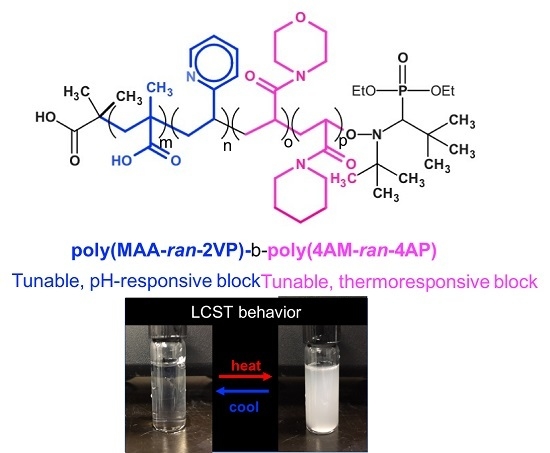
Poly(methacrylic acid-ran-2-vinylpyridine) Statistical Copolymer and Derived Dual pH-Temperature Responsive Block Copolymers by Nitroxide-Mediated Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Statistical Copolymerization of tert-Butyl Methacrylate (tBMA) and 2-Vinylpyridine (2VP)
2.1.2. Chain Extension of Poly(tert-butyl methacrylate-ran-2-vinylpyridine)(tBMA-ran-2VP) Macroinitiator with 4-acryloylmorpholine/4acryloylpiperidine (4AM/4AP) Mixtures
2.1.3. Hydrolysis of tert-Butyl Groups in the tBMA/2VP Statistical Copolymers
2.1.4. Gel Permeation Chromatography
2.1.5. Titration of MAA/2VP Statistical Copolymers
2.1.6. Particle Size Measurements of the Aqueous Solutions of MAA/2VP Statistical Copolymers
3. Results
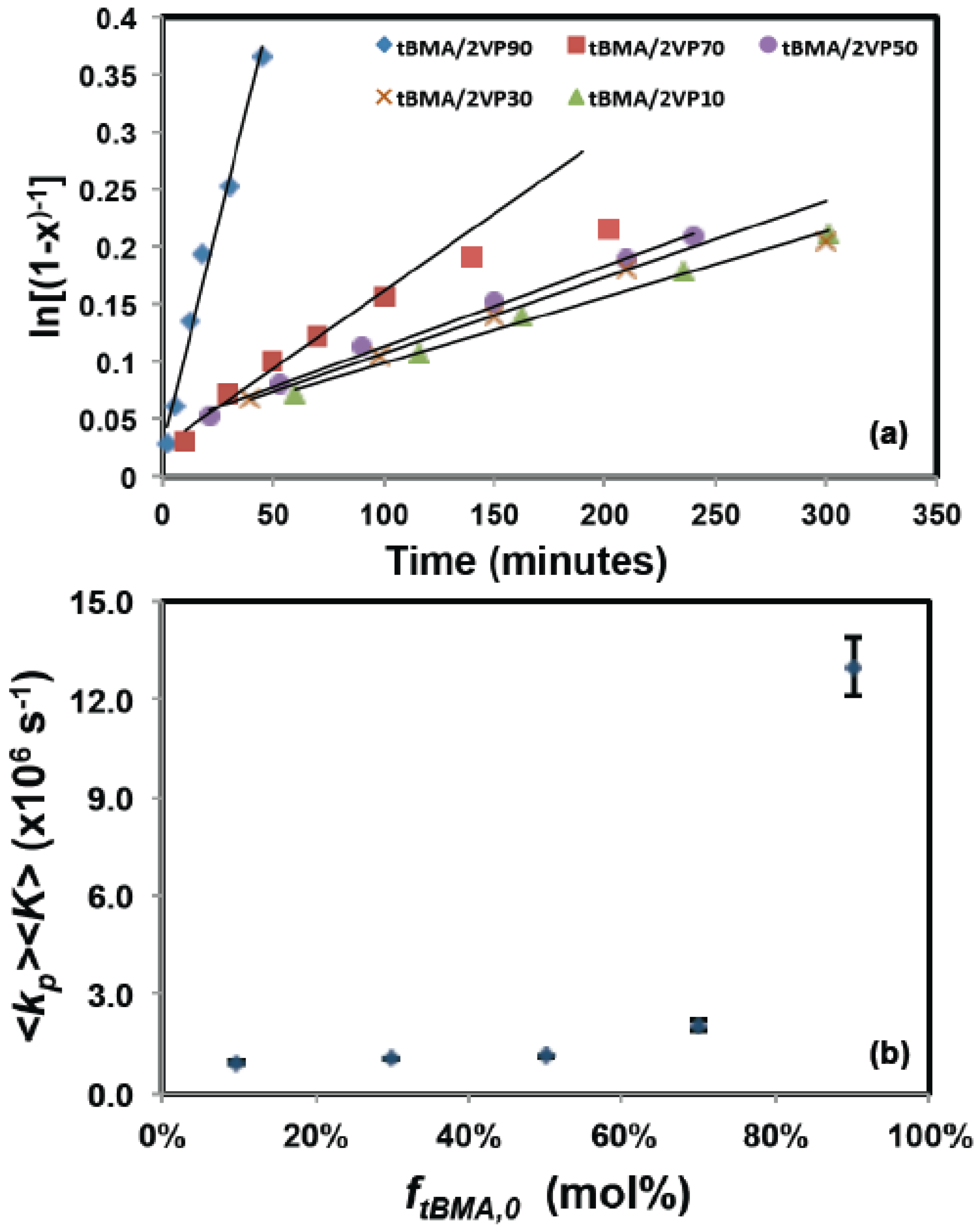
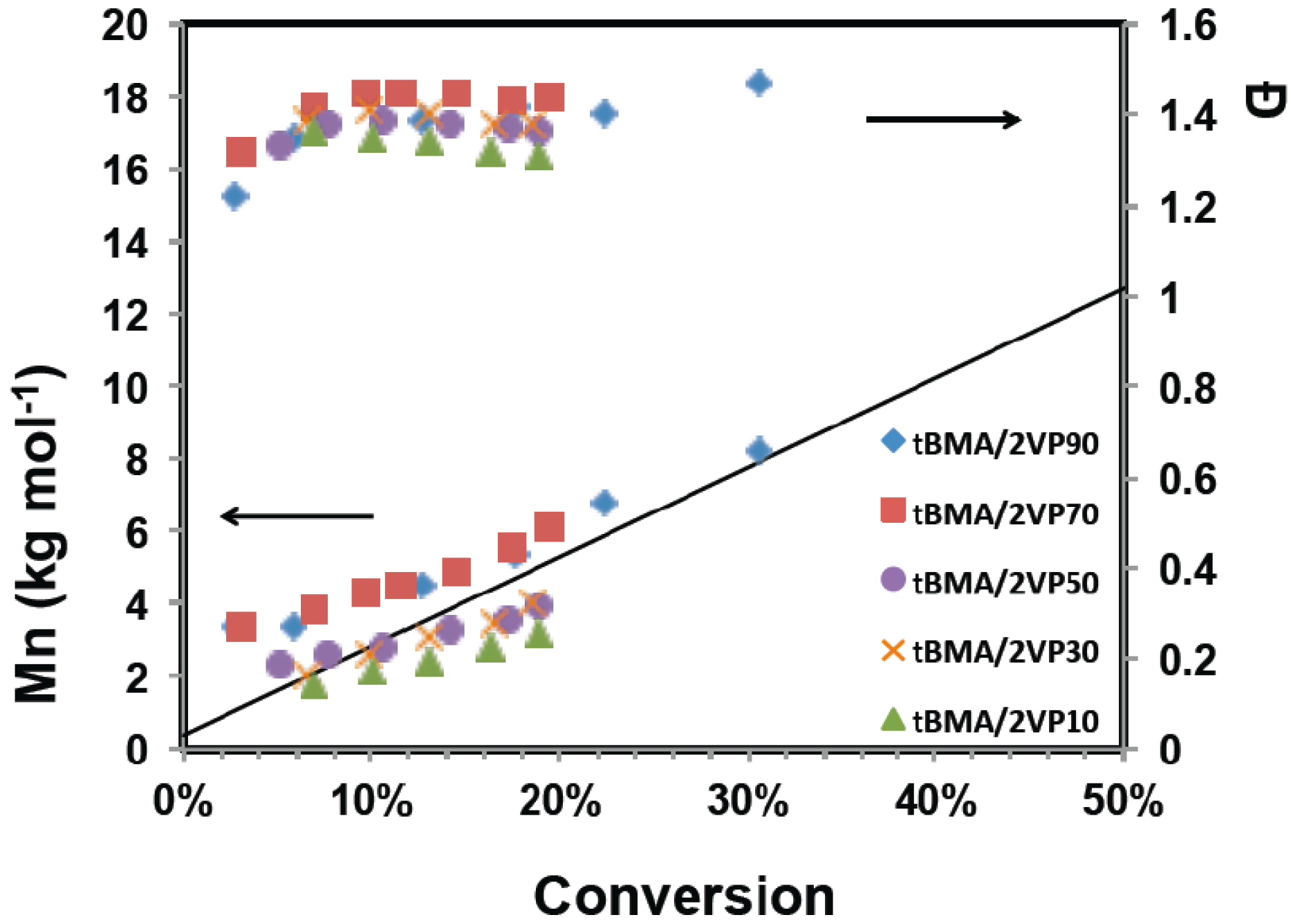
3.1. Statistical Copolymerization of tert-Butyl Methacrylate (tBMA) and 2-Vinylpyridine (2VP) Using NHS-BlocBuilder
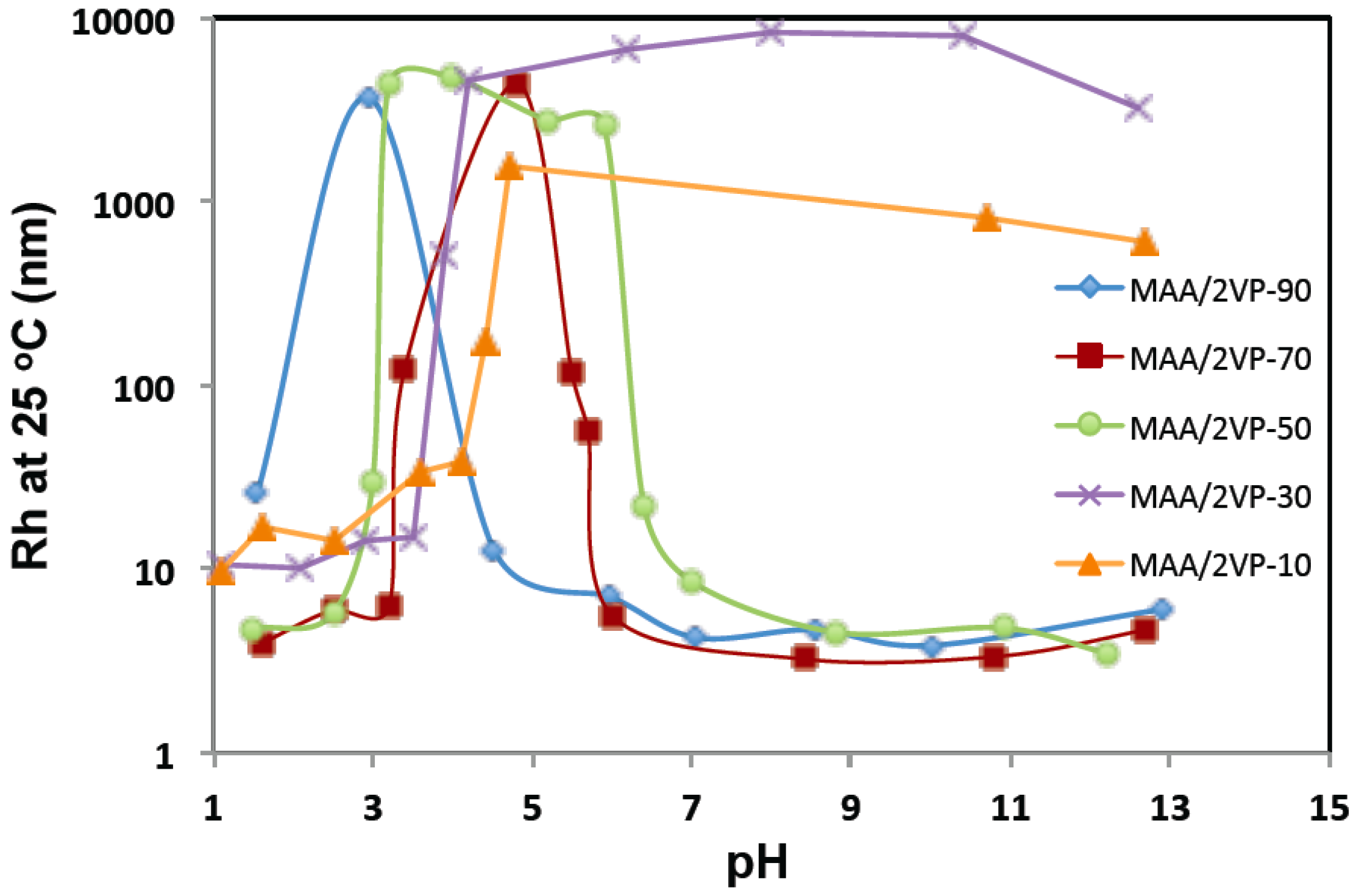
3.2 pH Sensitivity of the Methacrylic Acid/2-Vinyl Pyridine (MAA/2VP) Copolymers
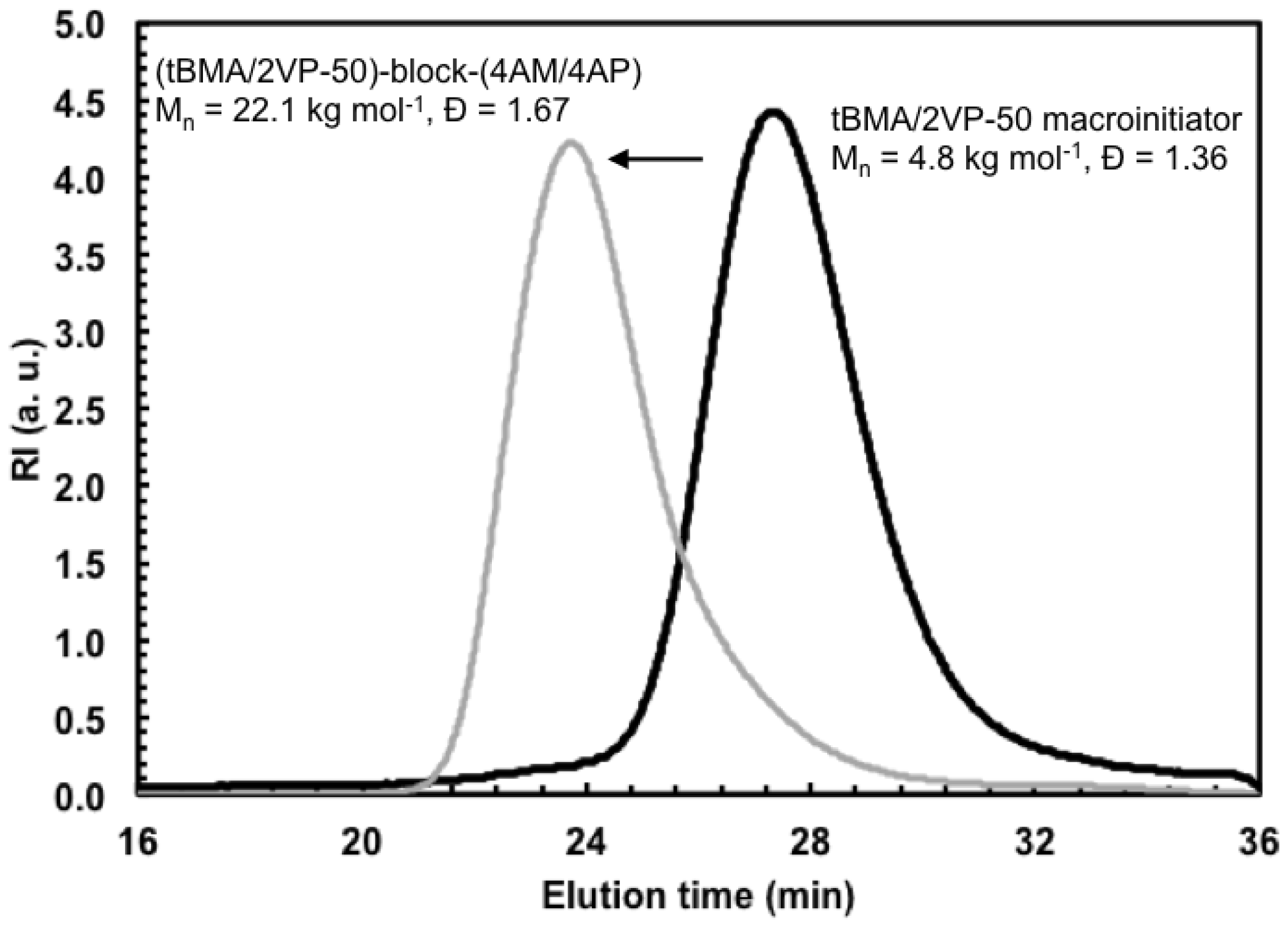
3.3. Chain Extension of Poly(tert-butyl methacrylate (tBMA)/2-vinylpyridine (2VP)) Macroinitiator with a Mixture of 4-acryloylmorpholine (4AM)/4-acryloylpiperidine (4AP)
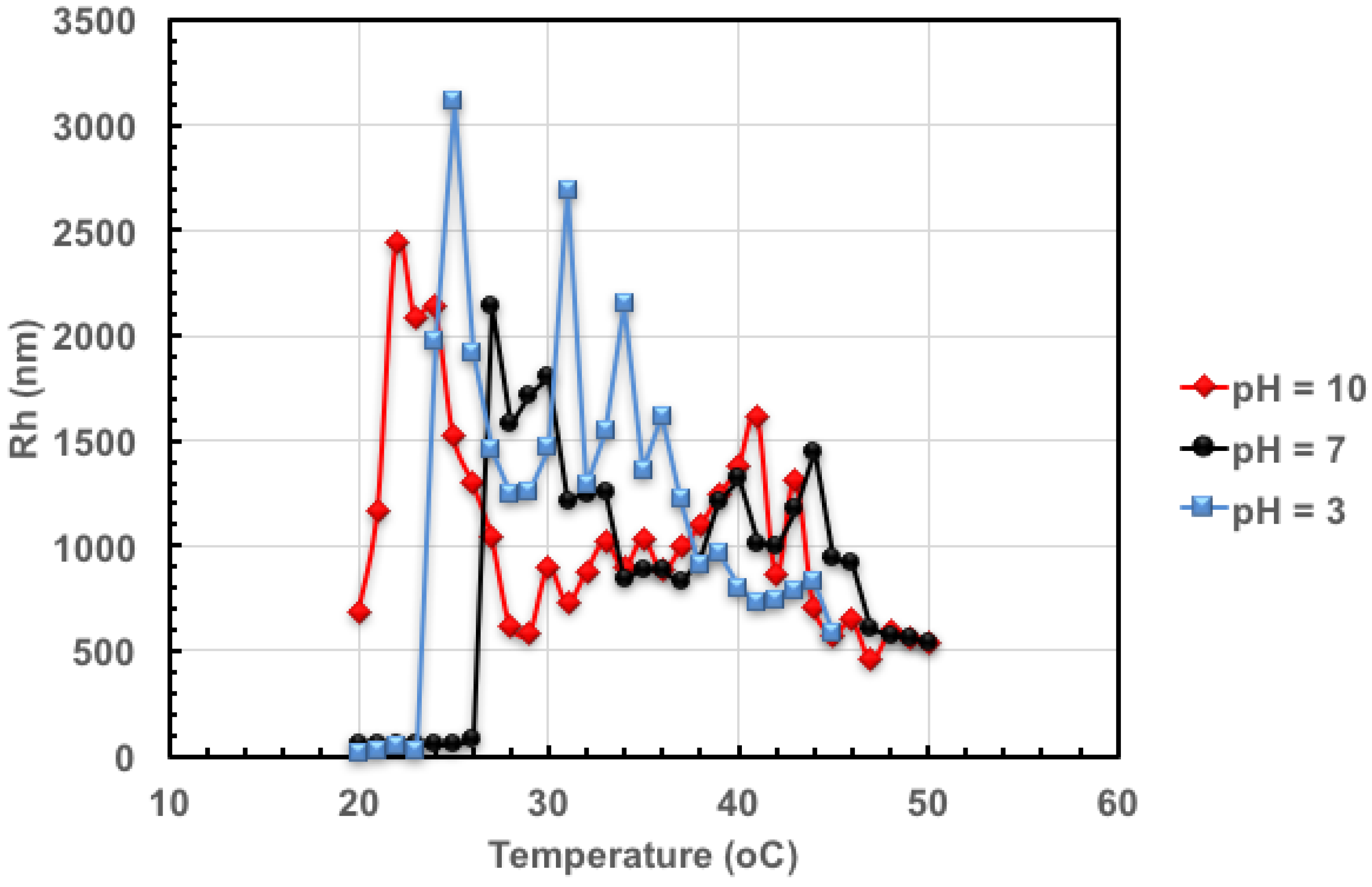
3.4. Cloud Point Temperature Measurement of Poly(methacrylic acid-stat-2-vinyl pyridine)-b-poly(4-acryloylmorpholine-stat-4-acryloylpiperidine) (Poly(MAA-stat-2VP)-b-poly(4AM-stat-4AP)) Block Copolymer at Various pH
4. Discussion
4.1 Nitroxide Mediated Polymerization
4.2 pH Sensitivity of the Methacrylic Acid/2-Vinyl Pyridine (MAA/2VP) Copolymers
4.3 Block Copolymers with Both pH and Temperature Sensitivities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, T., Jr.; Morawetz, W. Amphoteric Polyelectrolytes. I. 2-Vinylpyridine—Methacrylic Acid Copolymers 1, 2. J. Am. Chem. Soc. 1952, 74, 436–438. [Google Scholar] [CrossRef]
- Ehrlich, P.; Doty, J. The Solution Behavior of a Polymeric Ampholyte1. J. Am. Chem. Soc. 1954, 76, 3764–3777. [Google Scholar] [CrossRef]
- Tan, B.H.; Ravi, P.; Tan, L.N.; Tam, K.C. Synthesis and aqueous solution properties of sterically stabilized pH-responsive polyampholyte microgels. J. Colloid Interface Sci. 2007, 309, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, T.; Pinner, S.H. Preparation and titration of amphoteric polyelectrolytes. J. Polym. Sci. 1957, 23, 533–547. [Google Scholar] [CrossRef]
- Kamachi, M.; Kurihara, M.; Stille, J.K. Synthesis of block polymers for desalination membranes. Preparation of block copolymers of 2-vinylpyridine and methacrylic acid or acrylic acid. Macromolecules 1972, 5, 161–167. [Google Scholar] [CrossRef]
- Kurihara, M.; Kamachi, M.; Stille, J.K. Synthesis of ionic block polymers for desalination membranes. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 587–610. [Google Scholar] [CrossRef]
- Varoqui, R.; Tran, Q.; Pefferkorn, E. Polycation-polyanion complexes in the linear diblock copolymer of poly(styrene sulfonate)/poly(2-vinylpyridinium) salt. Macromolecules 1979, 12, 831–835. [Google Scholar] [CrossRef]
- Gotzamanis, G.; Tsitsilianis, C. Stimuli-responsive A-b-(B-co-C) diblock terpolymers bearing polyampholyte sequences. Macromol. Rapid. Commun. 2006, 27, 1757–1763. [Google Scholar] [CrossRef]
- André, X.; Zhang, M.; Müller, A.H.E. Thermo- and pH-responsive micelles of poly(acrylic acid)-block-poly (N,N-diethylacrylamide). Macromol. Rapid Commun. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Stavrouli, N.; Bocharova, V.; Angelopoulos, S.; Kiriy, A.; Katsampas, I.; Stamm, M. Stimuli responsive associative polyampholytes based on ABCBA pentablock terpolymer architecture. Polymer 2008, 49, 2996–3006. [Google Scholar] [CrossRef]
- Bütün, V.; Billingham, N.C.; Armes, S.P. Unusual aggregation behavior of a novel tertiary amine methacrylate-based diblock copolymer: Formation of micelles and reverse micelles in aqueous solution. J. Am. Chem. Soc. 1998, 120, 11818–11819. [Google Scholar] [CrossRef]
- Webster, O.W. The discovery and commercialization of group transfer polymerization. J. Polym. Sci. A Polym. Chem. 2000, 38, 2855–2860. [Google Scholar] [CrossRef]
- Bütün, V.; Weaver, J.V.M.; Bories-Azeau, X.; Cai, Y.; Armes, S.P. A brief review of ‘schizophrenic’ block copolymers. React. Funct. Polym. 2006, 66, 157–165. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Hertler, W.R.; Abbott, N.L.; Hatton, T.A. Diblock, Abc triblock, and random methacrylic polyampholytes-synthesis by group-transfer polymerization and solution behavior. Macromolecules 1994, 27, 930–937. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Alexandridis, P.; Su, C.-K.; Patrickios, C.S.; Hertler, W.R.; Hatton, T.A. Effect of block size and sequence on the micellization of ABC triblock methacrylic polyampholytes. Macromolecules 1995, 28, 8604–8611. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Polyampholytes. J. Polym. Sci. B Polym. Phys. 2004, 42, 3513–3538. [Google Scholar] [CrossRef]
- Kudabergnor, S. Polyampholytes. In Encyclopedia of Polymer Science and Technology; Mark, H.F., Ed.; Wiley: New York, NY, USA, 2014; Volume 10, pp. 297–325. [Google Scholar]
- Hadjichristidis, N.; Pispas, S.; Floudas, G.A. Block Copolymers: Synthetic Strategies, Physical Properties and Applications; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Georges, M.K.; Veregin, R.P.N.; Kazmaier, P.M.; Hamer, G.K. Narrow molecular weight distribution resins by a free radical polymerization process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine) ruthenium (II)/methylaluminum bis (2, 6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. ontrolled/"living" radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Čadová, E.; Konečný, J.; Kříž, J.; Svitáková, R.; Holler, P.; Genzer, J.; Vlček, P. ATRP of 2-vinylpyridine and tert-butyl acrylate mixtures giving precursors of polyampholytes. J. Polym. Sci. A Polym. Chem. 2010, 48, 735–741. [Google Scholar] [CrossRef]
- Jhon, Y.K.; Arifuzzaman, S.; Ozcam, A.E.; Kiserow, D.J.; Genzer, J. Formation of polyampholyte brushes via controlled radical polymerization and their assembly in solution. Langmuir 2012, 28, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Armes, S.P. Synthesis and aqueous solution behavior of a pH-responsive schizophrenic diblock copolymer. Langmuir 2003, 19, 4432–4438. [Google Scholar] [CrossRef]
- Cai, Y.; Armes, S.P. A zwitterionic ABC triblock copolymer that forms a “trinity” of micellar aggregates in aqueous solution. Macromolecules 2004, 37, 7116–7122. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Yang, S.; Xu, J. “Schizophrenic” micellization of poly(acrylic acid)-b-poly(2-dimethylamino)ethyl methacrylate and responsive behavior of the micelles. Soft Mater. 2013, 11, 394–402. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; Kirkland-York, S.E.; Savin, D.A.; McCormick, C.L. “Schizophrenic” self-assembly of block copolymers synthesized via aqueous RAFT polymerization: From micelles to vesicles. Paper number 143 in a series on water-soluble polymers. Macromolecules 2010, 43, 1210–1217. [Google Scholar] [CrossRef]
- Du, J.; O’Reilly, R.K. pH-responsive vesicles from a schizophrenic diblock copolymer. Macromol. Chem. Phys. 2010, 211, 1530–1537. [Google Scholar] [CrossRef]
- Savoji, M.T.; Strandman, S.; Zhu, X.X. Switchable vesicles formed by diblock random copolymers with tunable pH-and thermo-responsiveness. Langmuir 2013, 29, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.; Grimaldi, S.; Robin, S.; Finet, J.-P.; Tordo, P.; Gnanou, Y. Kinetics and mechanism of controlled free-radical polymerization of styrene and n-butyl acrylate in the presence of an acyclic β-phosphonylated nitroxide. J. Am. Chem. Soc. 2000, 122, 5929–5939. [Google Scholar] [CrossRef]
- Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C.J. Development of a universal alkoxyamine for “living” free radical polymerizations. J. Am. Chem. Soc. 1999, 121, 3904–3920. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E. The History of Nitroxide Mediated Polymerization. In Nitroxide Mediated Polymerization: From Fundamentals to Applications in Materials Science; Gigmes, D., Ed.; Royal Society of Chemistry: London, UK, 2015; pp. 1–44. [Google Scholar]
- Magee, C.; Sugihara, Y.; Zetterlund, P.B.; Aldabbagh, F. Chain transfer to solvent in the radical polymerization of structurally diverse acrylamide monomers using straight-chain and branched alcohols as solvents. Polym. Chem. 2014, 5, 2259–2265. [Google Scholar] [CrossRef]
- Götz, H.; Harth, E.; Schiller, S.M.; Frank, C.W.; Knoll, W.; Hawker, C.J. Synthesis of lipo-glycopolymer amphiphiles by nitroxide-mediated living free-radical polymerization. J. Polym. Sci. A Polym. Chem. 2002, 40, 3379–3391. [Google Scholar] [CrossRef]
- Schierholz, K.; Givehchi, M.; Fabre, P.; Nallet, F.; Papon, E.; Guerret, O.; Gnanou, Y. Acrylamide-based amphiphilic block copolymers via nitroxide-mediated radical polymerization. Macromolecules 2003, 36, 5995–5999. [Google Scholar] [CrossRef]
- Phan, T.N.T.; Maiez-Tribut, S.; Pascault, J.-P.; Bonnet, A.; Gerard, P.; Guerret, O.; Bertin, D. Synthesis and characterizations of block copolymer of poly(n-butyl acrylate) and gradient poly(methyl methacrylate-co-N,N-dimethyl acrylamide) made via nitroxide-mediated controlled radical polymerization. Macromolecules 2007, 40, 4516–4523. [Google Scholar] [CrossRef]
- Delaittre, G.; Rieger, J.; Charleux, B. Nitroxide-mediated living/controlled radical polymerization of N,N-diethylacrylamide. Macromolecules 2011, 44, 462–470. [Google Scholar] [CrossRef]
- Shipman, P.O.; Cui, C.; Lupinska, P.; Lalancette, R.A.; Sheridan, J.B.; Jäkle, F. Nitroxide-mediated controlled free radical polymerization of the chelate monomer 4-styryl-tris(2-pyridyl)borate (StTpyb) and supramolecular assembly via metal complexation. ACS Macro Lett. 2013, 2, 1056–1060. [Google Scholar] [CrossRef]
- Lang, A.S.; Neubig, A.; Sommer, M.; Thalakkat, M. NMRP versus “Click” Chemistry for the Synthesis of Semiconductor Polymers Carrying Pendant Perylene Bisimides. Macromolecules 2010, 43, 7001–7010. [Google Scholar] [CrossRef]
- Deng, L.; Furuta, P.T.; Garon, S.; Li, J.; Kavulak, D.; Thompson, M.E.; Fréchet, J.M.J. Living radical polymerization of bipolar transport materials for highly efficient light emitting diodes. Chem. Mater. 2006, 18, 386–395. [Google Scholar] [CrossRef]
- Lessard, B.; Graffe, A.; Maric, M. Styrene/tert-butyl acrylate random copolymers synthesized by nitroxide-mediated polymerization: Effect of free nitroxide on kinetics and copolymer composition. Macromolecules 2007, 40, 9284–9292. [Google Scholar] [CrossRef]
- Lessard, B.; Schmidt, S.C.; Maric, M. Styrene/acrylic acid random copolymers synthesized by nitroxide-mediated polymerization: Effect of free nitroxide on kinetics and copolymer composition. Macromolecules 2008, 41, 3446–3454. [Google Scholar] [CrossRef]
- Charleux, B.; Nicolas, J.; Guerret, O. Theoretical expression of the average activation-deactivation equilibrium constant in controlled/living free-radical copolymerization operating via reversible termination. Application to a strongly improved control in nitroxide-mediated polymerization of methyl methacrylate. Macromolecules 2005, 38, 5485–5492. [Google Scholar]
- Nicolas, J.; Brusseau, S.; Charleux, B. A minimal amount of acrylonitrile turns the nitroxide-mediated polymerization of methyl methacrylate into an almost ideal controlled/living system. J. Polym. Sci. A Polym. Chem. 2010, 48, 34–47. [Google Scholar] [CrossRef]
- Dire, C.; Charleux, B.; Magnet, S.; Couvreur, L. Nitroxide-mediated copolymerization of methacrylic acid and styrene to form amphiphilic diblock copolymers. Macromolecules 2007, 40, 1897–1903. [Google Scholar] [CrossRef]
- Nicolas, J.; Couvreur, P.; Charleux, B. Comblike polymethacrylates with poly(ethylene glycol) side chains via nitroxide-mediated controlled free-radical polymerization. Macromolecules 2008, 41, 3758–3761. [Google Scholar] [CrossRef]
- Lessard, B.; Maric, M. Incorporating glycidyl methacrylate into block copolymers using poly(methacrylate-ran-styrene) macroinitiators synthesized by nitroxide-mediated polymerization. J. Polym. Sci. A Polym. Chem. 2009, 47, 2574–2588. [Google Scholar] [CrossRef]
- Lessard, B.; Tervo, C.; De Wahl, S.; Clerveaux, F.J.; Tang, K.K.; Yasmine, S.; Andjelic, S.; D’Alessandro, A.; Maric, M. Poly(tert-butyl methacrylate/styrene) macroinitiators as precursors for organo-and water-soluble functional copolymers using nitroxide-mediated controlled radical polymerization. Macromolecules 2010, 43, 868–878. [Google Scholar] [CrossRef]
- Moayeri, A.; Lessard, B.; Maric, M. Nitroxide mediated controlled synthesis of glycidyl methacrylate-rich copolymers enabled by SG1-based alkoxyamines bearing succinimidyl ester groups. Polym. Chem. 2011, 2, 1121–1129. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Min, E.-H.; Escale, P.; Save, M.; Billon, L.; Stenzel, M.H. Lectin recognizable biomaterials synthesized via nitroxide-mediated polymerization of a methacryloyl galactose monomer. Macromolecules 2009, 42, 9422–9434. [Google Scholar] [CrossRef]
- Lessard, B.H.; Ling, E.Y.J.; Maric, M. Fluorescent, thermoresponsive oligo(ethylene glycol) methacrylate/9-(4-vinylbenzyl)-9H-carbazole copolymers designed with multiple LCSTs via nitroxide mediated controlled radical polymerization. Macromolecules 2012, 45, 1879–1891. [Google Scholar] [CrossRef]
- Lessard, B.H.; Maric, M. “Smart” poly(dimethylaminoethyl methacrylate-ran-9-(4-vinylbenzyl)-9H-carbazole) copolymers synthesized by nitroxide mediated radical polymerization. J. Polym. Sci. A Polym. Chem. 2011, 49, 5270–5283. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Maric, M. Synthesis of narrow molecular weight distribution norbornene-lactone functionalized polymers by nitroxide-mediated polymerization: Candidates for 193 nm photoresist materials. Polymers 2014, 6, 565–577. [Google Scholar] [CrossRef]
- Chenal, M.; Mura, S.; Marchal, C.; Gigmes, D.; Charleux, B.; Fattal, E.; Couvreur, P.; Nicolas, J. Facile synthesis of innocuous comb-shaped polymethacrylates with PEG side chains by nitroxide-mediated radical polymerization in hydroalcoholic solutions. Macromolecules 2010, 43, 9291–9303. [Google Scholar] [CrossRef]
- Brusseau, S.; Belleney, J.; Magnet, S.; Couvreur, L.; Charleux, B. Nitroxide-mediated copolymerization of methacrylic acid with sodium 4-styrene sulfonate: Towards new water-soluble macroalkoxyamines for the synthesis of amphiphilic block copolymers and nanoparticles. Polym. Chem. 2010, 1, 720–729. [Google Scholar] [CrossRef]
- Zhang, C.; Maric, M. pH-and temperature-sensitive statistical copolymers poly[2-(dimethylamino) ethyl methacrylate-stat-2-vinylpyridine] with Functional succinimidyl-ester chain ends synthesized by nitroxide-mediated polymerization. J. Polym. Sci. A Polym. Chem. 2012, 50, 4341–4357. [Google Scholar] [CrossRef]
- Consolante, V.; Maric, M. Nitroxide-mediated polymerization of an organo-soluble protected styrene sulfonate: Development of homo and random copolymers. Macromol. React. Eng. 2011, 5, 575–586. [Google Scholar] [CrossRef]
- Couvreur, L.; Lefay, C.; Belleney, J.; Charleux, B.; Guerret, O.; Magnet, S. First nitroxide-mediated controlled free-radical polymerization of acrylic acid. Macromolecules 2003, 36, 8260–8267. [Google Scholar] [CrossRef]
- Couvreur, L.; Charleux, B.; Guerret, O.; Magnet, S. Direct synthesis of controlled poly(styrene-co-acrylic acid)s of various compositions by nitroxide-mediated random copolymerization. Macromol. Chem. Phys. 2003, 204, 2055–2063. [Google Scholar] [CrossRef]
- Savelyeva, X.; Li, L.; Bennett, I.; Maric, M. Stimuli-responsive 4-acryloylmorpholine/4-acryloylpiperidine copolymers via nitroxide mediated polymerization. 2016; Submitted. [Google Scholar]
- Vinas, J.; Chagneux, N.; Gigmes, D.; Trimaille, T.; Favier, A.; Bertin, D. SG1-based alkoxyamine bearing a N-succinimidyl ester: A versatile tool for advanced polymer synthesis. Polymer 2008, 49, 3639–3647. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Controll. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Darabi, A.; Shirin-Abadi, A.R.; Jessop, P.G.; Cunningham, M.F. Nitroxide-mediated polymerization of 2-(diethylamino)ethyl methacrylate (DEAEMA) in water. Macromolecules 2015, 48, 72–80. [Google Scholar] [CrossRef]
- Driva, P.; Bexis, P.; Pitsikalis, M. Radical copolymerization of 2-vinyl pyridine and oligo(ethylene glycol) methyl ether methacrylates: Monomer reactivity ratios and thermal properties. Eur. Polym. J. 2011, 47, 762–771. [Google Scholar] [CrossRef]
- Mori, H.; Müller, A.H.E. New polymeric architectures with (meth)acrylic acid segments. Prog. Polym. Sci. 2003, 28, 1403–1439. [Google Scholar] [CrossRef]
- Merle, Y. Synthetic polyampholytes. V: Influence of nearest-neighbor interactions on potentiometric curves. J. Phys. Chem. 1987, 91, 3092–3098. [Google Scholar] [CrossRef]
- Ho, B.S.; Tan, B.H.; Tan, J.P.K.; Tam, K.C. Inverse microemulsion polymerization of sterically stabilized polyampholyte microgels. Langmuir 2008, 24, 7698–7703. [Google Scholar] [CrossRef] [PubMed]
- McParlane, J.; Dupin, D.; Saunders, J.M.; Lally, S.; Armes, S.P.; Saunders, B.R. Dual pH-triggered physical gels prepared from mixed dispersions of oppositely charged pH-responsive microgels. Soft Matter 2012, 8, 6239–6247. [Google Scholar] [CrossRef]
- Dupin, D.; Fujii, S.; Armes, S.P.; Reeve, P.; Baxter, S.M. Efficient synthesis of sterically stabilized pH-responsive microgels of controllable particle diameter by emulsion polymerization. Langmuir 2006, 22, 3381–3387. [Google Scholar] [CrossRef] [PubMed]
- Iatridi, Z.; Mattheolabakis, G.; Avgoustakis, K.; Tsitsilianis, C. Self-assembly and drug delivery studies of pH/thermo-sensitive polyampholytic (A-co-B)-b-C-b-(A-co-B) segmented terpolymers. Soft Matter 2011, 7, 11160–11168. [Google Scholar] [CrossRef]
- Gotzamanis, G.; Papadimitriou, K.; Tsitsilianis, C. Design of a C-b-(A-co-B)-b-C telechelic polyampholyte pH-responsive gelator. Polym. Chem. 2016, 7, 2121–2129. [Google Scholar] [CrossRef]
- Corten, C.; Kretschmer, K.; Kuckling, D. Novel multi-responsive P2VP-block-PNIPAAm block copolymers via nitroxide-mediated radical polymerization. Beilstein J. Organ. Chem. 2010, 6, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Schilli, C.M.; Zhang, M.; Rizzardo, E.; Thang, S.H.; Chong, Y.K.; Edwards, K.; Karlsson, G.; Müller, A.H.E. A new double-responsive block copolymer synthesized via RAFT polymerization: poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules 2004, 37, 7861–7866. [Google Scholar] [CrossRef]
- Delaittre, G.; Save, M.; Gaborieau, M.; Castignolles, P.; Rieger, J.; Charleux, B. Synthesis by nitroxide-mediated aqueous dispersion polymerization, characterization, and physical core-crosslinking of pH-and thermoresponsive dynamic diblock copolymer micelles. Polym. Chem. 2012, 3, 1526–1538. [Google Scholar] [CrossRef]
- Liu, S.; Billingham, N.C.; Armes, S.P. A schizophrenic water-soluble diblock copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328–2331. [Google Scholar] [CrossRef]
- Arotcarena, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef] [PubMed]

| Experiment ID a | [BlocBuilder] | [SG1] | [tBMA] | [2VP] | ftBMA,0 b | Mn,target c |
|---|---|---|---|---|---|---|
| mol·L−1 | mol·L−1 | mol·L−1 | mol·L−1 | mol% | kg·mol−1 | |
| tBMA/2VP-90 | 0.036 | 0.004 | 5.733 | 0.631 | 90% | 24.8 |
| tBMA/2VP-70 | 0.036 | 0.004 | 4.789 | 2.046 | 70% | 25.1 |
| tBMA/2VP-50 | 0.037 | 0.004 | 3.692 | 3.622 | 50% | 24.9 |
| tBMA/2VP-30 | 0.038 | 0.004 | 2.399 | 5.629 | 30% | 25.2 |
| tBMA/2VP-10 | 0.038 | 0.004 | 0.858 | 7.939 | 10% | 25.5 |
| Experiment ID a | Reaction Time (min) | Conversion b | FtBMA c (mol%) | d (kg·mol−1) | Đ d |
|---|---|---|---|---|---|
| tBMA/2VP-90 | 45 | 31% | 84% | 10.4 | 1.38 |
| tBMA/2VP-70 | 202 | 19% | 65% | 7.2 | 1.41 |
| tBMA/2VP-50 | 240 | 19% | 48% | 4.8 | 1.36 |
| tBMA/2VP-30 | 300 | 18% | 25% | 4.9 | 1.35 |
| tBMA/2VP-10 | 301 | 19% | 13% | 3.8 | 1.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marić, M.; Zhang, C.; Gromadzki, D. Poly(methacrylic acid-ran-2-vinylpyridine) Statistical Copolymer and Derived Dual pH-Temperature Responsive Block Copolymers by Nitroxide-Mediated Polymerization. Processes 2017, 5, 7. https://doi.org/10.3390/pr5010007
Marić M, Zhang C, Gromadzki D. Poly(methacrylic acid-ran-2-vinylpyridine) Statistical Copolymer and Derived Dual pH-Temperature Responsive Block Copolymers by Nitroxide-Mediated Polymerization. Processes. 2017; 5(1):7. https://doi.org/10.3390/pr5010007
Chicago/Turabian StyleMarić, Milan, Chi Zhang, and Daniel Gromadzki. 2017. "Poly(methacrylic acid-ran-2-vinylpyridine) Statistical Copolymer and Derived Dual pH-Temperature Responsive Block Copolymers by Nitroxide-Mediated Polymerization" Processes 5, no. 1: 7. https://doi.org/10.3390/pr5010007