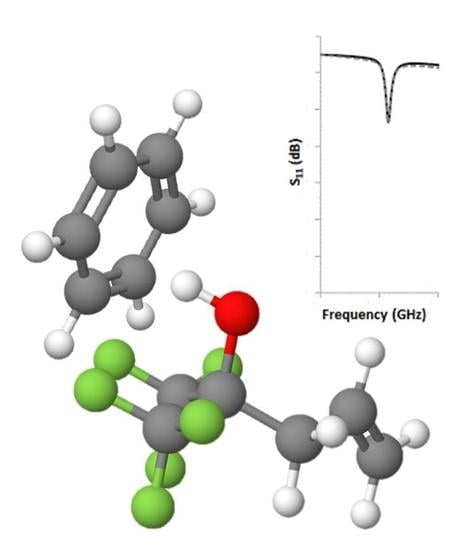
Investigation of the Interaction between Benzene and SXFA Using DFT
Abstract
:1. Introduction
2. Methodology
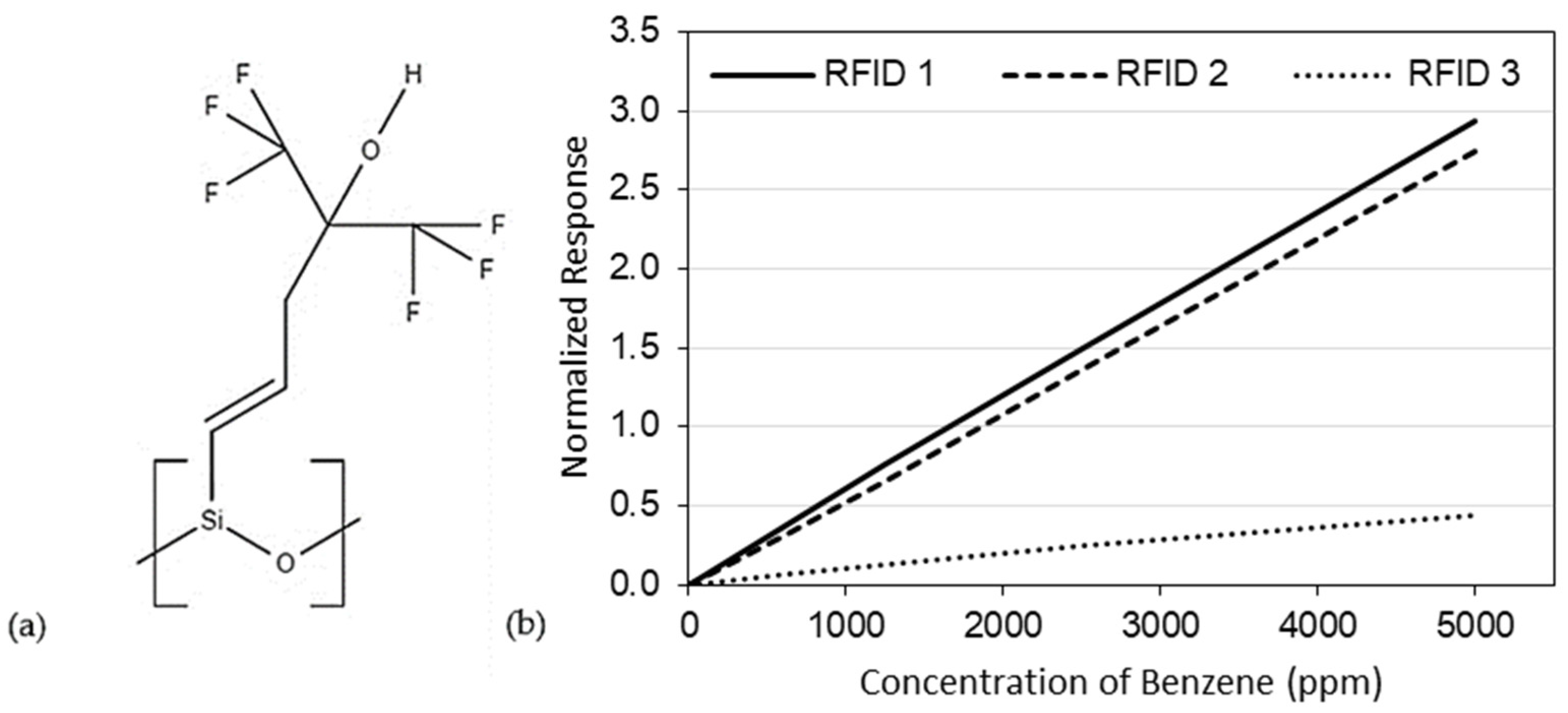
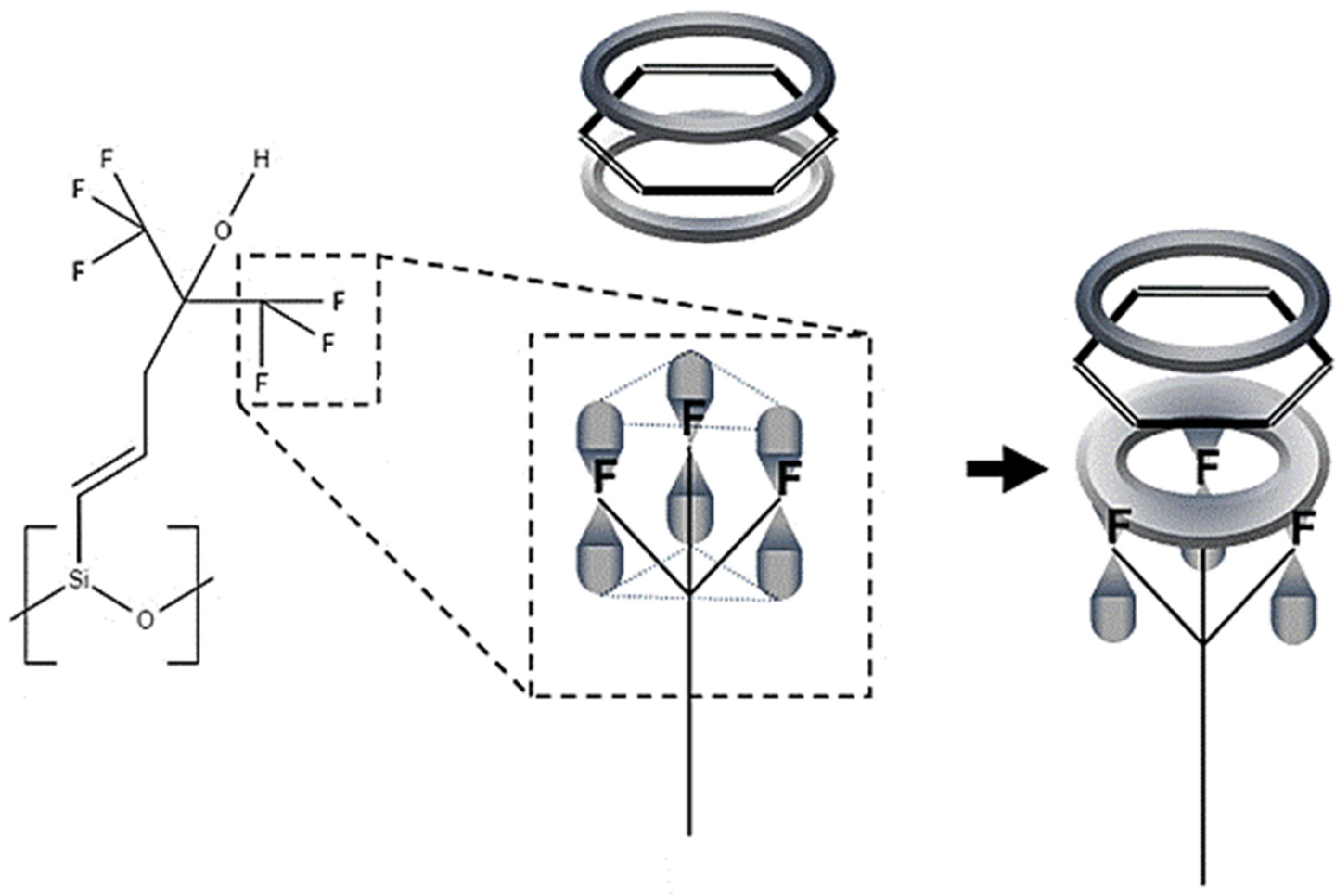
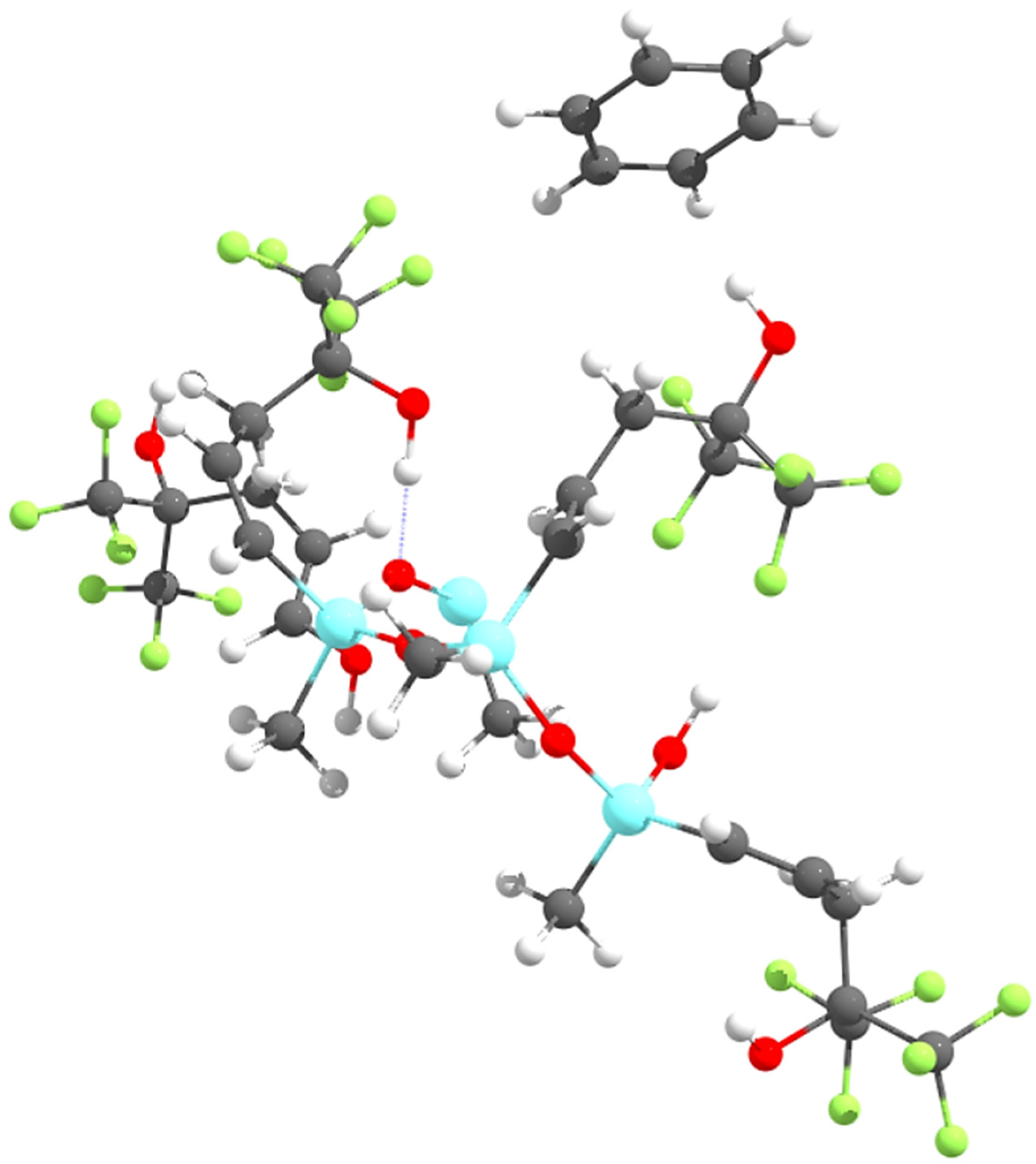
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, S.C.; Chiu, M.Y.; Ho, K.F.; Zou, S.C.; Wang, X. Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong. Chemosphere 2002, 48, 375–382. [Google Scholar] [CrossRef]
- World Health Organization. Exposure to Benzene: A Major Public Health Concern; Public Health and Environment, World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Mondal, R.K.; Dubey, K.A.; Bhardwaj, Y.K.; Varshney, L. Novel hybrid nanocarbons/poly (dimethylsiloxane) composites based chemiresistors for real time detection of hazardous aromatic hydrocarbons. Carbon 2016, 100, 42–51. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, M.C.; Severin, E.J.; Doleman, B.J.; Beaber, S.A.; Grubbs, R.H.; Lewis, N.S. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistors. Chem. Mater. 1996, 8, 2298–2312. [Google Scholar] [CrossRef]
- Mabrook, M.; Hawkins, P. A rapidly-responding sensor for benzene, methanol, and ethanol vapours based on films of titanium dioxide dispersed in a polymer operating at room temperature. Sens. Actuators B 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Talwar, V.; Singh, O.; Singh, R.C. ZnO assisted polyaniline nanofibers and its application as ammonia gas sensor. Sens. Actuators B 2014, 191, 276–282. [Google Scholar] [CrossRef]
- Stewart, K.M.E.; Penlidis, A. Designing polymeric sensing materials: What are we doing wrong? Polym. Adv. Technol. 2007, 28, 319–344. [Google Scholar] [CrossRef]
- Avramov, P.V.; Kudin, K.N.; Scuseria, G.E. Single wall carbon nanotubes density of states: Comparison of experiment and theory. Chem. Phys. Lett. 2003, 370, 597–601. [Google Scholar] [CrossRef]
- Tolmachev, A.M.; Firsov, D.A.; Kuznetsova, T.A.; Anuchin, K.M. DFT modeling of the adsorption of benzene, methanol, and ethanol molecules in activated carbon nanopores. Mol. Supramol. Struct. Interfaces 2009, 45, 177–183. [Google Scholar] [CrossRef]
- Pannopard, P.; Khongpracha, P.; Probst, M.; Limtrakul, J. Gas sensing properties of platinum derivatives of single-walled carbon nanotubes: A DFT analysis. J. Mol. Graph. Model. 2009, 28, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Shah, A.A.; Bilal, S.; Ayab, K. DFT Study of Polyaniline NH3, CO2, and CO Gas Sensors: Comparison with Recent Experimental Data. J. Phys. Chem. C 2013, 117, 23701–23711. [Google Scholar] [CrossRef]
- Tran, H.T.; Spencer, M.J. Zinc oxide for gas sensing of formaldehyde: Density functional theory modelling of the effect of nanostructure morphology and gas concentration on the chemisorption reaction. Mater. Chem. Phys. 2017, 193, 274–284. [Google Scholar] [CrossRef]
- Chen, W.T.; Stewart, K.M.E.; Yang, C.K.; Mansour, R.R.; Carroll, J.; Penlidis, A. Wearable RF Sensor Array Implementing Coupling-Matrix Readout Extraction Technique. IEEE Trans. Microw. Theory Tech. 2015, 63, 4157–4168. [Google Scholar] [CrossRef]
- Chen, W.T.; Stewart, K.M.E.; Mansour, R.R.; Penlidis, A. Novel underloaded radio-frequency (RF) resonant sensor for gaseous ethanol and interferents detection. Sens. Actuators A 2015, 230, 63–73. [Google Scholar] [CrossRef]
- Stewart, K.M.E.; Penlidis, A. Novel test system for gas sensing materials and sensors. Macromol. Symp. 2013, 324, 11–18. [Google Scholar] [CrossRef]
- Lazzaroni, R.; Calderone, A.; Bredas, J.; Rabe, J. Electronic structure of molecular van der Waals complexes with benzene: Implications for the contrast in scanning tunneling microscopy of molecular adsorbates on graphite. J. Chem. Phys. 1997, 107, 99–105. [Google Scholar] [CrossRef]
- Menapace, J.; Bernstein, E. Van der Waals modes of solute/solvent clusters: Benzene-methane, -deuteriomethane, and -carbon tetrafluoride. J. Phys. Chem. 1987, 91, 2843–2848. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Jurecka, P.; Tarakeshwar, P.; Hobza, P.; Kim, K. Understanding of assembly phenomena by aromatic-aromatic interactions: Benzene dimer and the substituted systems. J. Phys. Chem. A 2007, 111, 3446–3457. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Green, P.G.; Bumgarner, R.E.; Dasgupta, S.; Goddard, W.A., III; Blake, G.A. Benzene forms hydrogen bonds with water. Science 1992, 257, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Wanna, J.; Menapace, J.; Bernstein, E. Hydrogen bonded and non-hydrogen bonded van der Waals clusters: Comparison between clusters of pyrazine, pyrimidine, and benzene with various solvents. J. Chem. Phys. 1986, 85, 1795–1805. [Google Scholar] [CrossRef]
- Dougherty, D.A. Cation-pi interactions in chemistry and biology: A new view of benzene, Phe, Tyr, and Trp. Science 1996, 271, 163–168. [Google Scholar] [CrossRef] [PubMed]
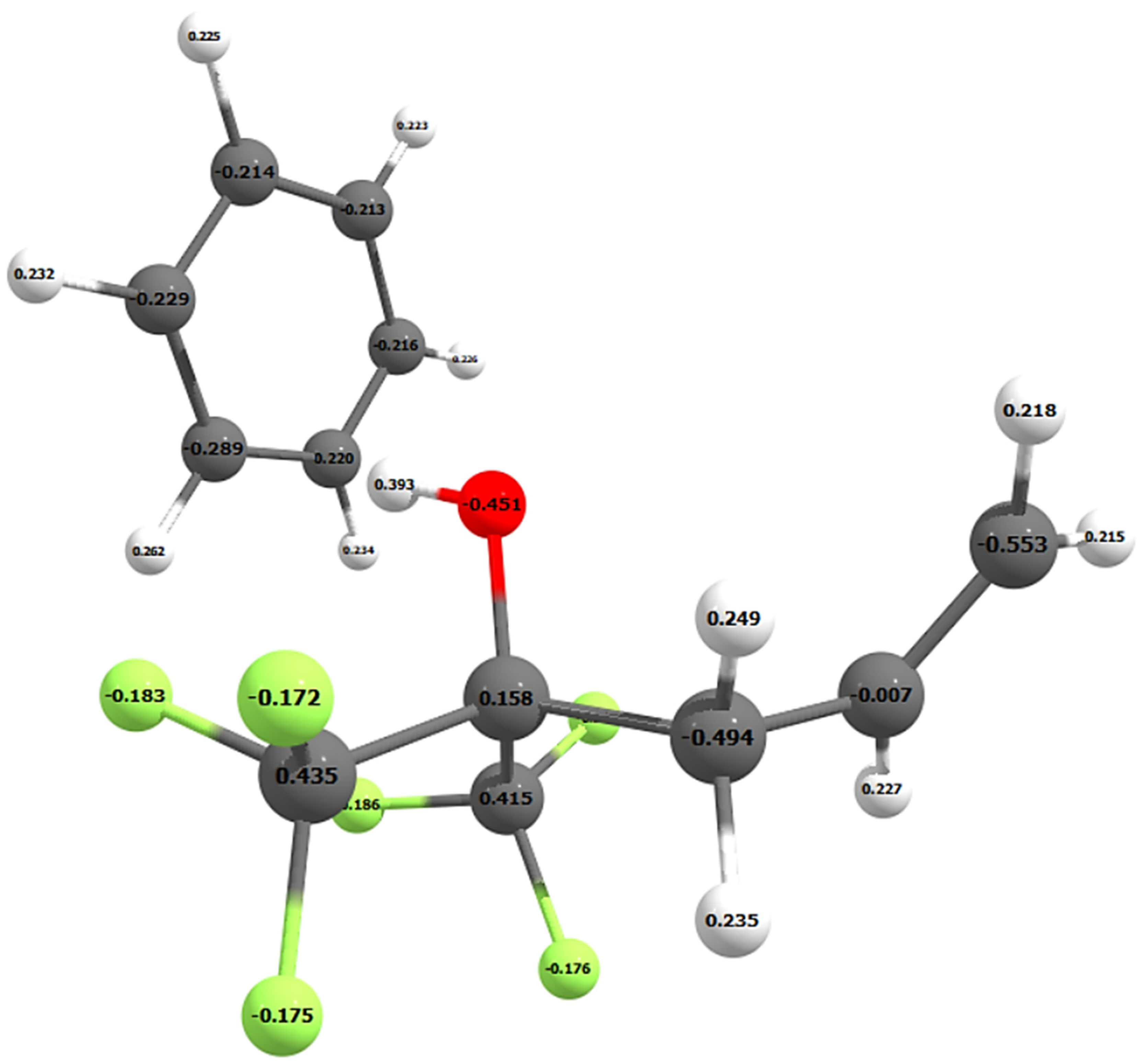
| Chain Length | Mulliken Atomic Charge on O | Mulliken Atomic Charge on H | NBO Atomic Charge on O | NBO Atomic Charge on H |
|---|---|---|---|---|
| 1 (Side chain only) | −0.451 | 0.393 | −0.785 | 0.529 |
| 2 | −0.447 −0.483 * | 0.388 0.459 * | −0.779 −0.781 * | 0.518 0.525 |
| 3 | −0.448 −0.452 −0.486 * | 0.387 0.381 0.454 * | −0.777 −0.781 −0.812 * | 0.521 0.524 0.544 * |
| 4 | −0.453 −0.454 −0.468 −0.497 * | 0.389 0.380 0.404 0.468 * | −0.765 −0.781 −0.807 −0.811 * | 0.512 0.524 0.492 0.495 * |
| Bond Lengths and Angles | SXFA | Adsorbed Benzene |
|---|---|---|
| C-O Length | 1.44438 Å | 1.43784 Å |
| O-H Length | 0.98162 Å | 0.98789 Å |
| C-O-H Angle | 111.294° | 112.839° |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, K.M.E.; Hamilton, I.P.; Penlidis, A. Investigation of the Interaction between Benzene and SXFA Using DFT. Processes 2018, 6, 10. https://doi.org/10.3390/pr6020010
Stewart KME, Hamilton IP, Penlidis A. Investigation of the Interaction between Benzene and SXFA Using DFT. Processes. 2018; 6(2):10. https://doi.org/10.3390/pr6020010
Chicago/Turabian StyleStewart, Katherine M. E., Ian P. Hamilton, and Alexander Penlidis. 2018. "Investigation of the Interaction between Benzene and SXFA Using DFT" Processes 6, no. 2: 10. https://doi.org/10.3390/pr6020010