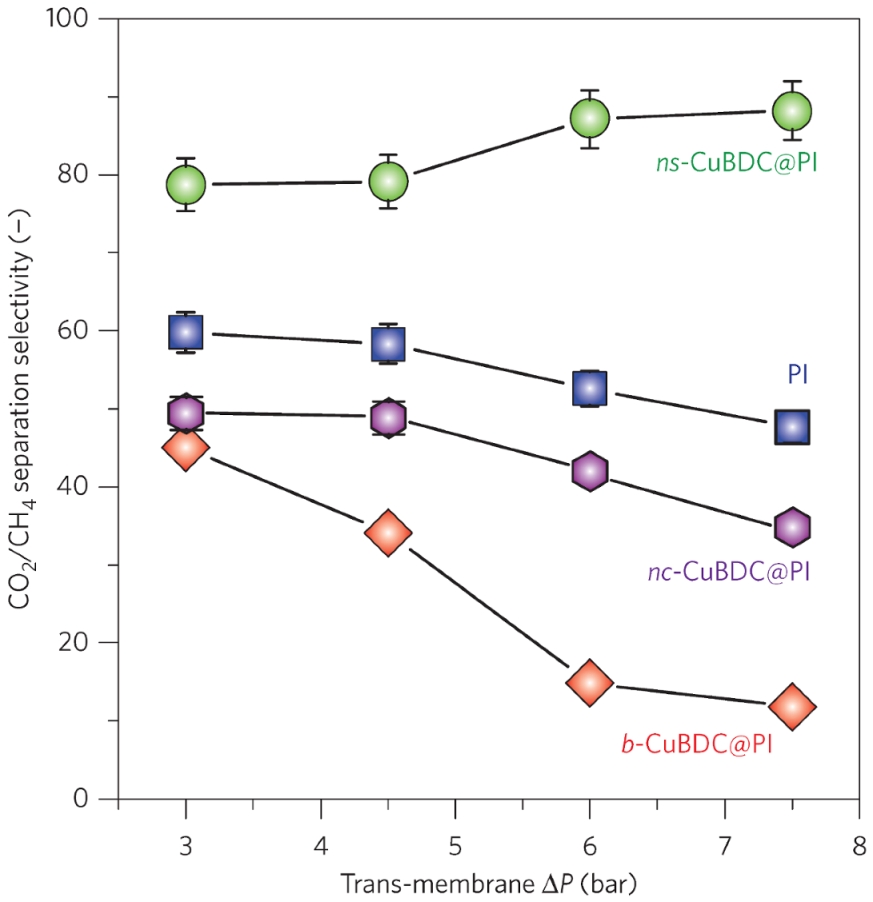
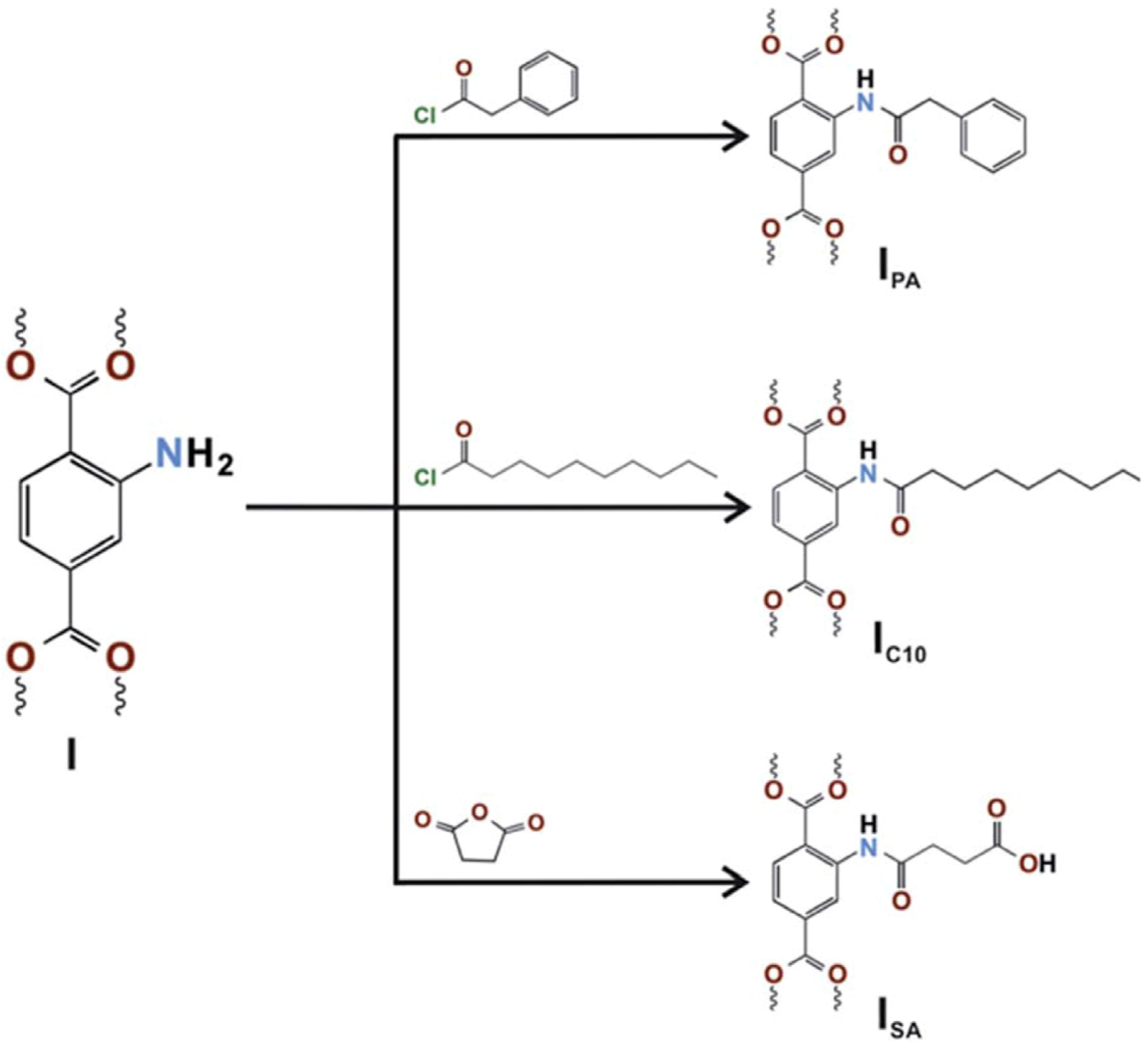
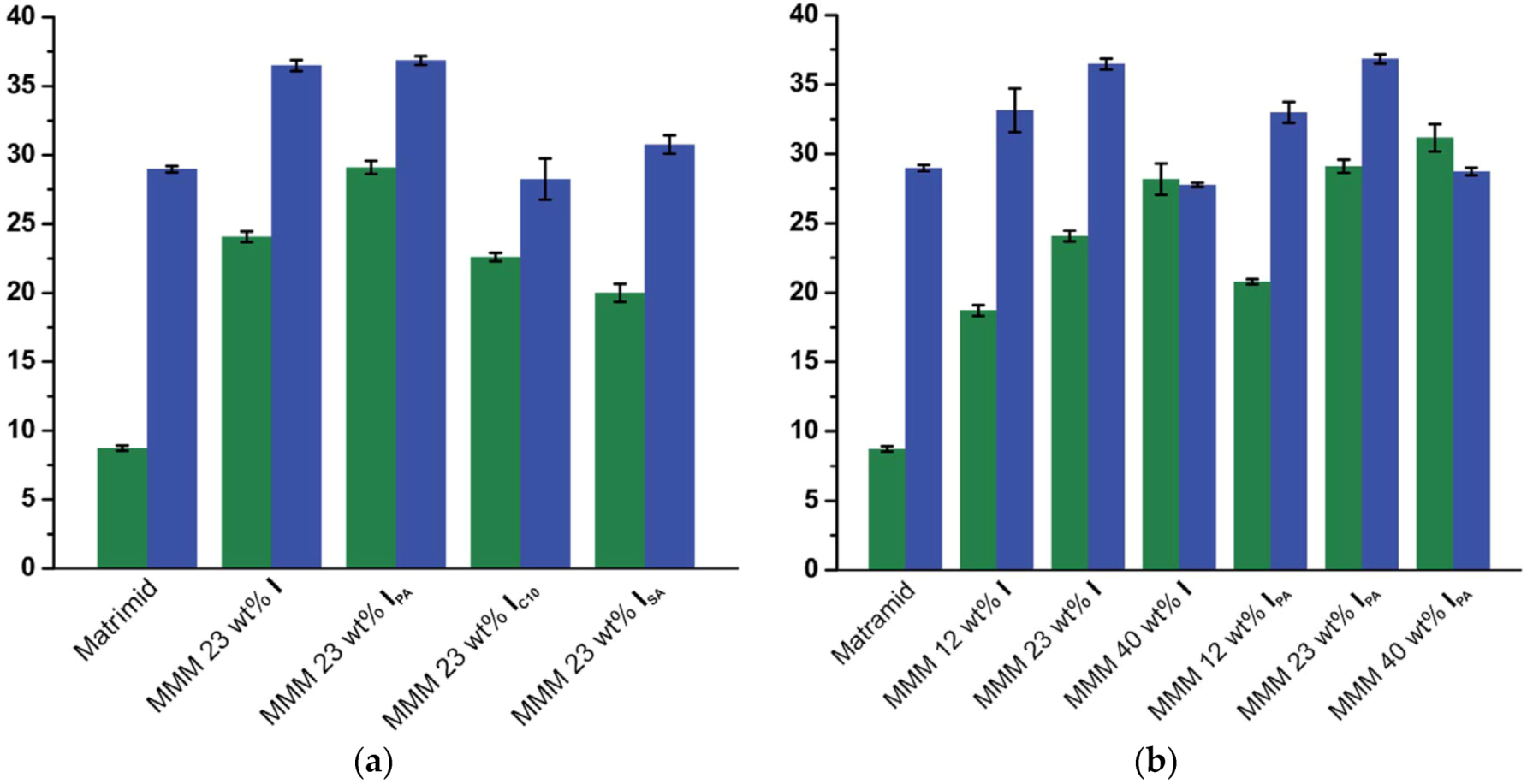
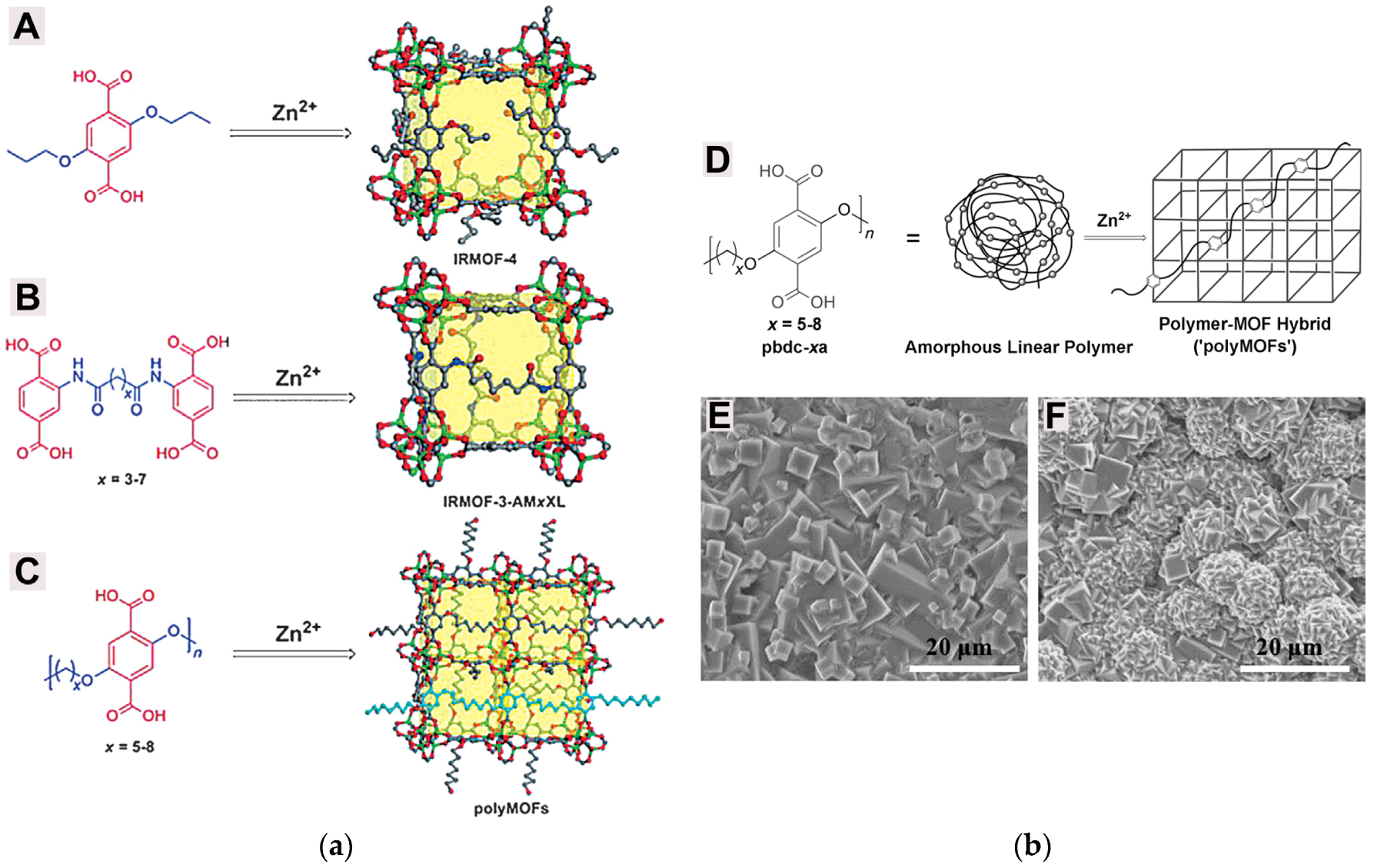
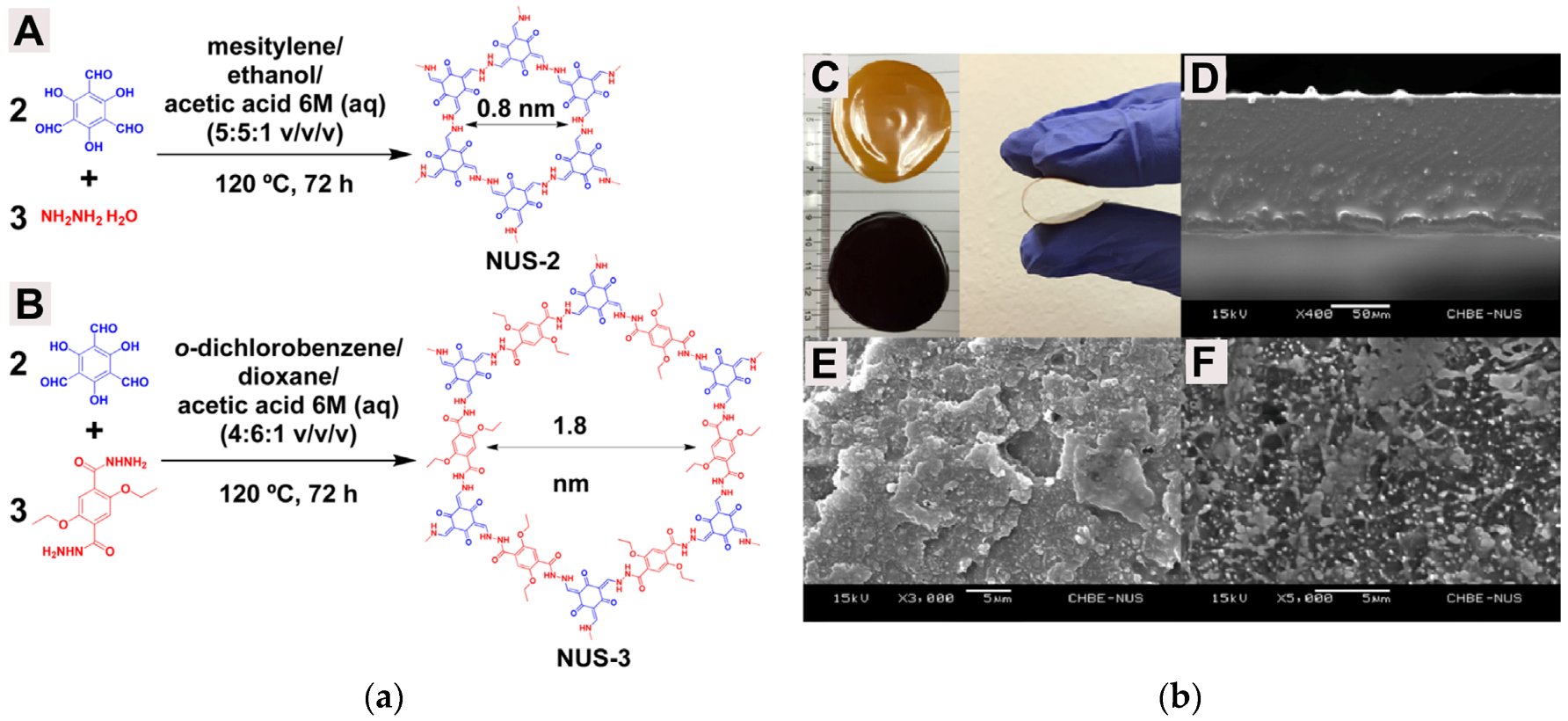
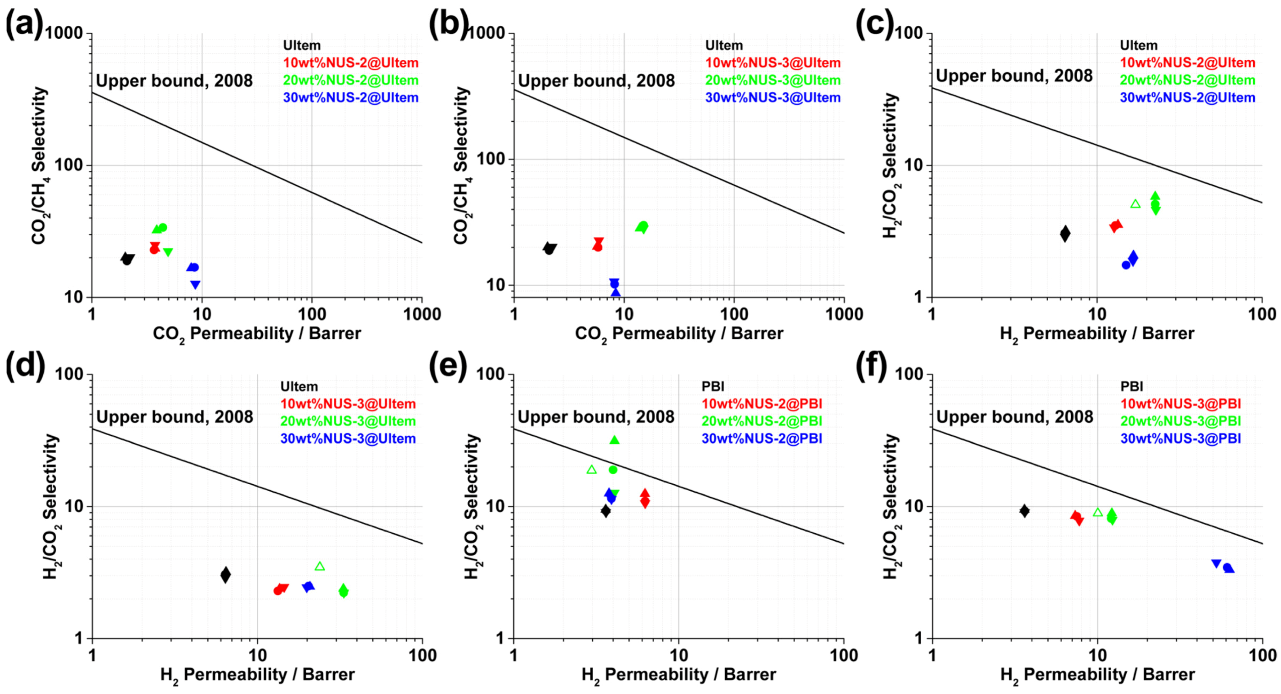
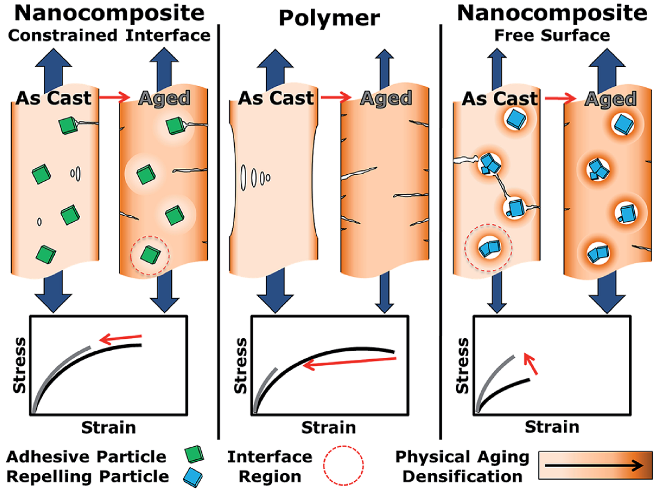
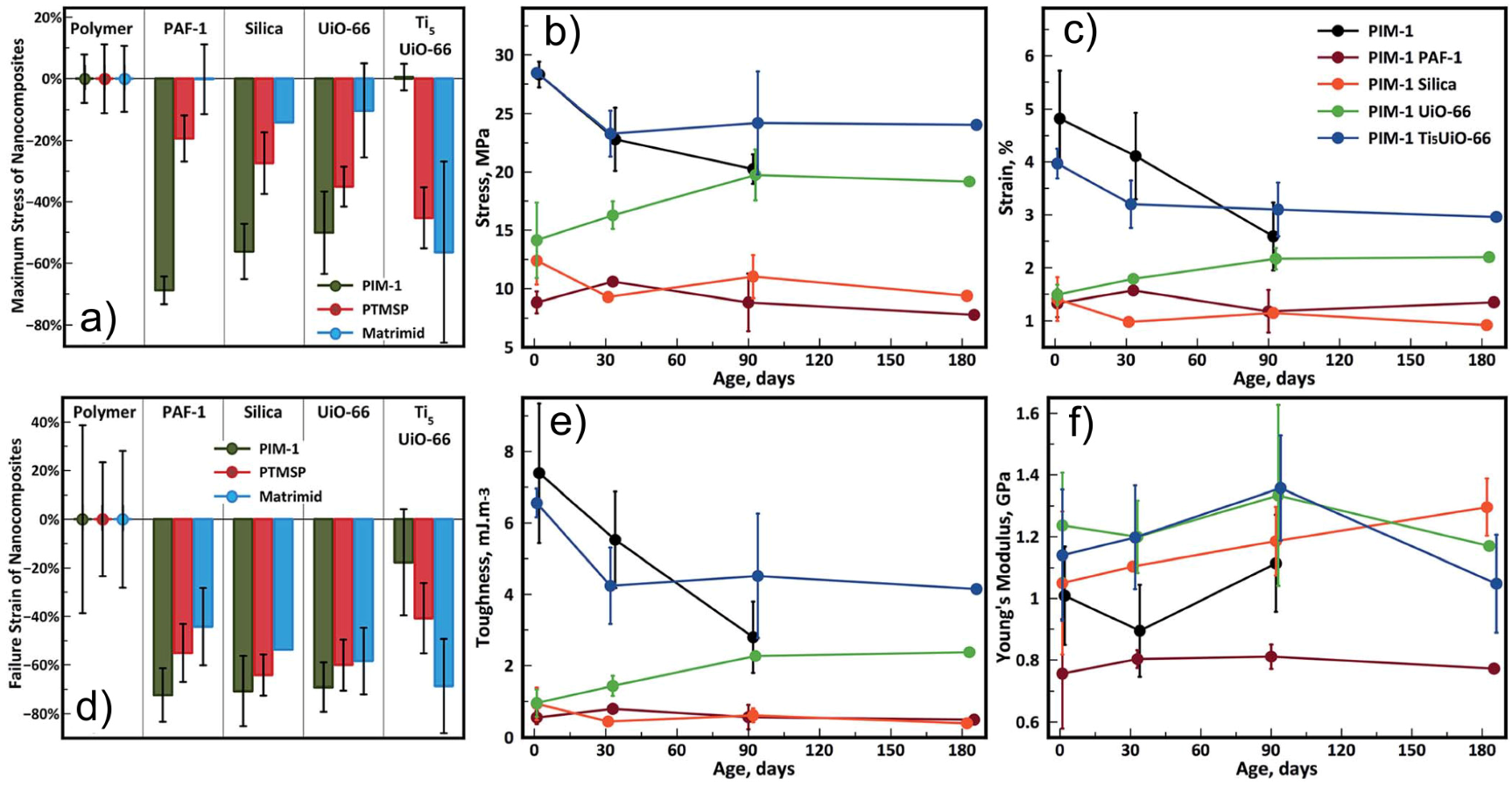

| Gas | Kinetic Diameter (Å) | Critical Temperature (°C) | Critical Pressure (Bar) | Vapor Pressure (Bar at (°C)) |
|---|---|---|---|---|
| C3H6 | 4.5 | 91.75 | 45.55 | 8.4 (21.1) |
| C3H8 | 4.3 | 96.74 | 42.51 | 8.0 (21.0) |
| CH4 | 3.8 | −82.59 | 45.99 | - |
| CO | 3.76 | −140.45 | 34.94 | - |
| N2 | 3.64 | −146.95 | 33.51 | - |
| O2 | 3.46 | −118.55 | 49.7 | - |
| CO2 | 3.3 | 30.98 | 73.0 | 60 (22.4) |
| H2 | 2.89 | −232.60 | 12.69 | 0.025 (21.0) |
| H2O | 2.65 | 373.80 | 217.7 | 8.76 (21.0) |
| NH3 | 2.6 | 132.25 | 113.3 | - |
| He | 2.6 | −267.96 | 2.24 | - |
| ZIF-8 (vol %) | Permeability (Barrer) | Selectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | He | O2 | N2 | CO2 | CH4 | H2/N2 | H2/CH4 | He/N2 | O2/N2 | CO2/CH4 | |
| 0 | 1630 | 760 | 580 | 180 | 4390 | 310 | 9.1 | 5.3 | 4.2 | 3.2 | 14.2 |
| 11 | 2560 | 1310 | 820 | 250 | 4815 | 320 | 10.2 | 8.0 | 5.2 | 3.3 | 15.0 |
| 28 | 2980 | 1430 | 870 | 195 | 4270 | 230 | 15.2 | 13.0 | 7.3 | 4.5 | 18.6 |
| 36 | 5745 | 2930 | 1640 | 380 | 6820 | 510 | 15.1 | 11.3 | 7.7 | 4.3 | 13.4 |
| 43 | 6680 | 3180 | 1680 | 350 | 6300 | 430 | 19.1 | 15.5 | 9.1 | 4.8 | 14.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, E.V.; Karunaweera, C.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Origins and Evolution of Inorganic-Based and MOF-Based Mixed-Matrix Membranes for Gas Separations. Processes 2016, 4, 32. https://doi.org/10.3390/pr4030032
Perez EV, Karunaweera C, Musselman IH, Balkus KJ, Ferraris JP. Origins and Evolution of Inorganic-Based and MOF-Based Mixed-Matrix Membranes for Gas Separations. Processes. 2016; 4(3):32. https://doi.org/10.3390/pr4030032
Chicago/Turabian StylePerez, Edson V., Chamaal Karunaweera, Inga H. Musselman, Kenneth J. Balkus, and John P. Ferraris. 2016. "Origins and Evolution of Inorganic-Based and MOF-Based Mixed-Matrix Membranes for Gas Separations" Processes 4, no. 3: 32. https://doi.org/10.3390/pr4030032