Recovery of Filtered Graphene Oxide Residue Using Elastic Gel Packed in a Column by Cross Flow
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Spherical Gel
2.3. Preparation of Graphene Oxide
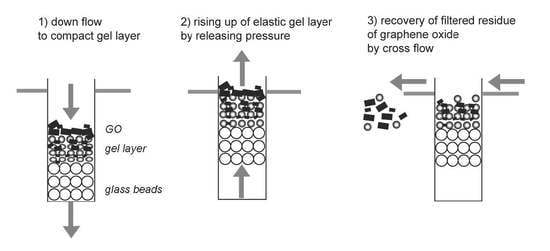
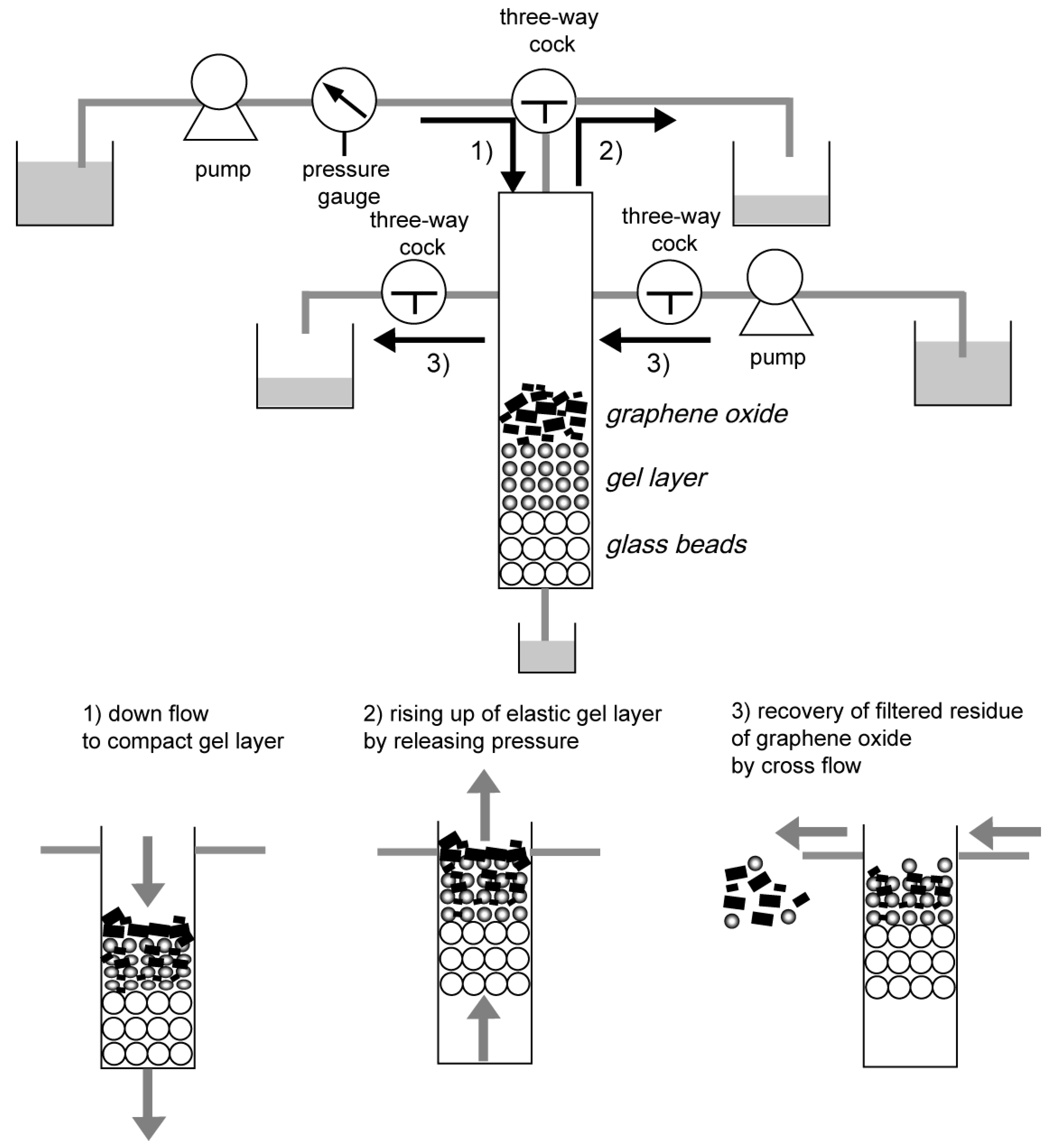
2.4. Filtration of Graphene Oxide and the Rising of the Gel Layer
2.5. Recovery of Filtered Graphene Oxide on the Rising Gel by Cross Flow
3. Results and Discussion
3.1. Dependence of the Height of the Rise of the Gel Layer on the Injected Volume of the Graphite Oxide Suspension
3.2. Recovery of the Filtered Graphene Oxide above the Gel Layer by Cross Flow
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Satyanarayana Chilukuri, V.V.; Marshall, A.D.; Munro, P.A.; Singh, H. Effect of sodium dodecyl sulphate and cross-flow velocity on membrane fouling during cross-flow microfiltration of lactoferrin solutions. Chem. Eng. Proc. 2001, 40, 321–328. [Google Scholar] [CrossRef]
- Makabe, R.; Akamatsu, K.; Nakao, S. Mitigation of particle deposition onto membrane surface in cross-flow microfiltration under high flow rate. Sep. Purif. Technol. 2016, 160, 98–105. [Google Scholar] [CrossRef]
- Himstedt, H.H.; Yang, Q.; Dasi, L.P.; Qian, X.; Wickramasinghe, S.R.; Ulbricht, M. Magnetically activated micromixers for separation membranes. Langmuir 2011, 27, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Uno, Y.; Morisada, S.; Ohto, K.; Kawakita, H. Filtration and recovery of starch granules using assembled magnetite filter. Chem. Eng. Proc. 2016, 110, 128–133. [Google Scholar] [CrossRef]
- Lu, W.-M.; Tung, K.-L.; Hung, S.-M.; Shiau, J.-S.; Hwang, K.-J. Constant pressure filtration of mono-dispersed deformable particle slurry. Sep. Sci. Technol. 2001, 36, 2355–2383. [Google Scholar] [CrossRef]
- Ueyama, K.; Furusaki, S. A theoretical study on the effect of compaction on the effectiveness factor of gel particles containing immobilized enzymes. Chem. Eng. Commun. 1985, 36, 299–316. [Google Scholar] [CrossRef]
- Takaoka, Y.; Morisada, S.; Ohto, K.; Kawakita, H. Filtration of colloidal particles using compacted-gel media packed in a column. J. Chem. Eng. Jpn. 2017, 50, 815–820. [Google Scholar] [CrossRef]
- Bidram, E.; Sulistio, A.; Amini, A.; Fu, Q.; Qjao, G.G.; Stewart, A.; Dunstan, D.E. Fractionation of graphene oxide single nano-sheets in water-glycerol solutions using gradient centrifugation. Carbon 2016, 103, 363–371. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Wirtz, C.; Duesberg, G.S.; Coleman, J.N. Lateral size selection of surfactant-stabilized graphene flakes using size exclusion chromatography. Chem. Phys. Lett. 2012, 531, 169–172. [Google Scholar] [CrossRef]
- Hirayama, C.; Yamaguchi, K.; Matsumoto, K.; Motozato, Y. Preparation of poly(N,N-dimethylamide) gels having a void in the center of the bead by suspension copolymerization. Kobunshi Ronbunshu 1983, 40, 547–553. [Google Scholar] [CrossRef]
- Gundogan, N.; Okay, O.; Oppermann, W. Swelling, elasticity and spatial inhomogeneity of poly(N,N-dimethylacrylamide) hydrogels formed at various polymer concentrations. Macromol. Chem. Phys. 2004, 205, 814–823. [Google Scholar] [CrossRef]
- Vallés, C.; Young, R.J.; Lomax, D.J.; Kinloch, I.A. The rheological behavior of concentrated dispersions of graphene oxide. J. Mater. Sci. 2014, 49, 6311–6320. [Google Scholar] [CrossRef]





| Chemicals | Amount of Chemicals | ||
|---|---|---|---|
| Mass (g) | Mass (mol) | ||
| monomer phase | N,N-dimethylacrylamide | 10 | 0.1 |
| N,N′-methylene bisacrylamide | 0.25 | 0.0016 | |
| azobis-iso-butyronitrile | 0.2 | 0.0012 | |
| water phase | sodium sulfate | 93 | 0.65 |
| sodium phosphate dodecahydrate | 10 | 0.026 | |
| carboxymethylcellulose sodium | 2.0 | - | |
| calcium carbonate | 5.0 | 0.05 | |
| distilled water | 400 | 22 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaoka, Y.; Miyoshi, M.; Sakaguchi, K.; Morisada, S.; Ohto, K.; Kawakita, H. Recovery of Filtered Graphene Oxide Residue Using Elastic Gel Packed in a Column by Cross Flow. Processes 2018, 6, 43. https://doi.org/10.3390/pr6050043
Takaoka Y, Miyoshi M, Sakaguchi K, Morisada S, Ohto K, Kawakita H. Recovery of Filtered Graphene Oxide Residue Using Elastic Gel Packed in a Column by Cross Flow. Processes. 2018; 6(5):43. https://doi.org/10.3390/pr6050043
Chicago/Turabian StyleTakaoka, Yuji, Manoka Miyoshi, Koichi Sakaguchi, Shintaro Morisada, Keisuke Ohto, and Hidetaka Kawakita. 2018. "Recovery of Filtered Graphene Oxide Residue Using Elastic Gel Packed in a Column by Cross Flow" Processes 6, no. 5: 43. https://doi.org/10.3390/pr6050043