1. Introduction
Packed-bed or fixed-bed reactors are ubiquitous in the chemical process industry [
1,
2,
3]. With the increasing popularity of flow chemistry-based synthesis, their use is likely to increase [
4,
5,
6]. A column is the most common configuration for a packed-bed reactor. It is a cylindrical vessel within which a bed of particles is tightly held in place under compression (see
Figure 1). In certain specialized forms of packed-beds, such as monoliths [
7,
8], the particles are fused together. Packed columns are also used for chemical separation techniques such as adsorption, distillation, extraction and chromatography [
9,
10,
11,
12].
A packed-column reactor generally facilitates contact between the surface of catalyst particles and reactant/s present in the fluid flowing through it. The most common example of the use of such catalytic packed-column reactors is ammonia synthesis. Columns packed with inert material could also be used to bring about intimate contact between reactants in a simple and continuous flow-through mode. In a separation process, the solid material comprising the packed column is generally the separation media, e.g., an adsorbent, being used to separate a solute of interest. Packed columns are also used to increase the interfacial area between immiscible fluids in processes such as distillation and extraction.
The residence time distribution or RTD is an important attribute in packed-bed reactors [
13,
14,
15]. By the rule of thumb, the wider the column, and the poorer (i.e., the broader) is the RTD. Therefore, wider columns are less efficient than longer columns. Also, with a column of a particular bed height, the heat transfer surface area to reactor volume ratio (i.e., specific surface area, excluding the headers) varies inversely as the radius. Therefore, when columns with large cross-sectional areas are used, the available heat transfer area could become a limiting factor [
16,
17,
18]. Where this is the case, multi-tube packed-column reactors are commonly used [
19,
20,
21,
22]. While this multi-tube configuration increases heat-transfer, there are penalties in the form of design complexity, and higher equipment cost.
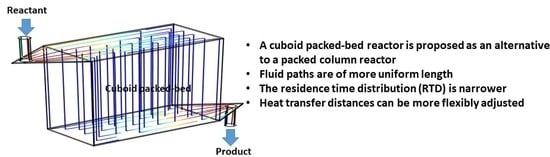
Chromatography is traditionally carried out using packed columns. In recent papers from my group, we have proposed the use of box-shaped or cuboid packed-bed devices as alternative to columns, for carrying out chromatographic separation of proteins [
23,
24,
25,
26]. The design of these cuboid packed-bed devices was inspired by laterally fed membrane chromatography (or LFMC) devices [
27,
28,
29,
30,
31], also developed in recent years in my research group. A diagrams of a cuboid packed-bed device is shown in
Figure 2. A set of lateral flow channels similar to those utilized in LFMC devices [
27,
28,
29,
30,
31] are used to distribute the feed liquid into the cuboid packed-bed and collect the effluent liquid emerging from it on the other side. Such flow arrangement ensures uniformity in solute flow path lengths, which in turn contributes towards the narrowing of the solute RTD within the device [
24,
29].
Figure 1 and
Figure 2 show images of a packed column and a cuboid packed-bed respectively, in each case, showing the idealized flow-paths within these devices. As mentioned earlier, a combination of variability in the flow path lengths and variability of velocity along these flow paths results in a significant broadening in solute RTD within a column [
23]. This contributes towards peak broadening and poor peak-resolution in multi-component separation. By contrast, the flow path lengths within a cuboid packed-bed are fairly uniform and the velocities along these do not vary that significantly [
23]. The cuboid packed-bed devices described in our papers outperformed their corresponding equivalent chromatography columns (i.e., packed with same media, and having the same bed height and area of cross-section) in terms of multiple separation metrics such as the number of theoretical plates, peak shape, peak width, peak asymmetry, and resolution in multi-component protein separation [
23,
24,
25,
26]. The dispersion effects in these cuboid packed-bed devices were significantly lower than those in their equivalent columns as evident from their greater numbers of theoretical plates per unit bed height. Based on the above considerations, could it be assumed that box-shaped or cuboid packed-bed reactors would outperform packed-column reactors, at least in terms of their hydraulic properties and RTD attributes?
This paper proposes the use of cuboid packed-beds as reactors for carrying out chemical and biochemical reactions. The rationale this is provided in terms of advantages resulting from superior system hydraulics and the resultant narrower RTD. The residence time distribution predictions are based on mathematical models discussed in our earlier papers [
24,
29]. Other potential advantages, such as superior heat transfer attributes are speculated based on geometric considerations.
2. Materials and Methods
Sodium chloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride solution was prepared using water obtained from a SIMPLICITY 185 water purification unit Millipore (Molsheim, France). All solutions were micro-filtered and degassed prior to use using PVDF micro-filter (VVLP04700, 0.1 µm pore size, Millipore, Billerica, MA, USA). HiTrap Capto S (5 mL, product number 17-5441-2) columns, and Capto S (product number 17-5441-01) chromatographic media were purchased from GE Healthcare Biosciences, QC, Canada. The HiTrap Capto S column was used as the surrogate packed-column reactor while a custom designed cuboid packed-bed device filled with Capto S resin particles served as the surrogate cuboid packed-bed reactor. The basic design of the cuboid packed-bed devices has been described in our previous papers [
23,
24,
25,
26]. The central housing for the cuboid packed-bed was made of polyvinyl chloride (PVC). The upper and lower plates were made of acrylic. The lateral channels engraved in the plates were pillared for efficient flow distribution and collection respectively. The dimensions of the cuboid packed-bed were respectively 25 mm (length) × 8 mm (width) × 25 mm (height), the effective bed volume being 5 mL. The packed-bed was separated from the lateral channels using nylon meshes (0.002 inch opening, product number 9318T48, McMaster Carr, Chicago, IL, USA) for retaining and holding the chromatographic media in place.
An AKTA prime liquid chromatography system (GE Healthcare Biosciences, Quebec, QC, Canada) was used for salt tracer experiments. E-curve type profiles for the packed column and the cuboid packed-bed device were obtained using 0.8 M sodium chloride as tracer and 0.4 M sodium chloride as base solution. A 0.1 mL sample loop was used for injecting the tracer pulse for characterizing the hydraulics of the column and the cuboid packed-bed.
3. Results and Discussion
In a previous paper [
23], the performance of a chromatographic column having 9 mL bed volume (30 mm diameter and 12.7 mm bed height) and packed with Capto S media was compared with an equivalent cuboid packed-bed (58.9 mm length × 12 mm width × 12.7 mm height). At a flow rate of 5 mL/min which corresponded to a superficial velocity of 42.4 cm/h, the number of theoretical plates per meter were found to be 2628 and 4651 respectively, for the column and the cuboid packed-bed. To verify whether such difference was due to flow maldistribution within the column, the RTD within the column and the cuboid packed-bed device were simulated using the using the methods described in our previous papers [
24,
29].
Figure 3 shows the RTD within the column as function of dimensionless radius (
r/
R) and dimensionless column volume (
V/
VT) at a superficial velocity of 42.4 cm/h. The radial distance step used in the simulation was 0.001 cm. The shortest residence time corresponded to
r = 0, i.e., the center of the column, and the value increased significantly towards the periphery. This was consistent with the idealized flow paths picturized in
Figure 1. In the top header, the liquid flowed in a radially outward direction while in the lower header, it flowed in a radially inward direction. This flow pattern resulted in significant variability in the path lengths, i.e., a flow path closer to the center was significantly shorter than one closer to the periphery. Also, as discussed in a previous paper [
23], the radial velocity in the top header decreased very significantly in an outward direction due to an increase in available cross-sectional area, and the loss of liquid due to entry in to the packed-bed.
In the lower header, the radial velocity increased in an inward direction due to a decrease in available cross-sectional area and cumulative collection of liquid from the packed-bed. Quite clearly, the increased transit time in the column headers for fluid elements traversing the peripheral regions of the column contributed significantly to the increase in residence time. Both profiles shown in
Figure 3 indicated the same trend, i.e., the residence time increased very significantly closer to the column periphery. However, this increase was a bit more pronounced when the residence time was plotted versus (
V/
VT). This reflected the fact that in a column, more media was located towards the periphery than near the center.
Figure 4 shows a plot of (
V/
VT) versus (
r/
R) which indicated that 50% of the media existed in the outer 29% radial locations of the column. Hence, the increased residence time closer to the periphery of the column affected a very significant volume fraction of media packed within the column.
Figure 5 shows the RTD within the cuboid packed-bed device as function of dimensionless length (
z/
l). The lateral distance step used in the simulation was 0.001 cm. In a recent paper (24) it has been shown that the residence time as function of length within a cuboid packed-bed could be obtained by:
When
is small:
Equation (3) implies that when the change in velocity within the lateral channels of the cuboid packed-bed is small, the residence time is largely independent of location.
Figure 5 shows that for the cuboid packed-bed device under consideration [
23], the residence time was slightly lower at the mid-point of the device. However, the difference in residence time between the mid-point and the ends was negligible compared to that observed with the packed column. From Equation (2), it could be shown that the minimum residence time corresponding to the mid-point of the cuboid packed-bed device could be obtained by [
24]:
Likewise, the maximum residence time corresponding to the ends could be obtained by [
24]:
For the optimum functioning of a cuboid packed-bed device, the difference between the maximum and minimum residence time should be as small as possible [
24]:
Tracer experiments using sodium chloride solution were carried out with the 5 mL HiTrap Capto S column (i.e., the surrogate packed-column reactor) and the 5 mL custom designed cuboid packed-bed device filled with Capto S resin particles (i.e., the surrogate cuboid packed-bed reactor). These experiments were performed in triplicate.
Figure 6 shows representative salt tracer peaks obtained from these experiments which were carried at 4 mL/min flow rate (150 cm/h) using 0.4 M sodium chloride as mobile phase and 100 μL of 0.8 M sodium chloride solution as the tracer pulse (i.e., 2% of the packed-bed volume). The peak obtained with the cuboid packed-bed device was higher, sharper and more symmetric than that obtained with the column. The calculated theoretical plate data (
N) obtained from these are summarized in
Table 1. The number of theoretical plates was calculated using the following equation [
32]:
The results shown in
Figure 6 and
Table 1 clearly demonstrate that the cuboid packed-bed device had vastly superior hydraulic attributes than the equivalent packed column. For the same bed height, the cuboid packed-bed had 3.8 times the number of theoretical plates. These new experimental results are consistent with the RTD results shown in
Figure 3 and
Figure 5. Overall, these results suggest that a reactor based on the cuboid packed-bed design would perform better than its equivalent packed column, at least in terms of the hydraulic properties and RTD. Future studies will involve detailed analysis of E-curves and F-curves obtained from such reactors. Also, the experiments discussed above were carried out using small reactors (i.e., 5 mL bed volume). Experimental work based on 200 mL scale reactors are currently being carried out in my laboratory. Computational fluid dynamics (CFD) studies which allow the study of larger reactors through simulations are also being carried out in parallel.
The design features of the cuboid packed-bed device also provide certain potential advantages from a heat transfer perspective. With a packed column of a given bed height, the heat transfer area per unit reactor volume (excluding the headers) varies inversely with the diameter, i.e., the larger the reactor diameter, the lower is the specific heat transfer area (HTA). This implies that removal or addition of heat to a packed column with a large diameter for endothermic and exothermic reactions respectively would be challenging. By contrast, the HTA of a cuboid packed-bed device could be changed in a very flexible manner by changing the length to width (L/W) ratio, to increase or decrease the values of HTA as required. To make an objective comparison, the HTA of a cuboid packed-bed device was compared with that of a packed column having the same bed height and bed-volume. For a packed column, the HTA is fixed based on the diameter.
Figure 7 shows a plot of the (HTA
cuboid/HTA
column) ratio for the two reactors as function of the (L/W) ratio of the cuboid packed-bed device. The minimum (HTA
cuboid/HTA
column) ratio corresponded to an (L/W) ratio of 1, i.e., when the cuboid packed-bed device had a square cross-sectional area. As the (L/W) ratio was increased, the (HTA
cuboid/HTA
column) ratio could be increased quite significantly. Therefore, a cuboid packed-bed reactor would have a significant advantage over a packed column for conducting endothermic and exothermic reactions.
The heat transfer advantage of the cuboid packed-bed device over a packed column could also be hypothesized from a heat transfer distance perspective. Heat transfer distance is a vital factor determining thermal gradients within packed-beds, i.e., the greater the distance, the greater is the likelihood of temperature variation. As shown in
Figure 8, the maximum heat transfer distance (MHTD) for a packed column corresponded to its radius. On the other hand, the average heat transfer distance (AHTD) corresponded to 29% of the radius, since 50% of the packed material existed within the zone extending up to 71% of the radius from the center. Therefore, both MHTD and AHTD for a packed column varied directly with the diameter, i.e., the greater the diameter, the greater these heat transfer distances. Therefore, for a packed column having a particular bed height and bed volume, both MHTD and AHTD are fixed. Once again, these heat transfer distances for a cuboid packed-bed could be adjusted in a flexible manner by changing the (L/W) ratio. To make an objective comparison, the MHTD and AHTD of a cuboid packed-bed device were compared with those of a packed column having the same bed height and the same bed volume. With the cuboid packed-bed device, the MHTD was equal to half the width while the AHTD was equal to one fourth the width.
Figure 8 shows a plot of the MHTD and AHTD ratios for the two reactors (cuboid/column) as function of the (L/W) ratio of the cuboid packed-bed device. The MHTD of the cuboid packed-bed device was consistently lower at all values of (L/W). The AHTD was greater with cuboid packed-bed device for (L/W) values lower than 2.4 but was lower are greater values of (L/W). Once again, these results suggest that a cuboid packed-bed reactor would have a significant advantage over a packed column for conducting endothermic and exothermic reactions.