Selected Phenomena of the In-Mold Nodularization Process of Cast Iron That Influence the Quality of Cast Machine Parts
Abstract
:1. Introduction
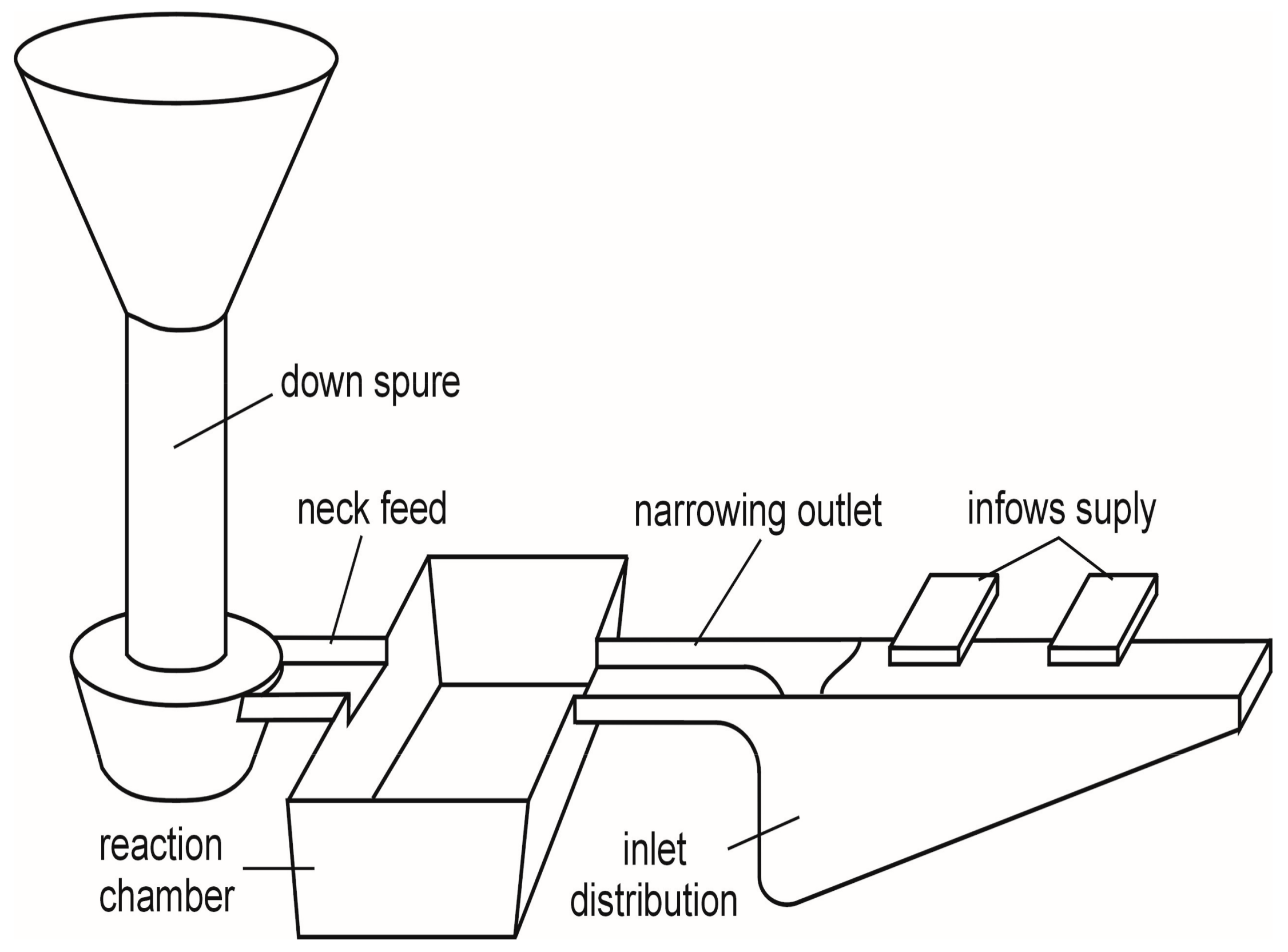
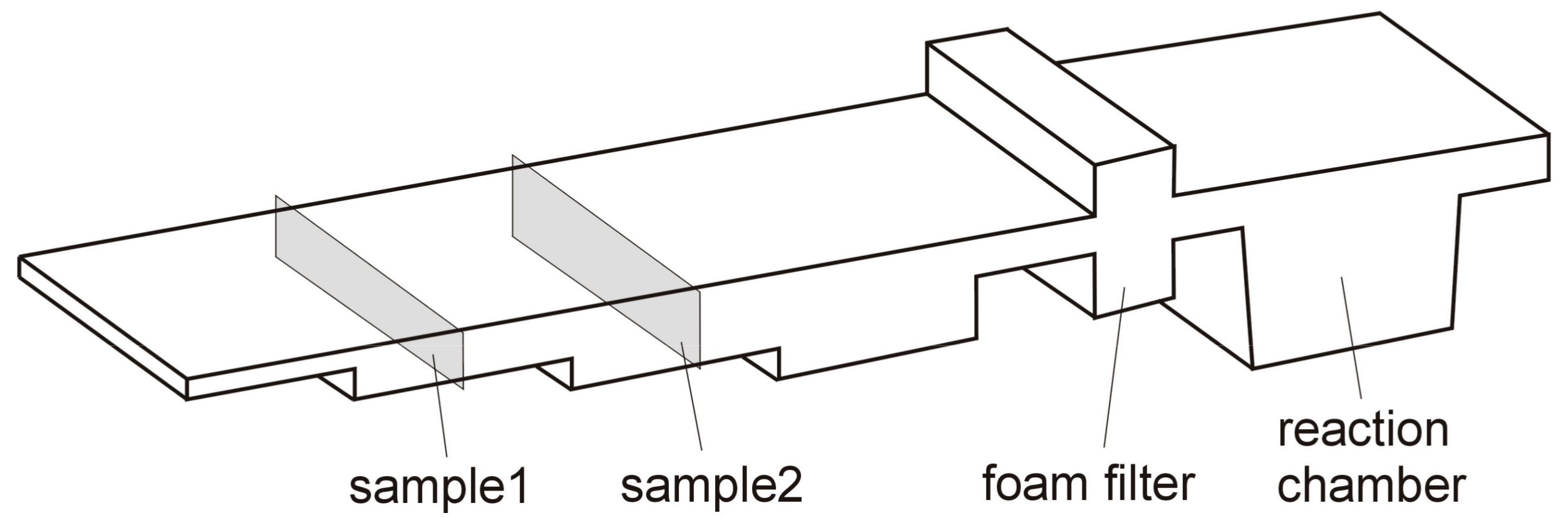
2. Experimental Details
3. Results
3.1. Chemical Composition Analysis
3.2. Metallographic Examinations
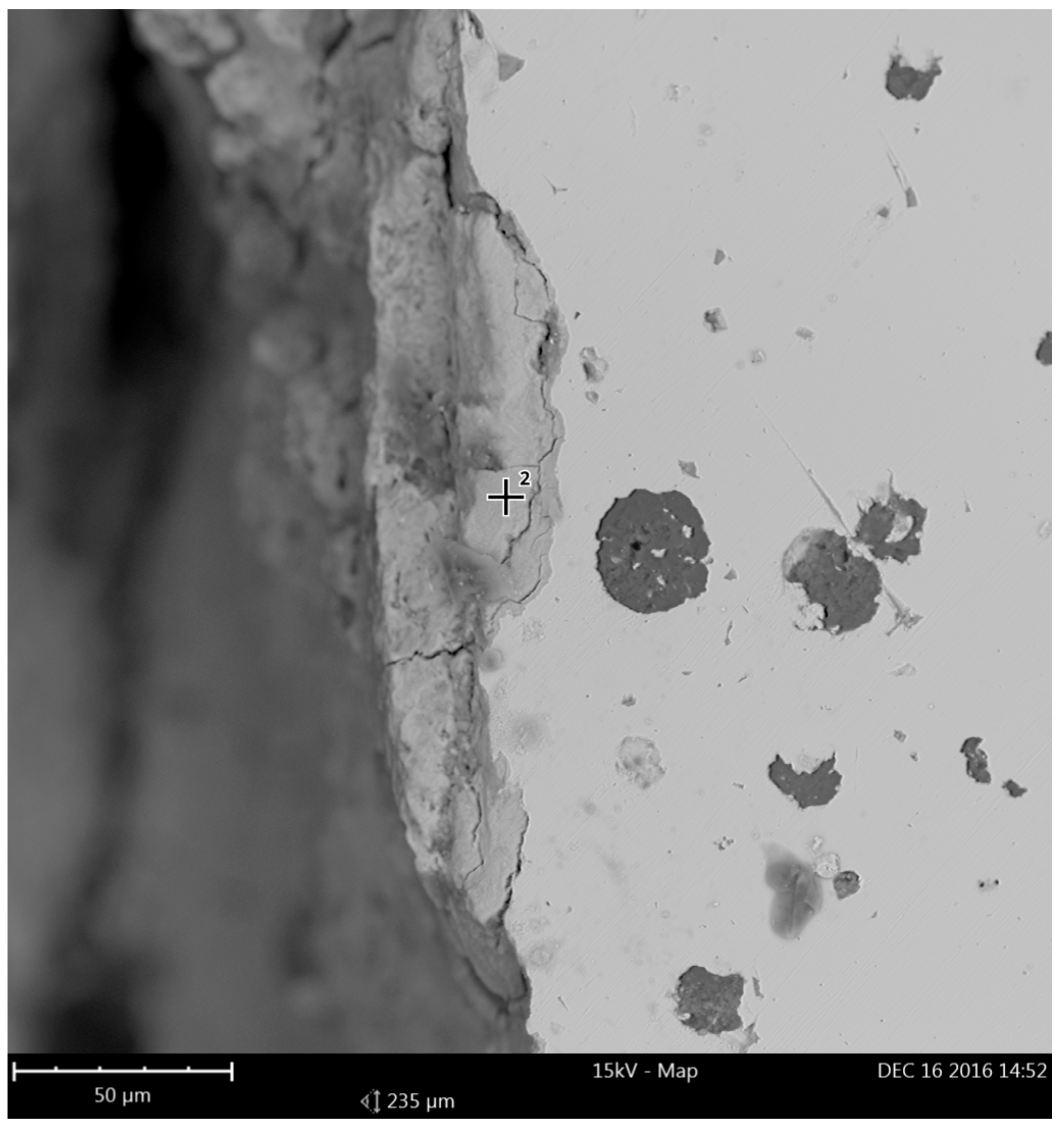
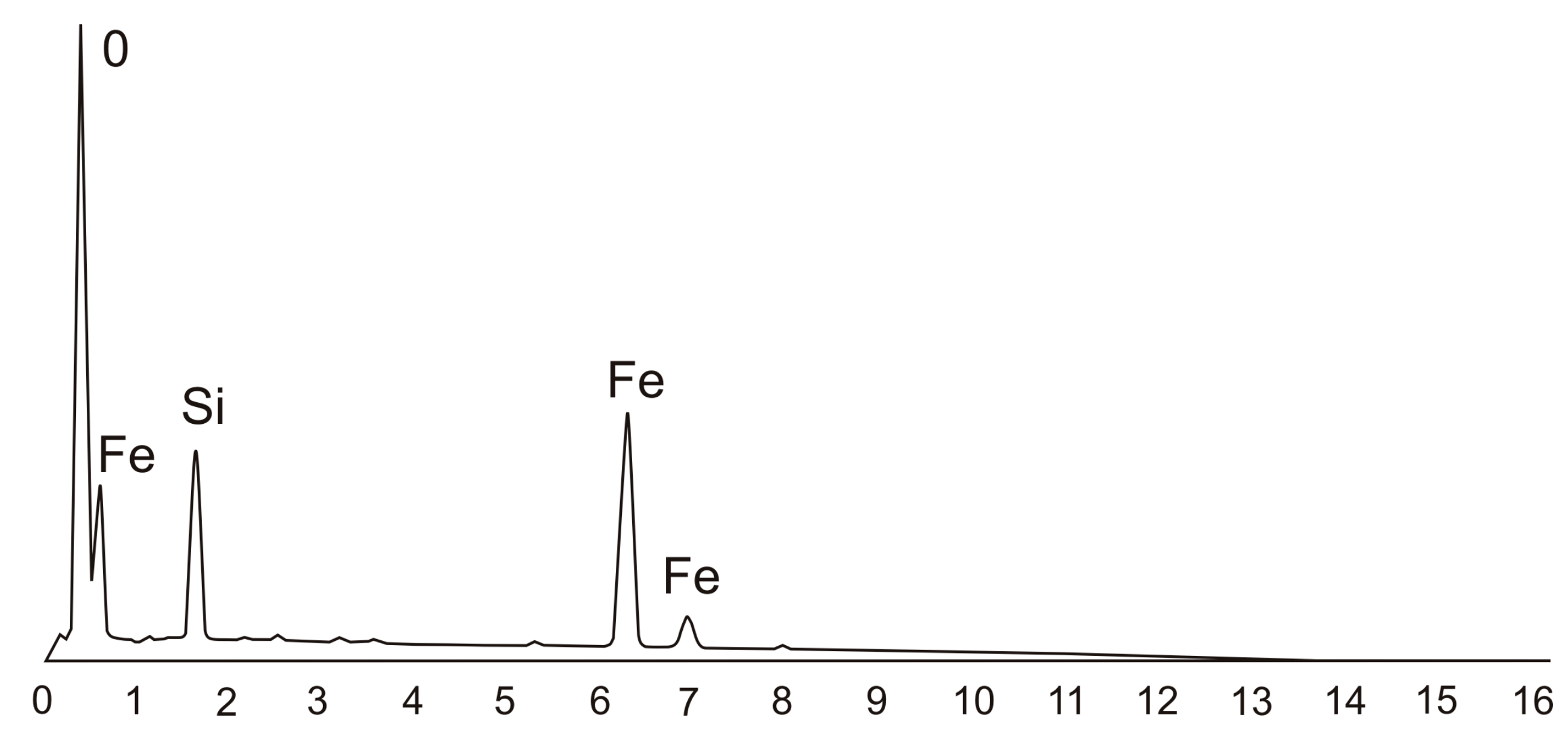
3.3. Corrosion of the Surface Layer
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Riposan, I.; Chisamera, M.; Kelley, R.; Barstow, M.; Naro, R.L. Magnesium-sulfur relationships in ductile and compacted graphite cast irons as influenced by late sulfur additions. Am. Foundry Soc. Trans. 2003, 869–884. [Google Scholar]
- Irons, G.A.; Guthrie, R.I.L. Kinetic aspects of magnesium desulfurization of blast furnace iron. Ironmark. Steelmark. 1981, 8, 114–121. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics, 84th ed.; CRC Press LLC: New York, NY, USA, 2004; p. 746. [Google Scholar]
- Campbell, J. A Hypothesis for cast iron microstructures. Met. Mater. Trans. Part B 2009, 40, 786–801. [Google Scholar] [CrossRef]
- Janerka, K.; Pawlyta, M.; Jezierski, J.; Szajnar, J.; Bartocha, D. Carburiser properties transfer into the structure of melted cast iron. J. Mater. Process. Technol. 2014, 214, 794–801. [Google Scholar] [CrossRef]
- Stawarz, M. SiMo ductile iron crystallization process. Arch. Foundry Eng. 2017, 1, 147–152. [Google Scholar] [CrossRef]
- Kajzer, A.; Kajzer, W.; Gołombek, K.; Knol, M.; Dzielicki, J.; Walke, W. Corrosion resistance, eis and wettability of the implants made of 316 LVM steel used in chest deformation treatment. Arch. Met. Mater. 2016, 61, 767–770. [Google Scholar] [CrossRef]
- Stawarz, M.; Kajzer, W.; Kajzer, A.; Dojka, M. Physicochemical properties of silicon cast iron. Arch. Foundry Eng. 2008, 17, 101–106. [Google Scholar] [CrossRef]
- Kopyciński, D.; Guzik, E. Effective inoculation of low-sulphur cast iron. Arch. Foundry Eng. 2008, 4, 77–80. [Google Scholar]
- Gumienny, G.; Kacprzyk, B.; Gawroński, J. Effect of copper on the crystallization process, microstructure and selected properties of CGI. Arch. Foundry Eng. 2017, 1, 51–56. [Google Scholar] [CrossRef]
- Trepczyńska-Łent, M. Solid-liquid interface morphology of white carbide eutectic during directional solidification. Arch. Met. Mater. 2017, 62, 365–368. [Google Scholar] [CrossRef]
- Zuk, M.; Gorka, J.; Dojka, R.; Czuprynski, A. Repair welding of cast iron coated electrodes. In IOP Conference Series-Materials Science and Engineering, Proceedings of the 5th International Conference on Modern Technologies in Industrial Engineering (ModTech), Sibiu, Romania, June 14–17 2017; Kifor, C., Naito, M., Carausu, C., Topala, P., Wrobel, A., Oanta, E., Schnakovszky, C., Paunoiu, V., Spanu, S., Nedelcu, D., Eds.; IOP Publishing Ltd.: Bristol, UK, 2017. [Google Scholar]
- Pacyniak, T.; Kaczorowski, R. Ductile cast iron obtaining by inmold method with use of lost foam process. Arch. Foundry Eng. 2010, 1, 101–104. [Google Scholar]
- Wilk-Kolodziejczyk, D.; Regulski, K.; Gumienny, G. Comparative analysis of the properties of the nodular cast iron with carbides and the austempered ductile iron with use of the machine learning and the support vector machine. Int. J. Adv. Manf. Techol. 2016, 87, 1077–1093. [Google Scholar] [CrossRef]
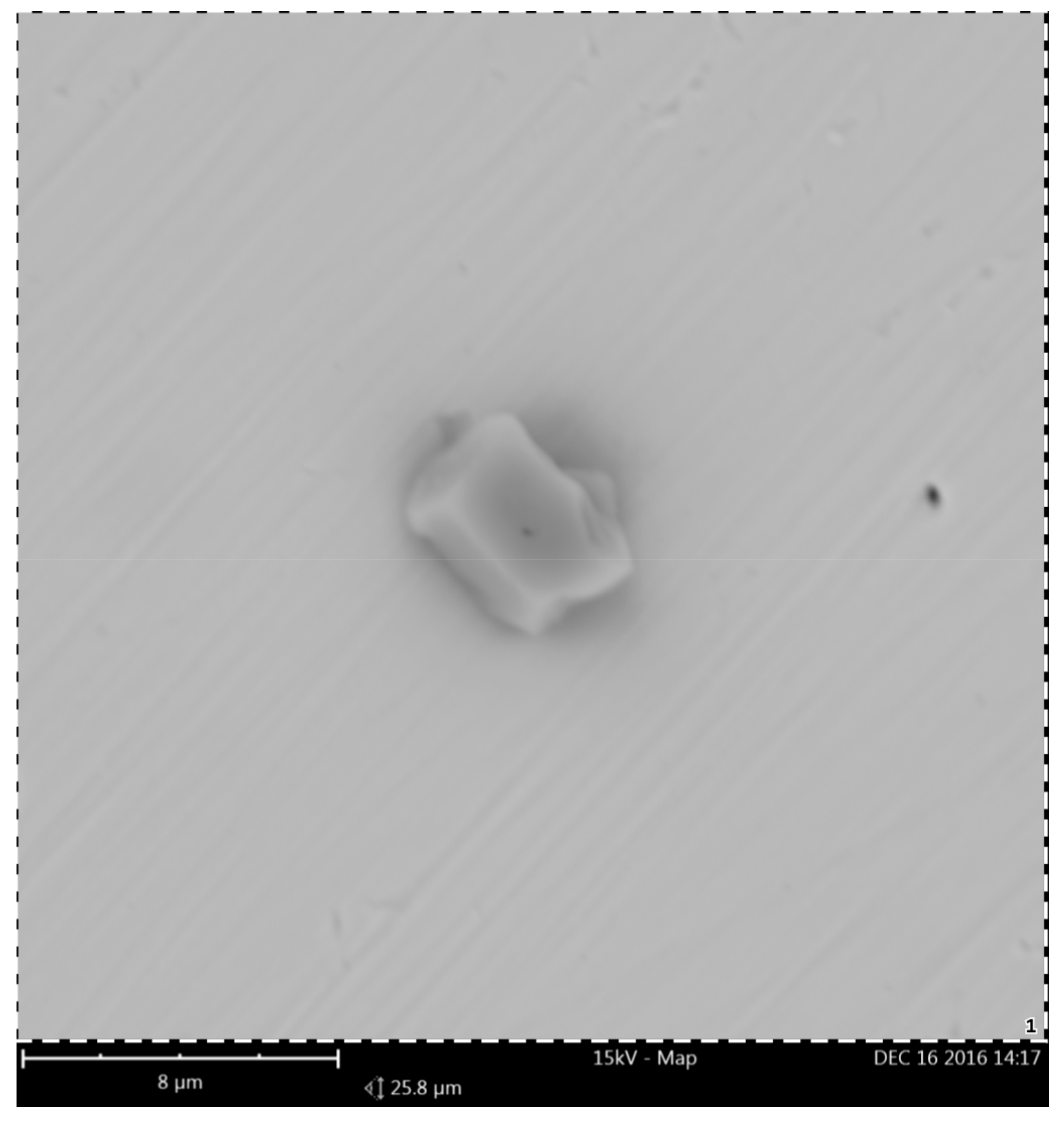
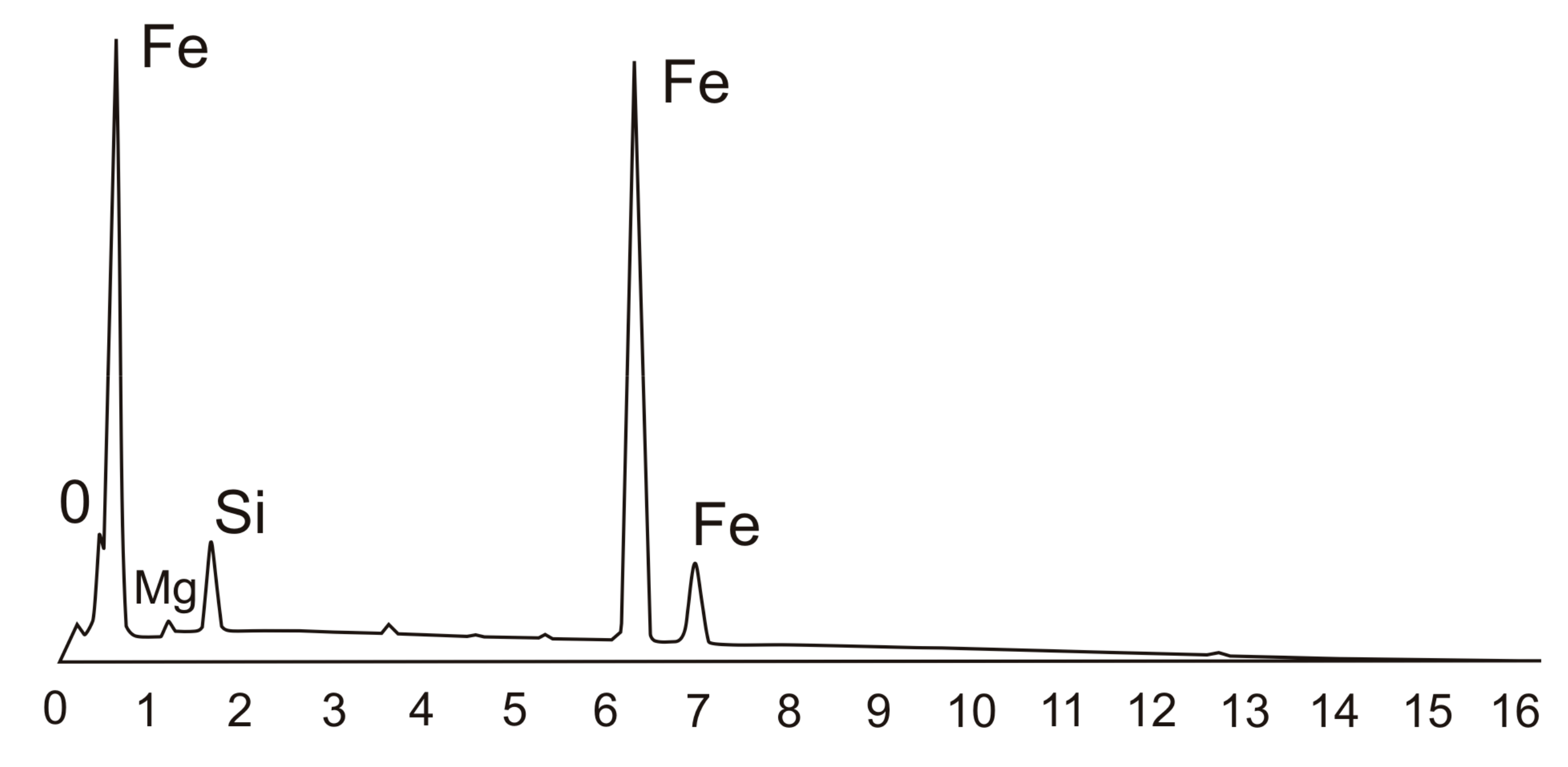
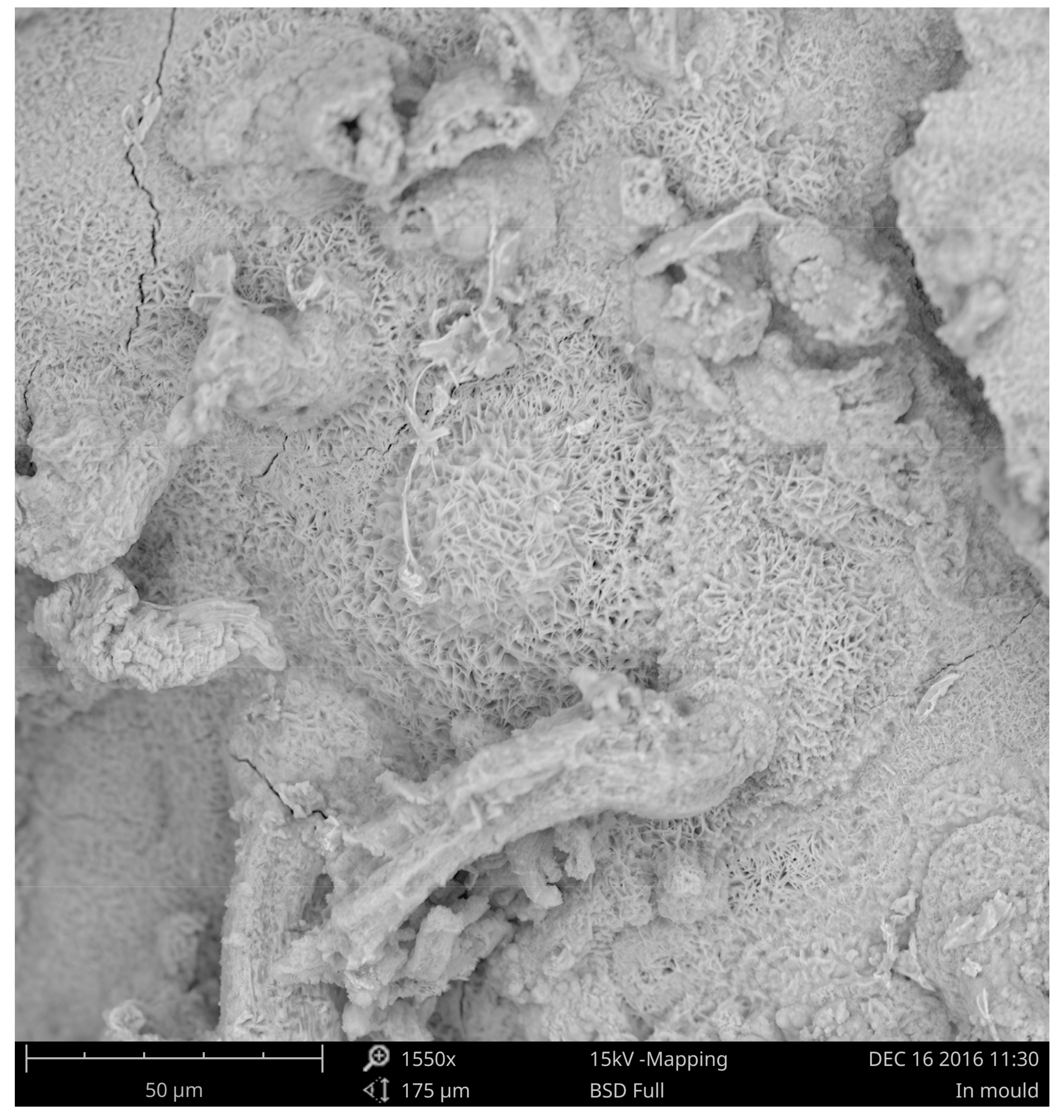
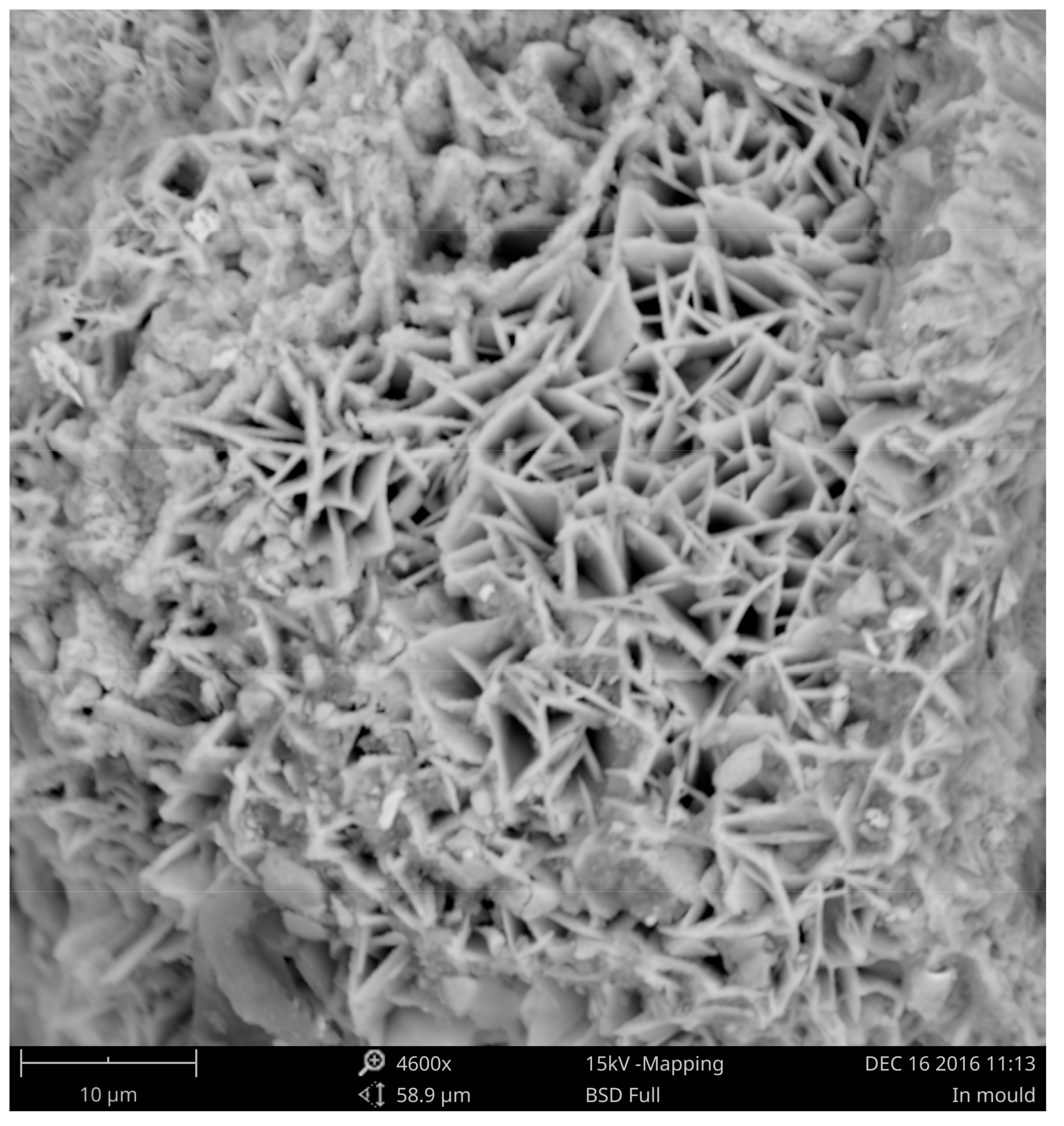
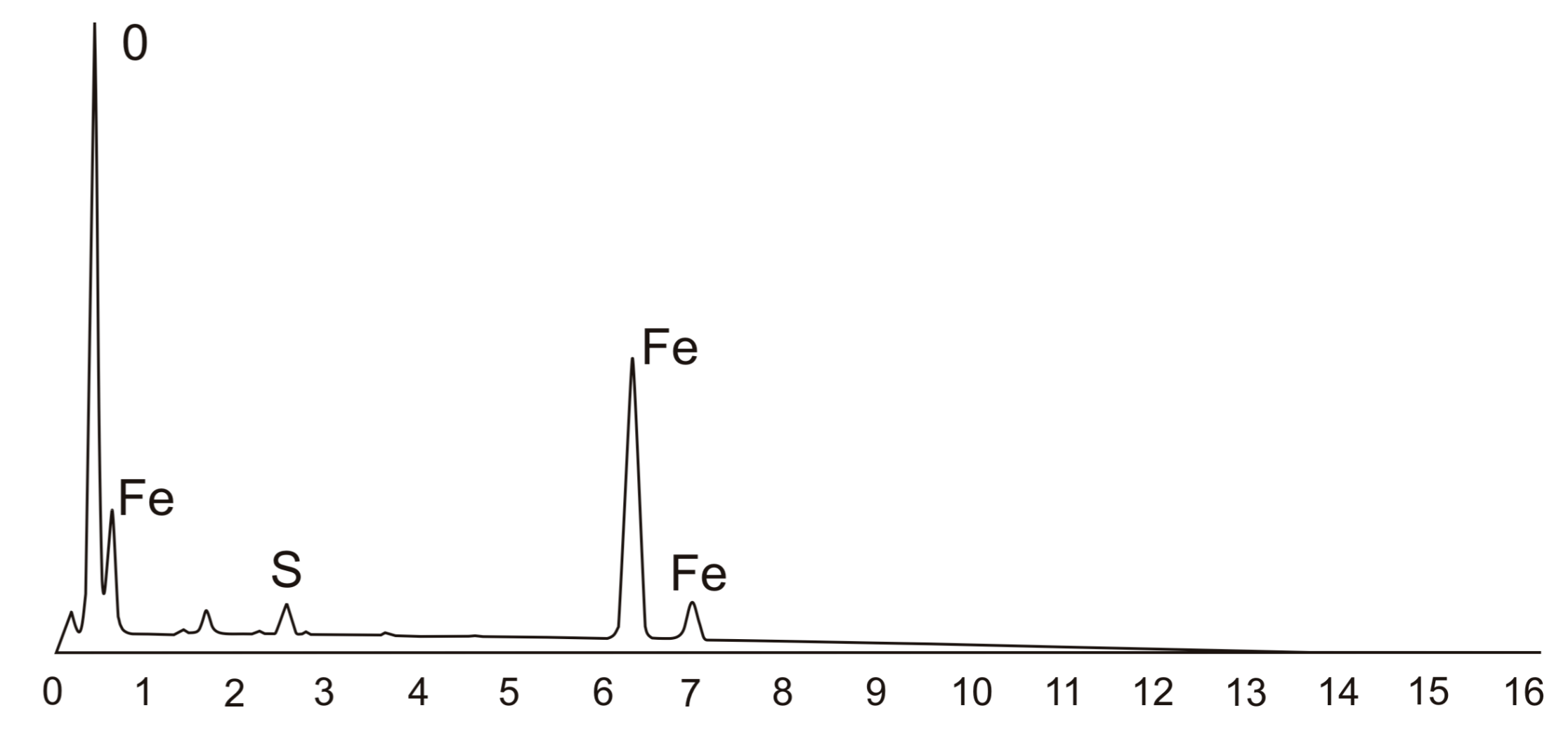
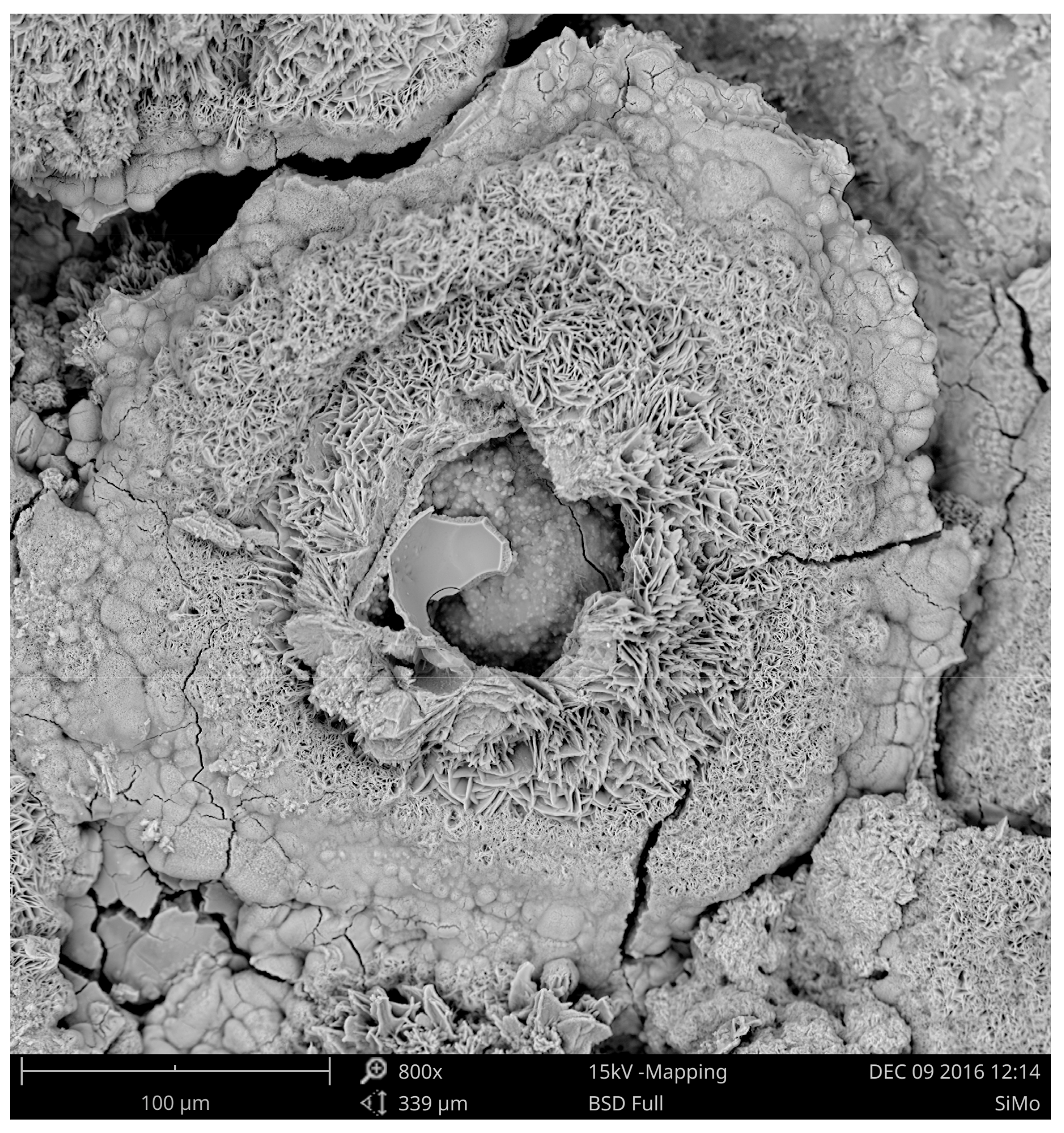
| Chemical Composition/wt.% | |||||||
|---|---|---|---|---|---|---|---|
| C | Mn | Si | P | Mo | S | Mg | |
| 1 * | 3.65 | 0.089 | 2.87 | 0.03 | 0.001 | 0.015 | - |
| 2 ** | 3.64 | 0.087 | 2.94 | 0.03 | 0.001 | 0.012 | 0.041 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarz, M.; Janerka, K.; Dojka, M. Selected Phenomena of the In-Mold Nodularization Process of Cast Iron That Influence the Quality of Cast Machine Parts. Processes 2017, 5, 68. https://doi.org/10.3390/pr5040068
Stawarz M, Janerka K, Dojka M. Selected Phenomena of the In-Mold Nodularization Process of Cast Iron That Influence the Quality of Cast Machine Parts. Processes. 2017; 5(4):68. https://doi.org/10.3390/pr5040068
Chicago/Turabian StyleStawarz, Marcin, Krzysztof Janerka, and Malwina Dojka. 2017. "Selected Phenomena of the In-Mold Nodularization Process of Cast Iron That Influence the Quality of Cast Machine Parts" Processes 5, no. 4: 68. https://doi.org/10.3390/pr5040068





