A Reaction Database for Small Molecule Pharmaceutical Processes Integrated with Process Information
Abstract
:1. Introduction
1.1. General Databases
1.2. Specialized Databases
- (i)
- Each reaction is an individual record in the database (detailed and graphical). The reaction must be able to be retrieved from the database as a detailed record (reagents, products, stoichiometry etc.). It can also be extracted as a graphical representation where the reaction scheme is shown. In many databases, the reaction is represented in a graphical form.
- (ii)
- Structural information for target product as well as substrates.
- (iii)
- Reaction centers. The reaction center of a reaction is the collection of atoms and bonds that are changed during the reaction [3].
- (iv)
- Reaction components must be searchable. Information for the components involved in the reaction such as reagent, catalysts, solvents etc.
- (v)
- Multistep reactions. In the case of multistep reactions, all reactions (individual and whole pathway) must be searchable.
- (vi)
- Reaction conditions. Conditions such as pH, temperature, pressure etc. should be searchable by exact and a suitable range of values.
- (vii)
- Reaction classification. The type of reaction (i.e., esterification) should be searchable.
- (viii)
- Post-processing of the database contents. Export of the retrieved reaction data in other tools (i.e., MS Excel).
- Identify reactions that are used to produce different types of products (Active Pharmaceutical Ingredients (API), Intermediates).
- Identify reactions to be utilized, for a given compound availability.
- Investigate the function of different type of solvents in single/multiphase reactive systems.
- Facilitate the choice of the reaction conditions.
- Evaluate the reaction pathway in terms of yield, cost and sustainability metrics.
- Facilitate the reactor design from available experimental data and kinetic models.
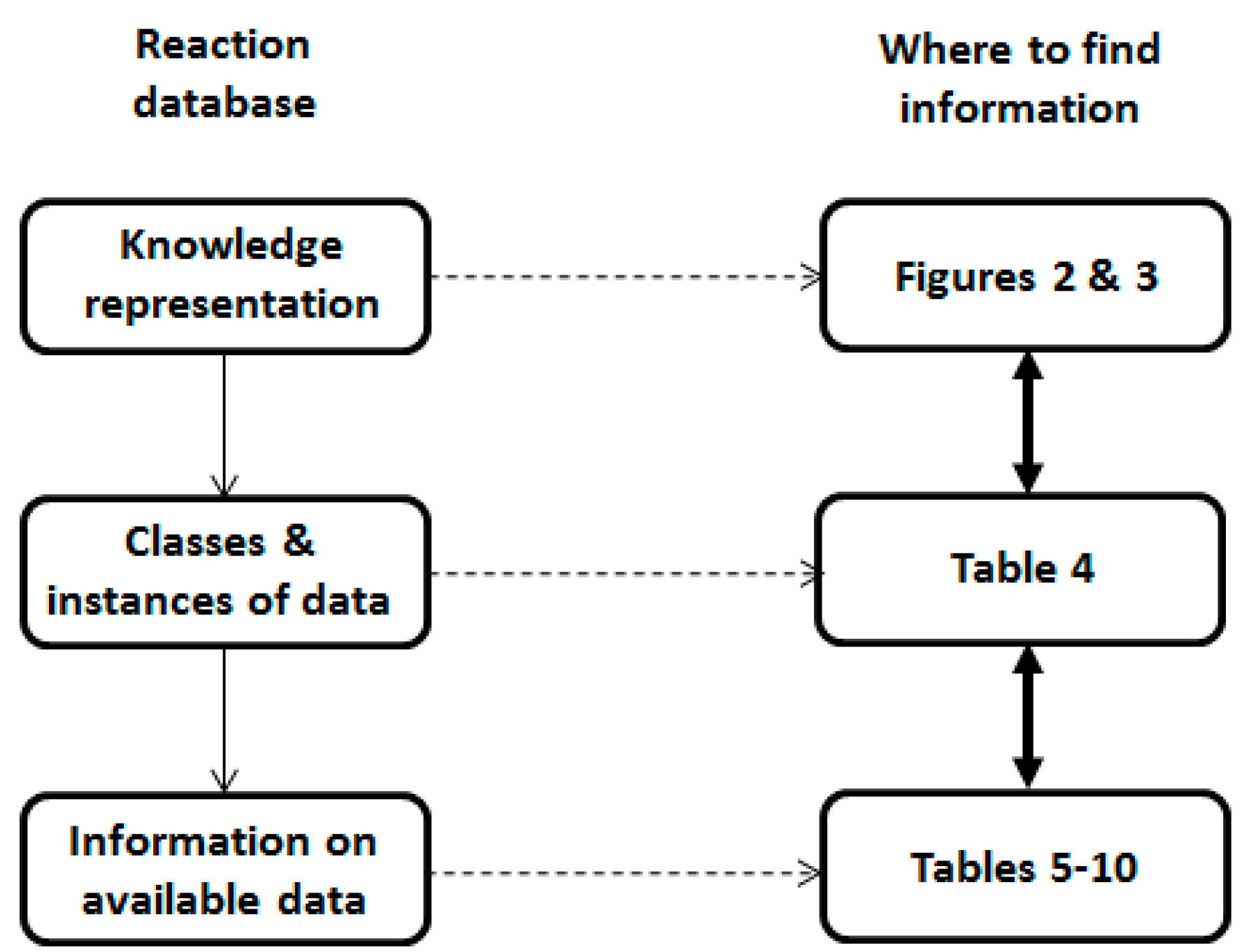
2. Reaction Database
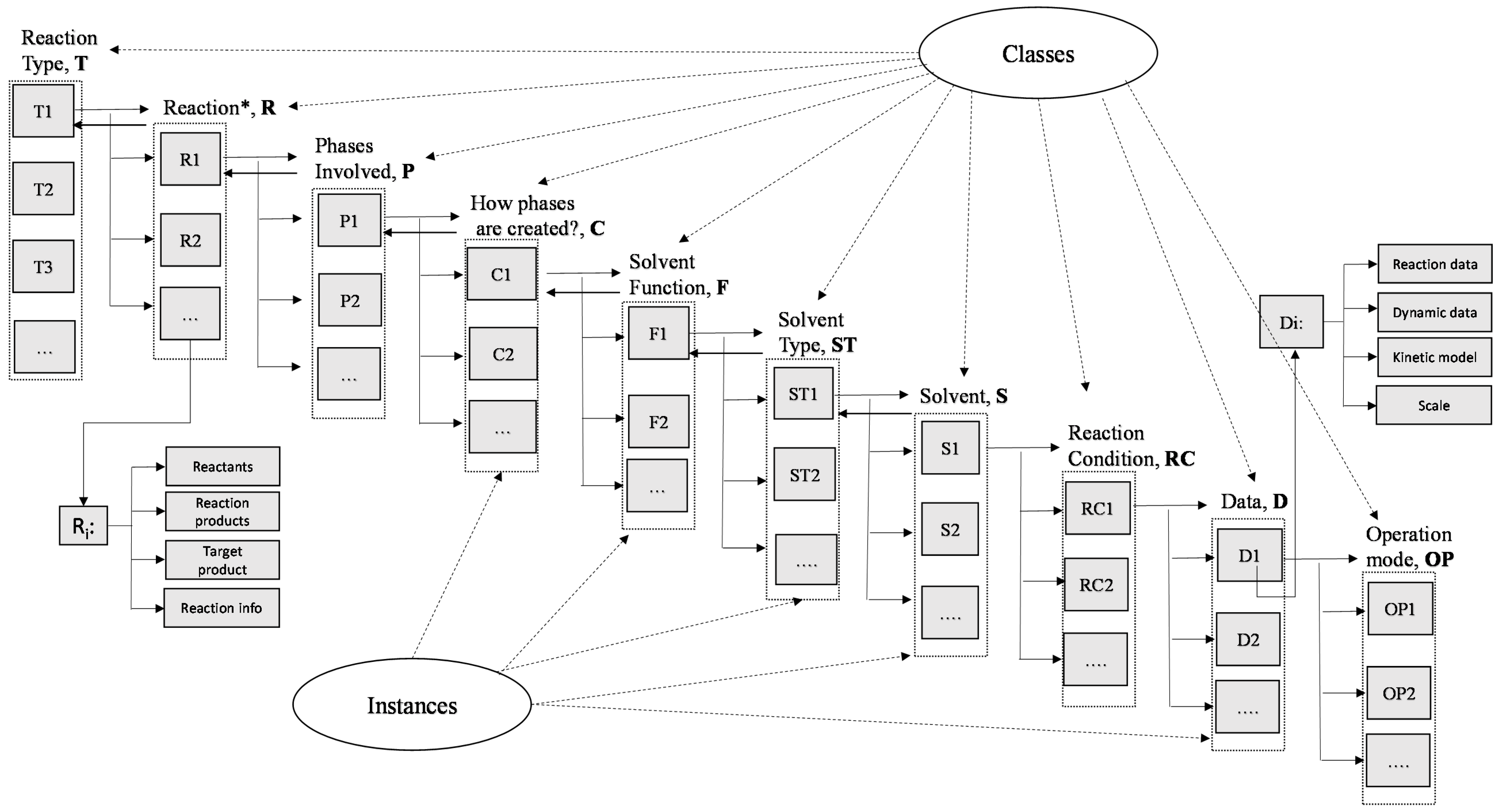
2.1. Knowledge Representation
2.2. Database Structure
3. Statistics of the Reaction Database
3.1. General Numbers
3.2. Reaction Types
3.3. Active Pharmaceutical Ingredients (APIs)
3.4. Reaction with Improved Reaction Performance When Solvent Is Used
- Reaction medium.
- Separation of the main product in order to shift the equilibrium reaction towards the product side in order to increase the yield and/or reduce the separation steps required.
- Separation of an inhibitory product to increase the productivity of the reaction.
- Controlled released of substrate, it might improve the process safety in case of hazardous compounds or increase selectivity towards the desired product.
- Reaction volume reduction.
- Dissolves reactants to increase the reaction rate and/or to avoid process complications when the reaction involves compounds in solid phase at the reaction conditions.
3.5. Kinetic Models Available
4. Reaction Database Application
4.1. Reaction Data
4.2. Organic Solvents
4.3. Search Options
- Search for reaction typesDifferent reaction types can be searched in the reaction database, the retrieved results provide information for the reaction (reactants, product and target product), the solvent role and how it improves the reaction, reaction conditions (i.e., temperature range, acid/base, different catalyst) and quantitative data (i.e., conversion, concentration vs. time), and finally applicability information such as scale or batch/continuous mode. The results can be used as similarity check, to identify reaction conditions, solvents and possibilities for improvement (i.e., equipment, production mode, technology) for quick reaction optimization.
- Search for main products (such as APIs or intermediates or type of products like chiral alcohols)Searching for main products or type of products, reactions that are used to synthesize this type of compound can be retrieved. The results are used to identify different ways for synthesis and to evaluate them in terms of reaction performance, cost, scalability and sustainability.
- Search for reactantsThe results obtained by searching reactants are used to identify ways for further utilizing them in case they have used or produced a product during a reaction.
- Multiphase reactionsMultiphase and single reactions where the solvent use has improved the reaction performance can be searched, the retrieved results are used to identify the role of the multiphase system, for example, solvent creates a second phase to remove inhibitory by-product and to quantify the improvement in reaction performance, for example, increased conversion.
- Identify reaction pathways, reaction types, reactants, catalysts, solvents and base/acid.
- Optimization reaction conditions.
- Investigate the solvent role in process improvement.
- Optimize the process development identified reactions in terms of cost, yield and time.
- Improve the overall process performance in terms of separation process, overall yield, sustainability, safety, scalability, controllability and utilized mass.
- Improve reactor design and evaluate different reactor designs.
- Establish operation procedure for the reactors.
- Assist in plant-wide design, simulation, and techno-economic optimization.
- Enhance process understanding.
5. Application Example: Ibuprofen Synthesis and Evaluation
5.1. Problem Definition
- To retrieve data relevant to the reaction pathway of Ibuprofen.
- Collect data related to individual reactions.
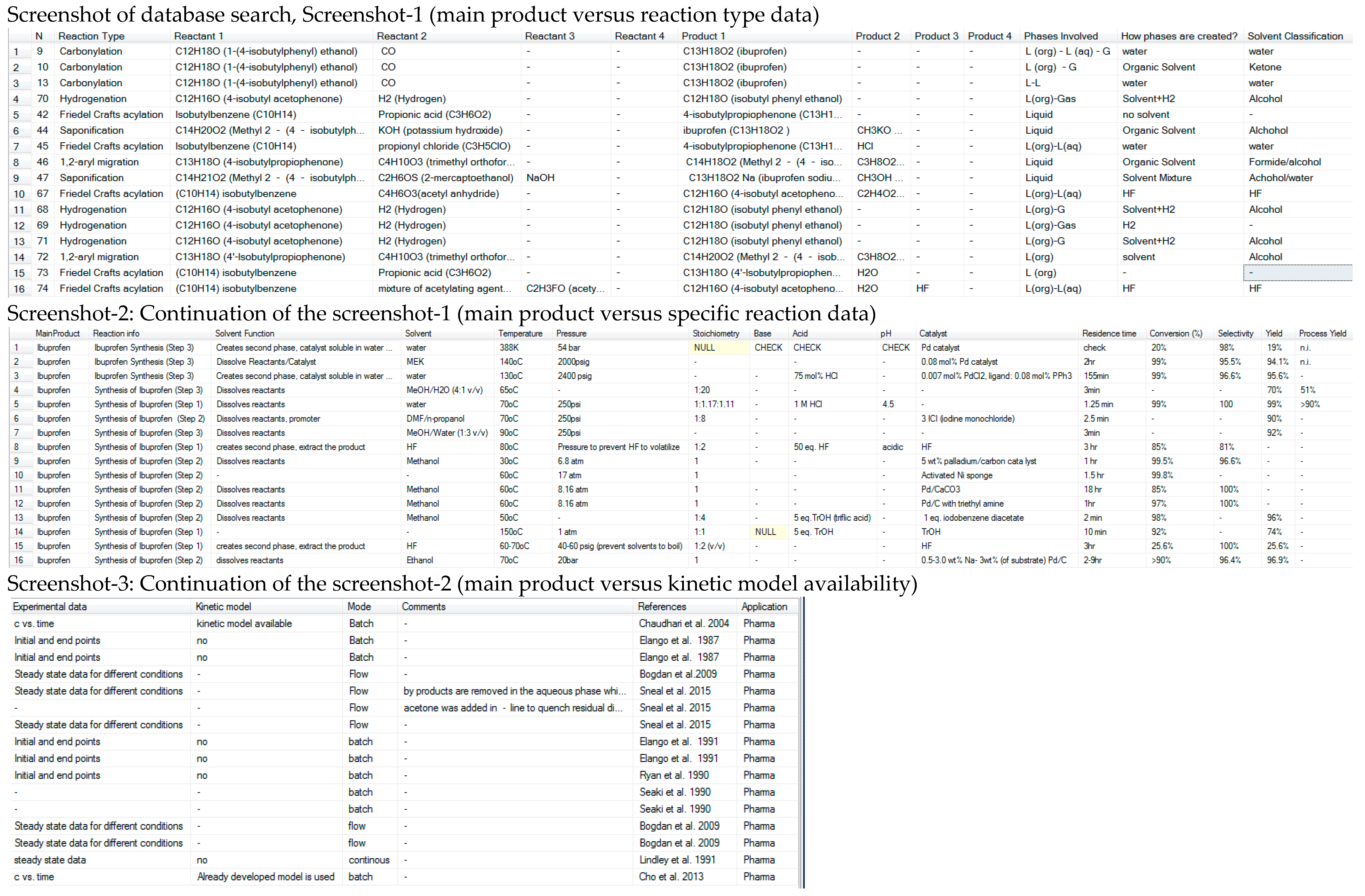
- Evaluate the alternatives based on green metrics.Database Search: “Main Product = ‘Ibuprofen’”.
5.1.1. Database Results
- Summary of the findings (reaction pathways, reaction types, operation mode, available data and reference).
- For each reaction pathway, each reaction is analyzed in terms of:
- Reactant, products, by-products, acids/base, solvents, catalysts.
- Then the reaction conditions for each reaction is presented.
- Finally, the reaction data is presented.
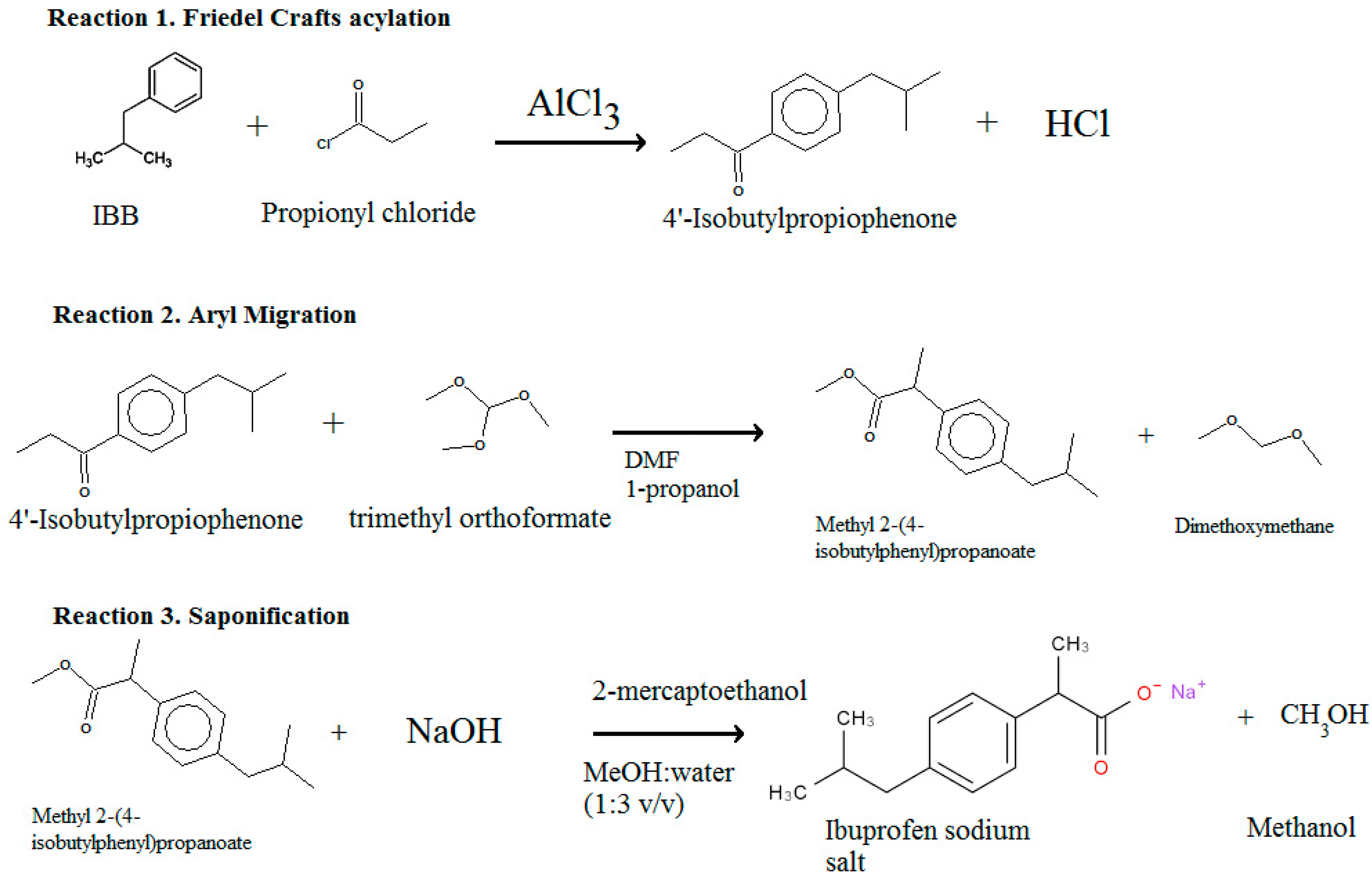
5.1.2. Pathway 2: Ibuprofen Synthesis
5.1.3. Reaction Pathways Evaluation through the “Green” Metrics
6. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Caron, S.; Thomson, N.M. Pharmaceutical process chemistry: Evolution of a contemporary data-rich laboratory environment. J. Org. Chem. 2015, 80, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J. Chemoinformatics: Achievements and challenges, a personal view. Molecules 2016, 21, 151. [Google Scholar] [CrossRef]
- Warr, W.A. A short review of chemical reaction database systems, computer-aided synthesis design, reaction prediction and synthetic feasibility. Mol. Inform. 2014, 33, 469–476. [Google Scholar] [CrossRef]
- Corey, E.J. General methods of synthetic analysis. Strategic bond disconnections for bridged polycyclic structures. J. Am. Chem. Soc. 1975, 97, 6116–6124. [Google Scholar] [CrossRef]
- Bersohn, M.; Esack, A. Computers and organic synthesis. Chem. Rev. 1976, 76, 269–282. [Google Scholar] [CrossRef]
- Corey, E.J.; Jorgensen, E.J. Computer-Assisted Synthetic Analysis. Synthetic Strategies Based on Appendages and the Use of Reconnective Transforms. J. Am. Chem. Soc. 1976, 98, 189–203. [Google Scholar] [CrossRef]
- Agarwal, K.K.; Larsen, T.D.L.; Gelernter, H.L. Application of chemical transforms in synchem2, a computer program for organic synthesis route discovery. Comput. Chem. 1978, 2, 75–84. [Google Scholar] [CrossRef]
- Wipke, T.W.; Ouchi, G.I.; Krishnan, S. Simulation and Evaluation of Chemical Synthesis—SECS: An Application of Artificial Intelligence Techniques. Artif. Intell. 1978, 11, 173–193. [Google Scholar] [CrossRef]
- Ravitz, O. Data-driven computer aided synthesis design. Drug Discov. Today Technol. 2013, 10, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bogevig, A.; Federsel, H.J.; Huerta, F.; Hutchings, M.G.; Kraut, H.; Langer, T.; Low, P.; Oppawsky, C.; Rein, T.; Saller, H. Route design in the 21st century: The IC SYNTH software tool as an idea generator for synthesis prediction. Org. Process Res. Dev. 2015, 19, 357–368. [Google Scholar] [CrossRef]
- Salatin, T.D.; Jorgensen, W.L. Computer-assisted mechanistic evaluation of organic reactions. 1. Overview. J. Org. Chem. 1980, 45, 2043–2051. [Google Scholar] [CrossRef]
- Chen, J.H.; Baldi, P. No electron left behind: A rule-based expert system to predict chemical reactions and reaction mechanisms. J. Chem. Inf. Model. 2009, 49, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Kayala, M.A.; Baldi, P. A Machine Learning Approach to Predict Chemical Reactions. Adv. Neural Inf. Process. Syst. 2011, 747–755. [Google Scholar]
- Kayala, M.A.; Azencott, C.A.; Chen, J.H.; Baldi, P. Learning to predict chemical reactions. J. Chem. Inf. Model. 2011, 51, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Gothard, C.M.; Soh, S.; Gothard, N.A.; Kowalczyk, B.; Wei, Y.; Baytekin, B.; Grzybowski, B.A. Rewiring chemistry: Algorithmic discovery and experimental validation of one-pot reactions in the network of organic chemistry. Angew. Chem. Int. Ed. 2012, 51, 7922–7927. [Google Scholar] [CrossRef] [PubMed]
- Science of Synthesis. Thieme Chemistry. Available online: https://www.thieme.de/en/thieme-chemistry/science-of-synthesis-54780.html (accessed on 7 July 2016).
- Zass, E. Databases of Chemical Reactions. In Handbook of Chemoinformatics: From Data to Knowledge in 4 Volumes; Gasteiger, J., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2003; pp. 667–699. [Google Scholar]
- Reactions-CASREACT. Available online: http://www.cas.org/content/reactions (accessed on 1 July 2016).
- Reaxys. Available online: https://www.elsevier.com/solutions/ (accessed on 7 July 2016).
- ChemInform. Available online: http://www.cheminform.com/ (accessed on 7 July 2016).
- Current Chemical Reaction. Available online: http://www.cheminform.com/ (accessed on 7 July 2016).
- The Chemogenesis web book. Available online: http://www.meta-synthesis.com/webbook.html (accessed on 7 July 2016).
- Organic Synthesis. Available online: http://www.orgsyn.org/ (accessed on 7 July 2016).
- Reaction Database. Available online: http://www.sigmaaldrich.com/help-welcome/product-search/reaction-database.html (accessed on 7 July 2016).
- Synthetic Pages. Available online: http://cssp.chemspider.com/ (accessed on 7 July 2016).
- The Chemical Thesaurus. Available online: http://www.chemthes.com/ (accessed on 7 July 2016).
- Webreactions. Available online: http://webreactions.net/index.html (accessed on 7 July 2016).
- SPRECI InfoChem. Available online: http://www.infochem.de/products/databases/spresiweb.shtml (accessed on 7 July 2016).
- Synthetic Reaction. Available online: http://pubs.rsc.org/lus/synthetic-reaction-updates (accessed on 7 July 2016).
- Biotage PathFinder. Available online: www.biotagepathfinder.com/index.jsp (accessed on 7 July 2016).
- e-EROS. Available online: http://onlinelibrary.wiley.com/book/10.1002/047084289X (accessed on 7 July 2016).
- FlowReact. Available online: www.flowreact.com/index.php/text/about (accessed on 7 July 2016).
- Protecting Groups. Available online: http://accelrys.com/products/datasheets/protecting-groups.pdf (accessed on 7 July 2016).
- Masek, B.B.; Baker, D.S.; Dorfman, R.J.; DuBrucq, K.; Francis, V.C.; Nagy, S.; Richey, B.L.; Soltanshahi, F. Multistep Reaction Based De Novo Drug Design: Generating synthetically feasible design ideas. J. Chem. Inf. Model. 2016, 56, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Databases M. Theilheimer. Available online: http://akosgmbh.de/pdf/overview.pdf (accessed on 7 July 2016).
- Butters, M.; Catterick, D.; Craig, A.; Curzons, A.; Dale, D.; Gillmore, D.; Green, S.P.; Marziano, I.; Sherlock, J.-P.; White, W. Critical assessment of pharmaceutical processes - A rationale for changing the synthetic route. Chem. Rev. 2006, 106, 3002–3027. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Padrell, A.E.; Skovby, T.; Kiil, S.; Gani, R.; Gernaey, K.V. Active pharmaceutical ingredient (API) production involving continuous processes--a process system engineering (PSE)-assisted design framework. Eur. J. Pharm. Biopharm. 2012, 82, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to “green” chemistry-which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Jolliffe, H.G.; Gerogiorgis, D.I. Plantwide design and economic evaluation of two continuous pharmaceutical manufacturing (CPM) cases: Ibuprofen and artemisinin. Comput. Chem. Eng. 2016, 91, 269–288. [Google Scholar] [CrossRef]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef]
- Jolliffe, H.G.; Gerogiorgis, D.I. Technoeconomic optimisation and comparative environmental impact evaluation of continuous crystallisation and antisolvent selection for artemisinin recovery. Comput. Chem. Eng. 2016, 91, 269–288. [Google Scholar] [CrossRef]
- Singh, R.; Gernaey, K.V.; Gani, R. An ontological knowledge-based system for the selection of process monitoring and analysis tools. Comput. Chem. Eng. 2010, 34, 1137–1154. [Google Scholar] [CrossRef]
- Pfruender, H.; Amidjojo, M.; Kragl, U.; Weuster-Botz, D. Efficient whole-cell biotransformation in a biphasic ionic liquid/water system. Angew. Chem. Int. Ed. 2004, 43, 4529–4531. [Google Scholar] [CrossRef] [PubMed]
- Kopach, M.E.; Murray, M.M.; Braden, T.M.; Kobierski, M.E.; Williams, O.L. Improved Synthesis of 1-(Azidomethyl)-3,5-bis-(trifluoromethyl)benzene: Development of Batch and Microflow Azide Processes. Org. Process Res. Dev. 2009, 13, 152–160. [Google Scholar] [CrossRef]
- Li, P.; Buchwald, S.L. Continuous-flow synthesis of 3,3-disubstituted oxindoles by a palladium-catalyzed α-arylation/alkylation sequence. Angew. Chem. Int. Ed. Engl. 2011, 50, 6396–6400. [Google Scholar] [CrossRef] [PubMed]
- Domier, R.C.; Moore, J.N.; Shaughnessy, K.H.; Hartman, R.L. Kinetic Analysis of Aqueous-Phase Pd-Catalyzed, Cu-Free Direct Arylation of Terminal Alkynes Using a Hydrophilic Ligand. Org. Process. Res. Dev. 2013, 17, 1262–1271. [Google Scholar] [CrossRef]
- Xin, J.Y.; Li, S.B.; Xu, Y.; Wang, L.L. Enzymatic resolution of (S)-(+)-naproxen in a trapped aqueous-organic solvent biphase continuous reactor. Biotechnol. Bioeng. 2000, 68, 78–83. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Xu, J.H.; Bao, D.; Qi, H. Enzymatic hydrolysis of penicillin G to 6-aminopenicillanic acid in cloud point system with discrete countercurrent experiment. Enzyme Microb. Technol. 2007, 41, 121–126. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, B.G. Transaminase-catalyzed asymmetric synthesis of L-2-aminobutyric acid from achiral reactants. Biotechnol. Lett. 2009, 31, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Jensen, J.S.; Al-Haque, N.; Neto, W.; Woodley, J.M. Process considerations for the asymmetric synthesis of chiral amines using transaminases. Biotechnol. Bioeng. 2011, 108, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Houng, J.Y.; Tseng, J.C.; Hsu, H.F.; Wu, J.Y. Kinetic investigation on asymmetric bioreduction of ethyl 4-chloro acetoacetate catalyzed by baker’s yeast in an organic solvent-water biphasic system. Korean J. Chem. Eng. 2008, 25, 1427–1433. [Google Scholar] [CrossRef]
- Houng, J.Y.; Liau, J.S. Applying slow-release biocatalysis to the asymmetric reduction of ethyl 4-chloroacetoacetate. Biotechnol. Lett. 2003, 25, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Papadogianakis, G.; Maat, L.; Sheldon, R.A. Catalytic Conversions in Water. Part 5: Carbonylation of 1- (4-Isobutylphenyl) ethanol to Ibuprofen Catalysed by Water-Soluble Palladium-Phosphine Complexes in a Two-Phase System. J. Chem. Technol. Biotechnol. 1997, 70, 83–91. [Google Scholar] [CrossRef]
- Chaudhari, R.V.; Mills, P.L. Multiphase catalysis and reaction engineering for emerging pharmaceutical processes. Chem. Eng. Sci. 2004, 59, 5337–5344. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Thakar, N.; Berger, R.J.; Kapteijn, F.; Moulijn, J.A. Modelling kinetics and deactivation for the selective hydrogenation of an aromatic ketone over Pd/SiO2. Chem. Eng. Sci. 2007, 62, 5322–5329. [Google Scholar] [CrossRef]
- Cho, H.-B.; Lee, B.U.; Ryu, C.-H.; Nakayama, T.; Park, Y.-H. Selective hydrogenation of 4-isobutylacetophenone over a sodium-promoted Pd/C catalyst. Korean J. Chem. Eng. 2013, 30, 306–313. [Google Scholar] [CrossRef]
- Cervera-Padrell, A.E. Moving from Batch towards Continuous Organic-Chemical Pharmaceutical Production. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, 2011. [Google Scholar]
- Bhatia, S.; Long, W.S.; Kamaruddin, A.H. Enzymatic membrane reactor for the kinetic resolution of racemic ibuprofen ester: Modeling and experimental studies. Chem. Eng. Sci. 2004, 59, 5061–5068. [Google Scholar] [CrossRef]
- Shankar, S.; Agarwal, M.; Chaurasia, S.P. Study of reaction parameters and kinetics of esterification of lauric acid with butanol by immobilized Candida antarctica lipase. Indian J. Biochem. Biophys. 2013, 50, 570–576. [Google Scholar] [PubMed]
- Shin, J.S.; Kim, B.G. Substrate inhibition mode of ω-transaminase from Vibrio fluvialis JS17 is dependent on the chirality of substrate. Biotechnol. Bioeng. 2002, 77, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Al-Haque, N.; Santacoloma, P.A.; Neto, W.; Tufvesson, P.; Gani, R.; Woodley, J.M. A robust methodology for kinetic model parameter estimation for biocatalytic reactions. Biotechnol. Prog. 2012, 28, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.B.; Straathof, A.J.J.; van der Does, T.; Ras, C.; Heijnen, J.J. Equilibrium Modeling of Extractive Enzymatic Hydrolysis of Penicillin G with Concomitant 6-Aminopenicillanic Acid Crystallization. Biotechnol. Bioeng. 2002, 78, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Den Hollander, J.L.; Zomerdijk, M.; Straathof, A.J.J.; Van Der Wielen, L.A.M. Continuous enzymatic penicillin G hydrolysis in countercurrent water-butyl acetate biphasic systems. Chem. Eng. Sci. 2002, 57, 1591–1598. [Google Scholar] [CrossRef]
- Jayasree, S.; Seayad, A.; Chaudhari, R.V. Novel palladium(II) complex containing a chelating anionic N-O ligand: efficient carbonylation catalyst. Org. Lett. 2000, 2, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Seayad, A.; Seayad, J.; Mills, P.L.; Chaudhari, R.V. Kinetic Modeling of Carbonylation of 1-(4-Isobutylphenyl)ethanol Using a Homogeneous PdCl2(PPh3)2/TsOH/LiCl Catalyst System. Ind. Eng. Chem. Res. 2003, 42, 2496–2506. [Google Scholar] [CrossRef]
- Seayad, A.; Kelkar, A.A.; Chaudhari, R.V. Carbonylation of p-isobutyl phenylethanol to ibuprofen using palladium catalyst: activity and selectivity studies. Stud. Surf. Sci. Catal. 1998, 113, 883–889. [Google Scholar]
- Rajashekharam, M.V.; Chaudhari, R.V. Kinetics of Hydrogenation of p-isobutyl acetophenone using a supported Ni catalyst in a slurry reactor. Chem. Eng. Sci. 1996, 51, 1663–1672. [Google Scholar] [CrossRef]
- Watson, D.; Dowdy, E.D.; Depue, J.S.; Kotnis, A.S.; Leung, S.; Reilly, B.C.O. Development of a Safe and Scalable Oxidation Process for the Preparation of 6-Hydroxybuspirone: Application of In-Line Monitoring for Process Ruggedness and Product Quality. Org. Process Res. Dev. 2004, 8, 616–623. [Google Scholar] [CrossRef]
- Elango, V.; Murphy, M.; Smith, B.L.; Davenport, K.G.; Mott, G.N.; Zey, E.G.; Moss, G.L. Method for Producing Ibuprofen. U.S. Patent 4,981,995 A, 1 January 1991. [Google Scholar]
- Lindley, D.D.; Curtis, T.A.; Ryan, T.R.; de la Garza, E.M.; Hilton, C.B.; Kenesson, T.M. Process for the Production of 4-Isobutylacetophenone. U.S. Patent 5,068,448 A, 26 November 1991. [Google Scholar]
- Snead, D.R.; Jamison, T.F. A three-minute synthesis and purification of ibuprofen: Pushing the limits of continuous-flow processing. Angew. Chem. Int. Ed. 2015, 54, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.R.; Poe, S.L.; Kubis, D.C.; Broadwater, S.J.; McQuade, D.T. The continuous-flow synthesis of Ibuprofen. Angew. Chem. Int. Ed. 2009, 8547–8550. [Google Scholar] [CrossRef] [PubMed]


| Database | Number of Reaction | Criteria [17] | Reference |
|---|---|---|---|
| CASREACT | >74.9 million (1840–present) | i, iv, v, vi | [18] |
| REAXYS (previously CrossFire Beilstein) | 40.7 million (1771–present) | i, ii, iv, vii | [19] |
| Theilheimer | >72200 (1946–1980) | i, v, vi, vii | [35] |
| ChemInform RX | >2 million (since 1990–present) | i, iv, vi | [20] |
| Current chemical reactions | 1,083,758 (1840–present) | i, vi | [21] |
| Methods in organic synthesis | 33,000 (1999–2014) | i, vii | [29] |
| Reference library of synthetic methodology | 209.800 (1946–2001) | i | [17] |
| ChemReact | 3.5 million reactions (1974–1998) | i, vii | [17] |
| Chemogenesis | - | ii, iii | [22] |
| Organic synthesis | >6000 (1921–present) | i, ii, v, vi, vii | [23] |
| Reaction Database-Chemical Synthesis | - | i, ii | [24] |
| Synthetic Pages | 292 | i, ii, vi, vii | [25] |
| The chemical thesaurus | 4000 | i, ii | [26] |
| WebReactions | >400,000 | i, ii, iii | [27] |
| Biotage Pathfinder (reaction assisted with microwave technology) | >1000 | i, vi, vii, viii | [30] |
| e-EROS Encyclopedia of Reagents for Organic Synthesis | >70,000 (4000 *) | i, ii | [31] |
| FlowReact Search | >2000 | i (reaction in flow) | [32] |
| Protecting groups | - | i | [33] |
| Science of Synthesis (previously Houben-Weyl) | 240,000 (early 1800s–present) | i, ii, iii | [16] |
| SPRESI | 4.6 million | i, ii, iii | [28] |
| Metric | Explanation | Equation |
|---|---|---|
| Effective Mass yield (EM) | The percentage of the mass of product over the overall mass of non-benign compounds used during the synthesis. | |
| E-factor | The mass of total waste produced for a given amount of produced product. | |
| Atom Economy | How much of the reactants remain in the product. | Where A, B, D, F, G, I: reactants; P: product |
| Mass Intensity (MI) | Total mass used to produce the product. | |
| Carbon efficiency | Percentage of carbon of the reactants that remain in the final product. | |
| Reaction mass efficiency (RME) | Mass of reactants remaining in the product. |
| Criterion | CASREACT | REAXYS | Theilheimer | ChemInform RX | Current Chemical reactions | Synthetic Reaction Updates | Reference Library of Synthetic methodology | ChemReact | Chemogenesis | Organic Synthesis | Reaction Database-Chemical Synthesis | Synthetic Pages | The Chemical Thesaurus | Webreactions | Biotage Pathfinder | e-EROS Encyclopedia of Reagents for Organic Synthesis | FlowReact Search | Protecting Groups | Science of Synthesis | SPRESI | This Work |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i. | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ii. | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||
| iii. | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| iv. | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| v. | ✔ | ✔ | ✔ | ✔ | |||||||||||||||||
| vi. | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||||
| vii. | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||||||
| viii. | ✔ | ✔ |
| Main Classes | Relation with Instances | Instances |
|---|---|---|
| Reaction Type, T | T = [T1, T2, ..., Ti, …, Tn] | Ti: reaction type in the knowledge base (i.e., acylation etc.) |
| Reaction, R | R = [R1, R2, ..., Ri, …, Rn] | Ri: reaction of the ith reaction type; for each reaction information about the reactants and reaction products are provided as well as information for the target product and process (for example: 1st step for production of an API) |
| Phases involved, P | P = [P1, P2, ..., Pi, …, Pn] | Pi: phase of the ith reaction (i.e., organic-aqueous, organic-gas etc.) |
| How phases are created, C | C = [C1, C2, ..., Ci, …, Cn] | Ci: (i.e., solvent etc.) |
| Solvent function, F | F = [F1, F2, ..., Fi, …, Fn] | Fi: (i.e., phase creation, carrier etc.) |
| Solvent type, ST | ST = [ST1, ST2, ..., STi, …, STn] | STi: (i.e., ether, alcohol etc.) |
| Solvent, S | S = [S1, S2, ..., Si, …, Sn] | Si: Solvents in ith reaction |
| Reaction condition, RC | RC = [RC1, RC2, ..., RCi, …, RCn] | RCi (i.e., Temperature, composition, cat, pH etc.) |
| Data, D | D = [D1, D2, ..., Di, …, Dn] | Di (reaction data: conversion, selectivity, reaction time, and dynamic data: concentration vs. time, scale information and kinetic models etc.) |
| Operation Mode, OP | OP = [OP1, OP2, ..., OPi, …, OPn] | OPi: batch, continuous, fed batch |
| Category | Number |
|---|---|
| Total number of reactions | 285 |
| Types of reactions | 44 |
| Number of multiphase reactions | 88 |
| Number of reaction with solvents | 226 |
| Solvent | Dissolve, Phase creation, Substrate/catalyst carrier, compound extraction |
| Number of APIs (with total synthesis pathway) | 21 |
| Number of building blocks (type of compounds) | 19 |
| Number of experimental data | 275 (conversion, selectivity, reaction yield, conditions), 32 (dynamic data), 11 (kinetic models) |
| Number of production mode data | 96 (in flow), 203 (in batch) |
| Number of application examples | 14 (chemicals), 16 (Fine chemicals), 251 (pharmaceuticals) |
| Reaction Type | Catalyst | Phases | Solvent Function |
|---|---|---|---|
| 1. Alkylation | Yes | Liquid (org.) | Dissolves reactants |
| Liquid (org.)—Liquid (aq.) | Creates second phase | ||
| 2. Hydrogenation | Yes | Liquid (org.)—Gas | Dissolves reactants |
| 3. Epoxidation | Liquid (org.)—Liquid (aq.) | Creates second phase Reactant/catalyst carrier | |
| 4. Carbonylation | Yes | Liquid (org.)—Liquid (aq)—Gas | Creates phase Carrier for catalyst |
| Yes | Liquid (org.)—Gas | Dissolves reactants | |
| 5. Hydroformulation | ? | Creates second phase Catalyst carrier | |
| 6. Enzymatic reduction | Yes | Liquid (org.)—Liquid (aq.) | Reactant carrier Creates second phase |
| 7. Arylation | Yes | Liquid (org.)—Liquid (aq.) | Creates second phase |
| Yes | Liquid (org.) | Dissolves reactants | |
| 8. Oxidation | Yes | Liquid (org.)—Gas | Dissolves reactants |
| 9. Transamination | yes | Liquid (org,)—Liquid (aq.) | Creates second phase Product removal |
| 10. Saponification | No | Liquid (org.) | Dissolves reactants |
| 11. Amidation | Yes/No | Liquid (org.)—liquid (aq.) | Creates second phase removes product |
| 12. Amination | Yes | Liquid (org.) | Dissolves reactants |
| 13. Esterification | Yes | Liquid (org.) | Solvent free |
| Liquid (org.) | Dissolves reactant | ||
| 14. Hydrolysis | Yes | Liquid (org.)—liquid (aq.) | Creates second phase removes product |
| Dissolves reactants | |||
| 15. Aminolysis | yes | Liquid (org.) | Dissolves reactants |
| 16 .Condensation | No | Liquid (org.) | Dissolves reactant |
| 17. Deprotection | No | Liquid (org.) | Dissolves reactant |
| 18. Protection | Yes | Liquid (org.) | Dissolves reactant |
| 19. Dehydration | Yes | Liquid (org.)—liquid (aq.) | Catalyst carrier Create second phase Product removal |
| 20. Cyclization | No | Liquid (org.)—liquid (aq.) | Dissolves reactant Product separation |
| 21. Lithiation | No | Liquid (org.) | Dissolves reactants |
| APIs | |
|---|---|
| 1. 6-aminopenicillanic acid | 12. Tramadol |
| 2. Zuchopenthixol | 13. Artemisinin |
| 3. 6-Hydroxybuspirone | 14. Saxagliptin |
| 4. aliskiren hemifumarate | 15. Atazanavir |
| 5. Ibuprofen | 16. PDE5 inhibitor * |
| 6. Meclinertant * | 17. Axitinib |
| 7. Rufinamide | 18. Olanzapine * |
| 8. Ciprofloxacin | 19. Amitriptyline |
| 9. Naproxen | 20. Tamoxifen |
| 10. OZ439 * (antimalarial drug candidate) | 21. Vildagliptin |
| 11. Efavirenz * | |
| Possible Improvements | |||||
|---|---|---|---|---|---|
| Productivity | Process safety | Separation steps | Waste reduction | ||
| Solvent Functions | Reaction medium | ✔ | ✔ | - | - |
| Product removal (phase creation) | ✔ | - | ✔ | ✔ | |
| Substrate carrier (phase creation) | ✔ | ✔ | - | ✔ | |
| Catalyst carrier (Phase creation) | ✔ | ✔ | ✔ | ✔ | |
| Reaction Type | Main Product | Phases | Solvent Function | Improvement |
|---|---|---|---|---|
| Amidation [1] | PDE5 inhibitor | Liquid(org.)—Solid | Product separation (Product not soluble in solvent) | Direct product separation |
| Enzymatic reduction [43] | Chiral alcohols | Liquid (aq.)—Ionic liquid | Substrate carrier | Increased productivity (82–92% yield) |
| Liquid (aq.) | - | Productivity (42–46% yield) | ||
| Liquid (aq.)—Organic solvent | Substrate carrier | Productivity (0% yield) | ||
| Alkylation [44] | Alyl azides | liquid (org. DMSO) | Dissolves reactants | High productivity (94% yield) but high waste generation |
| Liquid (aq.)—liquid (org. DMSO) | Dissolves reactants | High in productivity (94% yield) and lower waste generation | ||
| Liquid (aq.)—liquid (org. Isopropyl acetate) | Dissolves reactants | High productivity (96.5% yield) and lower waste generation | ||
| Liquid (aq.)—liquid (org. Isooctane) | Dissolves reactants | High productivity (91.4% yield) and lower waste generation | ||
| Arylation [45] | 3,3-disubstituted oxindoles | Liquid (aq.)—liquid (org. THF or Toluene) | Dissolve reactants | Increased reaction rate that leads to complete conversion and high yields compared to single phase systems |
| Arylation [46] | Arylation of Alkynes | Liquid (aq)—liquid (org.) | Catalysts dissolved in aq. Phase | Catalyst recovery while maintaining high yields |
| Hydrolysis [47] | Naproxen | Liquid (aq.)—liquid (org.; Hexane or isooctane or toluene) | Product removal (in organic phase) | Increased yield, enzyme stability increases |
| Hydrolysis [48] | 6-amino penicillanic acid | Liquid (aq.)—liquid (org.; butyl acetate) | Product removal in the organic phase | Productivity increases (product removal shifts the equilibrium reaction towards the product) |
| Transamination [49] | L-2 Aminobutyric acid | Liquid (aq.)—liquid (org.) | By-product inhibits the enzyme, removal in the organic phase | Increased conversion (96%) |
| Liquid (aq.) | - | Conversion (~40%) | ||
| Transamination [50] | Chiral amines | Liquid (aq.)—Resin | Product removal | Equilibrium shifts towards product side |
| Enzymatic Reduction [51,52] | S-4-Chloro-3-hydroxybutyric acid ethyl ester | Liquid (aq.)—liquid (org.) | Substrate controlled release | Increased reaction productivity |
| Carbonylation [53,54] | Ibuprofen | Gas—Liquid (org.)—Liquid (aq.) | Dissolves catalyst (aq.) | Less waste generated, same productivity, slightly lower reaction rates, reduction in the separation steps |
| Gas—Liquid (org.); MEK-Liquid (aq.) | Dissolves catalyst (aq.); Dissolves reactants (org.) | Increased reaction rates | ||
| Transamination [55,56] | Sitagliptin | Liquid (aq.); DMSO used as co-solvent | DMSO dissolves amine donor | Increased productivity; enantiomeric selectivity and less waste generated |
| Kinetic Models | Number | Reference |
|---|---|---|
| Dehydration | 1 | [59] |
| Enzymatic reduction | 3 | [51,60] |
| Esterification | 1 | [61] |
| Transamination | 3* | [50,62,63] |
| Hydrolysis | 2 | [64,65] |
| Carbonylation | 2* | [66,67,68] |
| Hydrogenation | 2* | [57,69] |
| Pathway | Reaction Steps | Database Entries | Operation | Reference |
|---|---|---|---|---|
| 1 | 1.1 Friedel Crafts acylation | 67 and 74 | Batch (67), continuous (74) | Elango et al. [71] |
| Lindley et al. [72] | ||||
| 1.2 Hydrogenation | 68–71 and 92 | Batch | Elango et al. [71] | |
| 1.3 Carbonylation | 9–13 | Batch | Elango et al. [71] | |
| 2 | 3.1 Friedel Crafts | 45 | Continuous | Snead et al. [73] |
| 3.2 1–2 aryl migration | 46 | Continuous | Snead et al. [73] | |
| 3.3 Saponification | 44 | Continuous | Snead et al. [73] | |
| 3 | 2.1 Friedel-Crafts | 73 | Continuous | Bogdan et al. [74] |
| 2.2 1–2,aryl migration | 72 | Continuous | Bogdan et al. [74] | |
| 2.3 Saponification | 44 | Continuous | Bogdan et al. [74] |
| Reaction Information | Reaction Step 1 | Reaction Step 2 | Reaction Step 3 |
|---|---|---|---|
| Friedel Crafts (Flow) | Aryl Migration (Flow) | Saponification (Flow) | |
| Reaction | Isobutylbenzene + propionyl chloride → 4-isobutylpropiophenone + HCl | C13H18O (4-isobutylpropiophenone) + C4H10O3 (trimethyl orthoformate) → C14H18O2 (Methyl 2-(4-isobutylphenyl) propanoate) + C3H8O2 (Dimethoxymethane) | C14H21O2 (Methyl 2-(4-isobutylphenyl) propanoate) + C2H6OS (2-mercaptoethanol) + NaOH→ C13H18O2 Na (ibuprofen sodium salt) + CH3OH ( MeOH ) |
| Composition (Reactant A: Reactant B, in moles eq.) | 1:1.17 | 1:8 | 1:8 |
| Solvent | Water | DMF/1-propanol | MeOH/H2O |
| Catalyst | AlCl3 | ICI | - |
| Acid/Base | HCl | - | - |
| By Products | - | - | - |
| Reaction Conditions | Reaction Step 1 | Reaction Step 2 | Reaction Step 3 |
|---|---|---|---|
| Friedel Crafts (Flow) | Aryl Migration (Flow) | Saponification (Flow) | |
| Temperature | 87 °C | 90 °C | 90 °C |
| Pressure | 17 atm | 14 atm | 14 atm |
| Residence time | 1.25 min | 1min | 1 min |
| Catalyst amount | 1.11 eq. AlCl3 | 3 eq. ICI | - |
| Solvent amount | - | 0.25 eq. DMF/0.71 eq. n-propanol | MeOH/H2O (1:3 v/v) |
| Type of Data | Reaction Step 1 | Reaction Step 2 | Reaction Step 3 |
|---|---|---|---|
| Friedel Crafts (Flow) | Aryl Migration (Flow) | Saponification (Flow) | |
| Conversion | 99% | 90% | 89%*(*yield) |
| Selectivity (main product; by-product) | - | - | - |
| Reaction Yield | - | - | - |
| Experimental | Steady state data for different residence times | Steady state data for different residence times | Steady state data for different residence times |
| Model | No | No | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, E.; Anantpinijwatna, A.; Woodley, J.M.; Gani, R. A Reaction Database for Small Molecule Pharmaceutical Processes Integrated with Process Information. Processes 2017, 5, 58. https://doi.org/10.3390/pr5040058
Papadakis E, Anantpinijwatna A, Woodley JM, Gani R. A Reaction Database for Small Molecule Pharmaceutical Processes Integrated with Process Information. Processes. 2017; 5(4):58. https://doi.org/10.3390/pr5040058
Chicago/Turabian StylePapadakis, Emmanouil, Amata Anantpinijwatna, John M. Woodley, and Rafiqul Gani. 2017. "A Reaction Database for Small Molecule Pharmaceutical Processes Integrated with Process Information" Processes 5, no. 4: 58. https://doi.org/10.3390/pr5040058





