Substrate Effect on Carbon/Ceramic Mixed Matrix Membrane Prepared by a Vacuum-Assisted Method for Desalination
Abstract
:1. Introduction
2. Experimental
2.1. Membrane Preparation and Characterisation
2.2. Membrane Testing and Characterisation
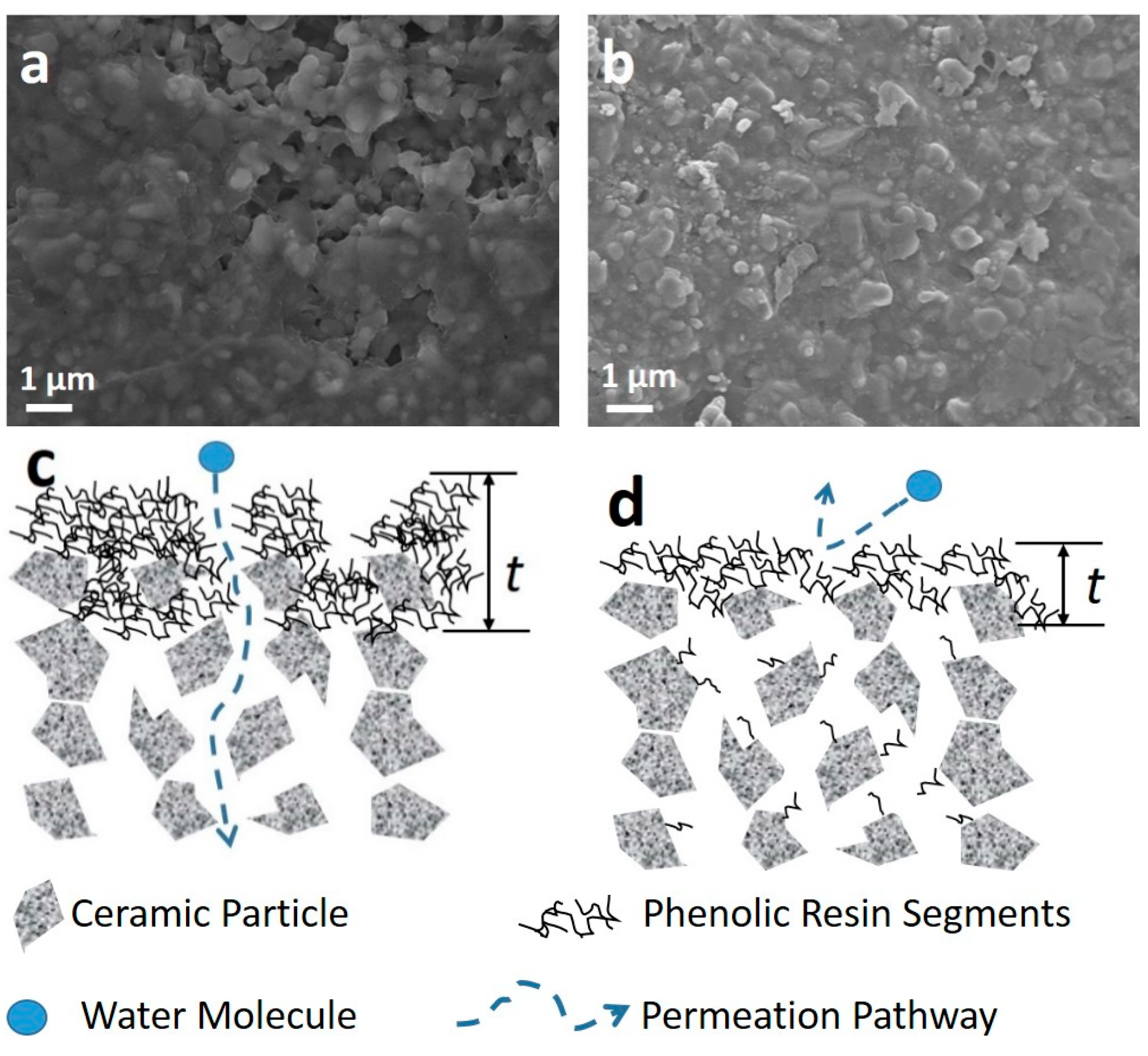
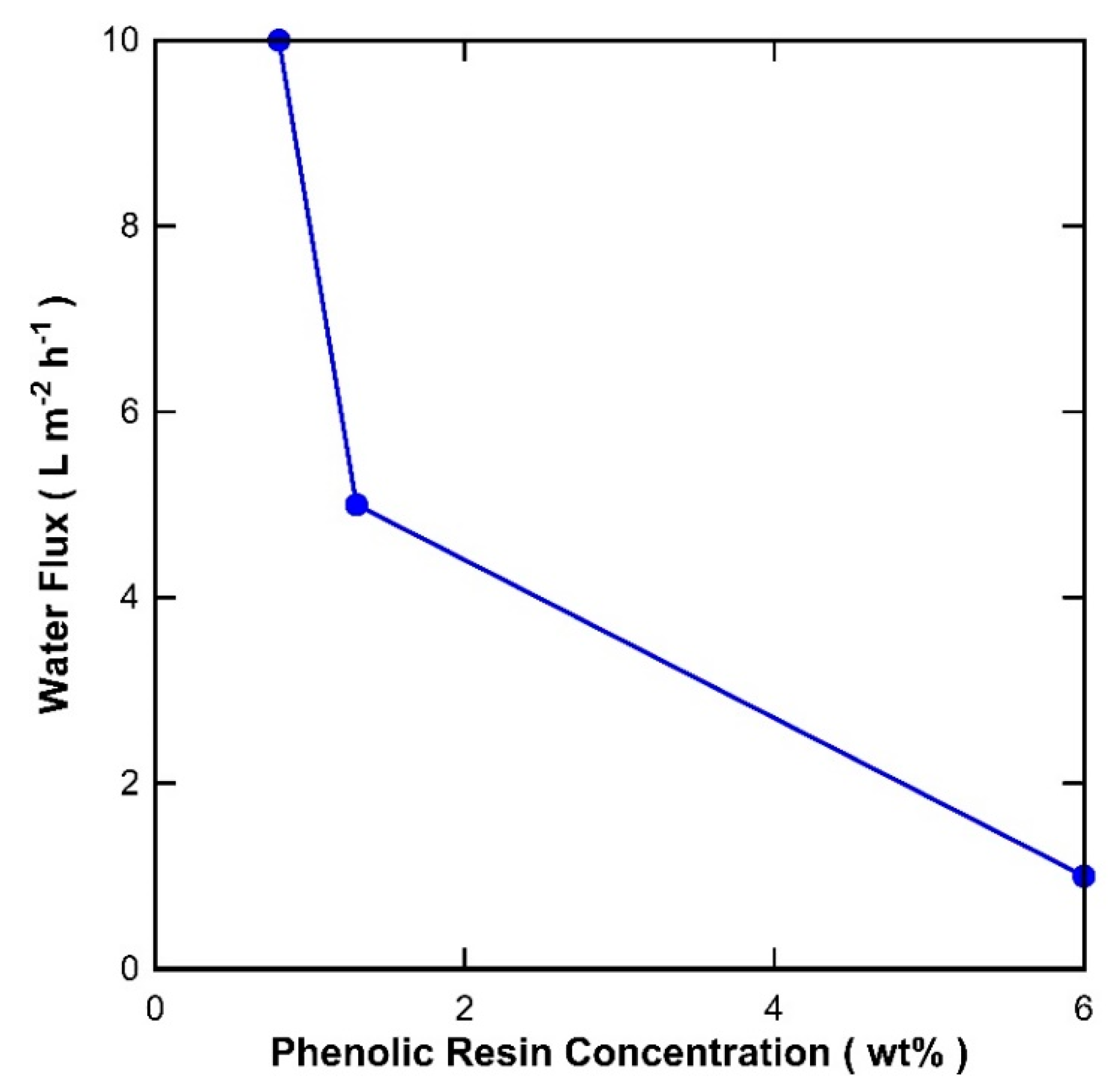
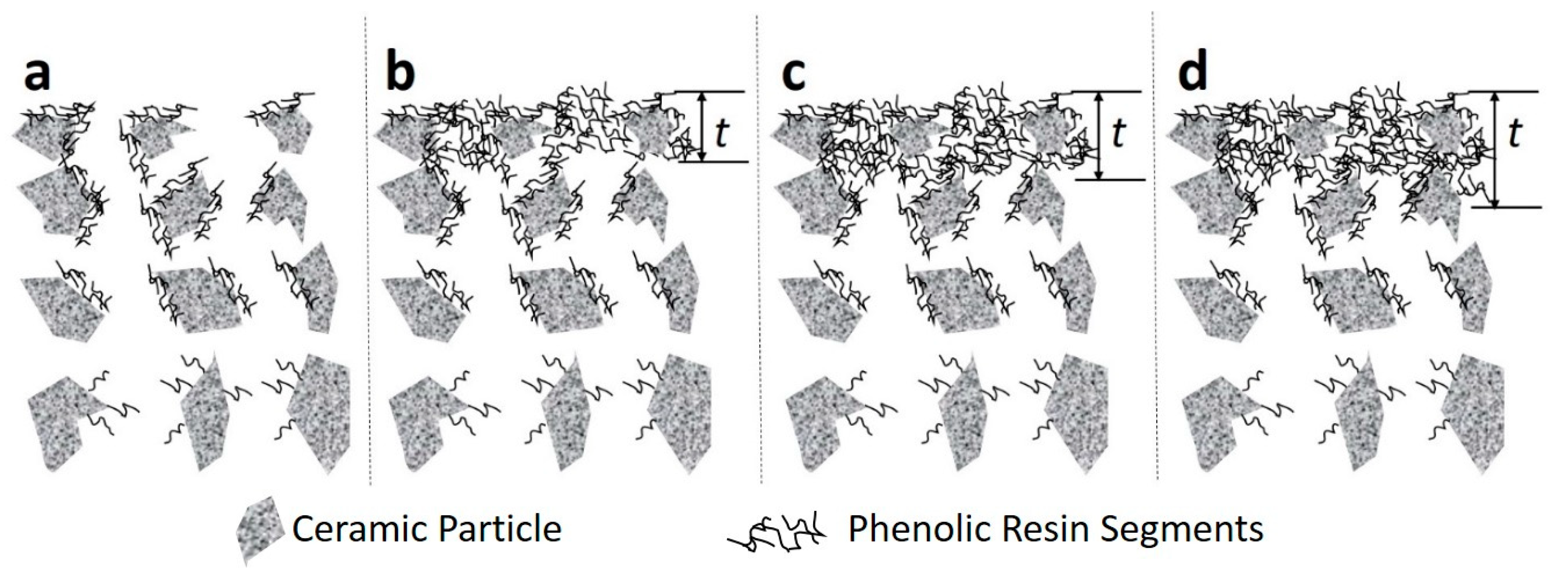
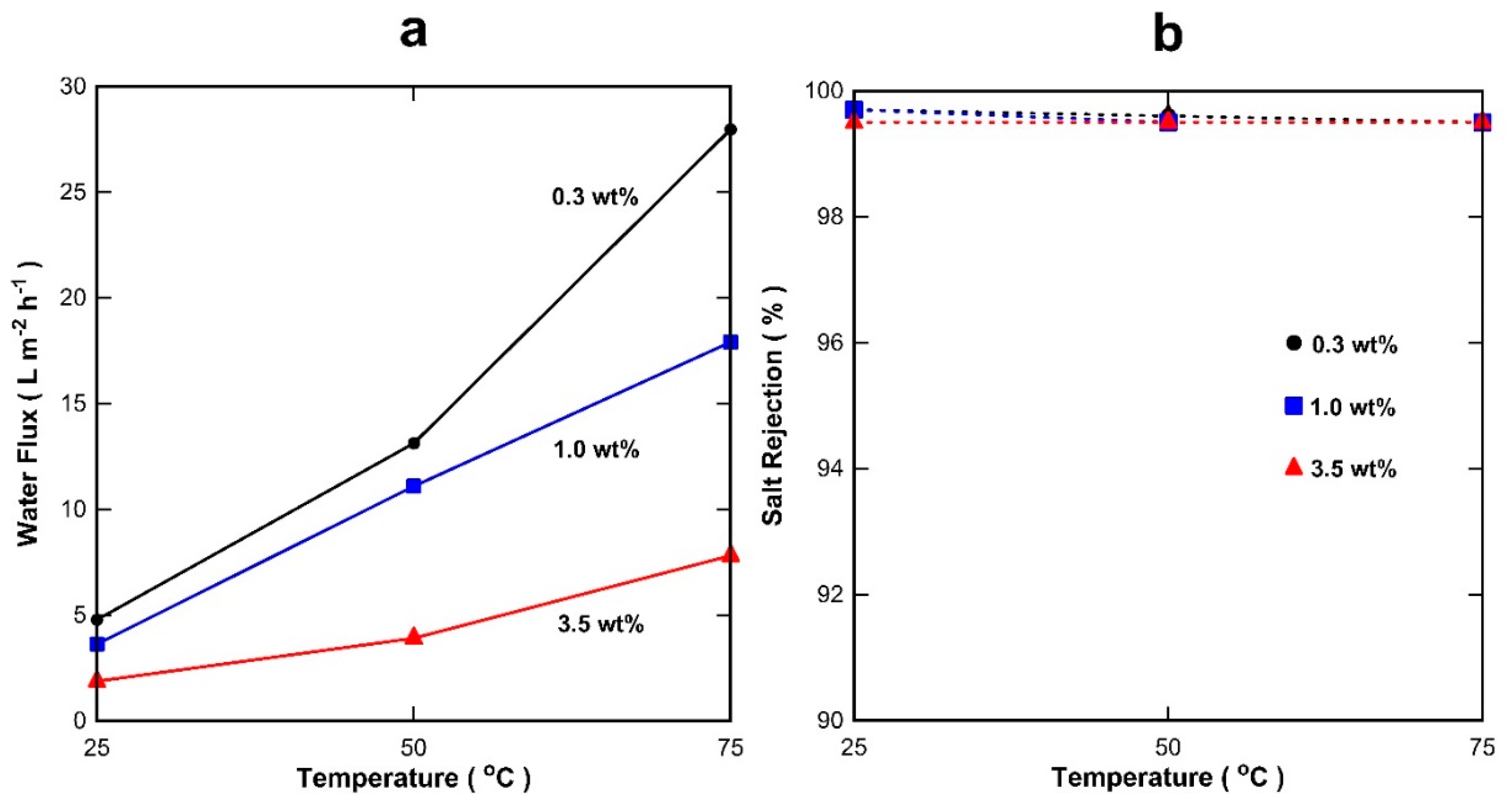
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thakur, J.K.; Mahesh Neupane, M.; Mohanan, A.A. Water poverty in upper Bagmati River Basin in Nepal. Water Sci. 2017, 31, 93–108. [Google Scholar] [CrossRef]
- Gumma, M.K.; Birhanu, B.Z.; Mohammed, I.A.; Tabo, R.; Whitbread, A.M. Prioritization of watersheds across Mali using remote sensing data and GIS techniques for agricultural development planning. Water 2016, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Al-Mashaqbeh, O.A.; Ghrair, A.M.; Megdal, S.B. Grey water reuse for agricultural purposes in the Jordan Valley: Household survey results in Deir Alla. Water 2012, 4, 580–596. [Google Scholar] [CrossRef]
- Sofroniou, A.; Bishop, S. Water scarcity in Cyprus: A review and call for integrated policy. Water 2014, 6, 2898–2928. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Mpandeli, S.; Madhlopa, A.; Modi, A.T.; Backeberg, G.; Nhamo, L. Southern Africa’s water—energy nexus: Towards regional integration and development. Water 2016, 8, 235. [Google Scholar] [CrossRef]
- Hamed, O.A.; Al-Sofi, M.A.K.; Imam, M.; Mustafa, G.M.; Mardouf, K.B.; Al-Washmi, H. Thermal performance of multi-stage flash distillation plants in Saudi Arabia. Desalination 2000, 128, 281–292. [Google Scholar] [CrossRef]
- Attia, A.A.A.; Abdel-Rehim, A.A. Thermal analysis for system uses pressurized hot water for seawater desalination (pressurized multistage). Desalination 2014, 346, 91–99. [Google Scholar] [CrossRef]
- Said, S.A.; Emtir, M.; Mujtaba, I.M. Flexible design and operation of multi-stage flash (MSF) desalination process subject to variable fouling and variable freshwater demand. Processes 2013, 1, 279–295. [Google Scholar] [CrossRef]
- Ullah, R.; Khraisheh, M.; Esteves, R.J.; McLeskey, J.T.; AlGhouti, M.; Gad-el-Hak, M.; Tafreshi, H.V. Energy efficiency of direct contact membrane distillation. Desalination 2018, 433, 56–67. [Google Scholar] [CrossRef]
- Silva, T.L.S.; Morales-Torres, S.; Esteves, C.M.P.; Ribeiro, A.R.; Nunes, O.C.; Figueiredo, J.L.; Silva, A.M.T. Desalination and removal of organic micropollutants and microorganisms by membrane distillation. Desalination 2018, 437, 121–132. [Google Scholar] [CrossRef]
- Singh, D.; Sirkar, K.K. Performance of PVDF flat membranes and hollow fibers in desalination by direct contact membrane distillation at high temperatures. Sep. Purif. Technol. 2017, 187, 264–273. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently combining water reuse and desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Roberts, D.A.; Johnston, E.L.; Knott, N.A. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Res. 2010, 44, 5117–5128. [Google Scholar] [CrossRef] [PubMed]
- Thiruvenkatachari, R.; Francis, M.; Cunnington, M.; Su, S. Application of integrated forward and reverse osmosis for coal mine wastewater desalination. Sep. Purif. Technol. 2016, 163, 181–188. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Marques, L.S.; Martins, S.S.; Reis, B.G.; Amaral, M.C.S. Assessment of the chemical stability of nanofiltration and reverse osmosis membranes employed in treatment of acid gold mining effluent. Sep. Purif. Technol. 2017, 174, 301–311. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C. Microporous Silica Based Membranes for Desalination. Water 2012, 4, 629–649. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, M.; Takeuchi, H. SWRO-PRO System in “Mega-ton Water System” for Energy Reduction and Low Environmental Impact. Water 2018, 10, 48. [Google Scholar] [CrossRef]
- Kurihara, M.; Sasaki, T.; Nakatsuji, K.; Kimura, M.; Henmi, M. Low pressure SWRO membrane for desalination in the “Mega-ton Water System”. Desalination 2015, 368, 135–139. [Google Scholar] [CrossRef]
- Fritzmann, C.; Lowenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnott, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Duke, M.C.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.S.; Diniz da Costa, J.C. Seawater desalination performance of MFI type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using NaA zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Memb. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]
- Drobek, M.; Yacou, C.; Motuzas, J.; Julbe, A.; Ding, L.; Diniz da Costa, J.C. Long term pervaporation desalination of tubular MFI zeolite membranes. J. Memb. Sci. 2012, 415, 816–823. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, J.; Huang, A. Seeding-free synthesis of zeolite FAU membrane for seawater desalination by pervaporation. Microporous Mesoporous Mater. 2016, 234, 377–383. [Google Scholar] [CrossRef]
- Malekpour, A.; Nasiri, H. High performance pervaporative desalination of saline waters using Na-X zeolite membrane. Membr. Water Treatm. 2017, 8, 437–448. [Google Scholar]
- Elma, M.; Yacou, C.; Diniz da Costa, J.C.; Wang, D.K. Performance and Long Term Stability of Mesoporous Silica Membranes for Desalination. Membranes 2013, 3, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.T.; Lin, C.X.C.; Kleitz, F.; Zhao, X.S.; Smart, S. Nanoporous organosilica membrane for water desalination. Chem. Commun. 2013, 49, 4534–4536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, D.K.; Motuzas, J.; Smart, S.; Diniz da Costa, J.C. Rapid Thermal Treatment of Interlayer-free Ethyl Silicate 40 Derived Membranes for Desalination. J. Memb. Sci. 2016, 516, 94–103. [Google Scholar] [CrossRef]
- Wang, S.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C. Improved stability of ethyl silicate interlayer-free membranes by the rapid thermal processing (RTP) for desalination. Desalination 2017, 402, 25–32. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; Diniz da Costa, J.C. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Ladewig, B.P.; Tan, Y.H.; Lin, C.X.C.; Ladewig, K.; Diniz da Costa, J.C.; Smart, S. Preparation, Characterization and Performance of Templated Silica Membranes in Non-Osmotic Desalination. Materials 2011, 4, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Elma, M.; Wang, D.K.; Yacou, C.; Diniz da Costa, J.C. Interlayer-Free P123 Carbonised Template Silica Membranes for Desalination with Reduced Salt Concentration Polarisation. J. Memb. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Yang, H.; Elma, M.; Wang, D.K.; Motuzas, J.; Diniz da Costa, J.C. Interlayer-free hybrid carbon-silica membranes for processing brackish to brine salt solutions by pervaporation. J. Memb. Sci. 2017, 523, 197–204. [Google Scholar] [CrossRef]
- Yang, H.; Wang, D.K.; Motuzas, J.; Diniz da Costa, J.C. Hybrid vinyl silane and P123 template sol−gel derived carbon silica membrane for desalination. J. Sol-Gel Sci. Technol. 2018, 85, 280–289. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.; Smart, S.K.; Diniz da Costa, J.C. Cobalt Oxide Silica Membranes for Desalination. J. Coll. Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Elma, M.; Wang, D.K.; Yacou, C.; Motuzas, J.; Diniz da Costa, J.C. High Performance Interlayer-Free Mesoporous Cobalt Oxide Silica Membranes for Desalination Applications. Desalination 2015, 365, 308–315. [Google Scholar] [CrossRef]
- Darmawan, A.; Karlina, L.; Astuti, Y.; Sriatun; Wang, D.K.; Diniz da Costa, J.C. Structural evolution of nickel oxide silica sol-gel for the preparation of interlayer-free membranes. J. Non-Cryst. Solids 2016, 447, 9–15. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; Diniz da Costa, J.C. Mesoporous TiO2 based membranes for water desalination and brine processing. Sep. Purif. Technol. 2015, 147, 166–171. [Google Scholar] [CrossRef]
- Song, Y.; Wang, D.K.; Birkett, G.; Martens, W.; Smart, S.; Diniz da Costa, J.C. Mixed Matrix Carbon Molecular Sieve and Alumina (CMS-Al2O3) Membranes for Desalination. Sci. Rep. 2016, 6, 30703. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, D.K.; Birkett, G.; Smart, S.; Diniz da Costa, J.C. Vacuum film etching effect of carbon alumina mixed matrix membranes. J. Memb. Sci. 2017, 541, 53–61. [Google Scholar] [CrossRef]
- De Lint, W.B.S.; Zivkovic, T.; Benes, N.E.; Bouwmeester, H.J.M.; Blank, D.H.A. Electrolyte retention of supported bi-layered nanofiltration membranes. J. Membr. Sci. 2006, 277, 18–27. [Google Scholar]
- Abd Jalil, S.N.; Wang, D.K.; Yacou, C.; Motuzas, J.; Smart, S.; Diniz da Costa, J.C. Vacuum-Assisted Tailoring of Pore Structures of Phenolic Resin Derived Carbon Membranes. J. Membr. Sci. 2017, 525, 240–248. [Google Scholar] [CrossRef]
- Abd Jalil, S.N.; Wang, D.K.; Yacou, C.; Motuzas, J.; Smart, S.; Diniz da Costa, J.C. Molecular Weight Cut-off and Structural Analysis of Vacuum-assisted Titania Membranes for Water Processing. Materials 2016, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Hamm, J.B.S.; Ambrosi, A.; Griebeler, J.G.; Marcilio, N.R.; Tessaro, I.C.; Pollo, L.D. Recent advances in the development of supported carbon membranes for gas separation. Int. J. Hydrog. Energy 2017, 42, 24830–24845. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Generalization of effect of oxygen exposure on formation and performance of carbon molecular sieve membranes. Carbon 2010, 48, 4442–4449. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, Y.I.; Nam, S.E.; Park, H.; Lee, P.S. Separations of gases from nitrogen through thin carbon membranes. Sep. Purif. Technol. 2016, 158, 108–114. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Nomura, T.; Sakoda, A.; Suzuki, M. Fabrication of carbon coated ceramic membranes by pyrolysis of methane using a modified chemical vapor deposition apparatus. J. Memb. Sci. 2002, 197, 23–35. [Google Scholar] [CrossRef]
- Schmeda-Lopez, D.R.; Smart, S.; Meulenberg, W.A.; Diniz da Costa, J.C. Mixed matrix carbon stainless steel (MMCSS) hollow fibres for gas separation. Sep. Purif. Technol. 2017, 174, 150–158. [Google Scholar] [CrossRef]
- Wei, W.; Qin, G.T.; Tang, P.J.; Wu, L.P. Effects of pyrolysis conditions on the permeance of phenol-formaldehyde resin based carbon membrane for CO2 separation. Adv. Mater. Res. 2011, 239–242, 1804–1808. [Google Scholar] [CrossRef]
- Wei, W.; Hu, H.; Qin, G.; You, L.; Chen, G. Pore structure control of phenol-formaldehyde based carbon microfiltration membranes. Carbon 2004, 42, 679–681. [Google Scholar] [CrossRef]
- Centeno, T.A.; Vilas, J.L.; Fuertes, A.B. Effects of phenolic resin pyrolysis conditions on carbon membrane performance for gas separation. J. Membr. Sci. 2004, 228, 45–54. [Google Scholar] [CrossRef]
- Teixeira, M.; Rodrigues, S.; Campo, M.C.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Madeira, L.; Sousa, J.; Mendes, A. Boehmite-phenolic resin carbon molecular sieve membranes—Permeation and adsorption studies. Chem. Eng. Res. Des. 2014, 92, 2668–2680. [Google Scholar] [CrossRef]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Rodrigues, S.C.; Teixeira, M.; Mendes, A. Composite-alumina-carbon molecular sieve membranes prepared from novolac resin and boehmite. Part I: Preparation, characterization and gas permeation studies. Int. J. Hydrog. Energy 2015, 40, 5653–5663. [Google Scholar] [CrossRef]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Mendes, A. Composite-alumina-carbon molecular sieve membranes prepared from novolac resin and boehmite. Part II: Effect of the carbonization temperature on the gas permeation properties. Int. J. Hydrog. Energy 2014, 40, 3485–3496. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.C.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Magen, C.; Mendes, A. Composite phenolic resin-based carbon molecular sieve membranes for gas separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.; Liu, L.; Wang, X. Fabrication of phenolic resin based desalting membrane with ordered mesostructure and excellent chlorine resistance. J. Membr. Sci. 2018, 550, 502–509. [Google Scholar] [CrossRef]
- Knop, A.; Scheib, W. Chemistry and Application of Phenolic Resins; Springer: New York, NY, USA, 1979. [Google Scholar]
- Knop, A.; Pilato, L.A. Phenolic Resins; Springer: New York, NY, USA, 1985. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Sparrow, B.S. Empirical equations for the thermodynamic properties of aqueous sodium chloride. Desalination 2003, 159, 161–170. [Google Scholar] [CrossRef]
- Edwi, F.; Chung, T.-S. Development of hollow fiber membranes for water and salt recovery from highly concentrated brine via direct contact membrane distillation and crystallization. J. Membr. Sci. 2012, 421, 111–123. [Google Scholar] [CrossRef]
- Ali, A.; Drioli, D.; Macedonio, F. Membrane engineering for sustainable development: a perspective. Appl. Sci. 2017, 7, 1026. [Google Scholar] [CrossRef]
- Department of Environment and Heritage Protection. Coal Seam Gas Water Management Policy; Department of Environment and Heritage Protection: Brisbane, Australia, 2012.
- Motuzas, J.; Yacou, C.; Madsen, R.S.K.; Fu, W.; Wang, D.K.; Julbe, A.; Vaughan, J.; Diniz da Costa, J.C. Novel inorganic membrane for the percrystallization of mineral, food and pharmaceutical compounds. J. Membr. Sci. 2018, 550, 407–415. [Google Scholar] [CrossRef]




| Timeline | Membrane | Flux (L m−2 h−1) | Rejection (%) | Reference |
|---|---|---|---|---|
| 2009 | Zeolite MFI-ZSM-5 | 0.2 | 99 | Duke et al. [24] |
| 2011 | Zeolite NaA | 0.4 | 99.9 | Cho et al. [25] |
| 2012 | Zeolite MFI-S1 | 1 | 99 | Drobek et al. [26] |
| 2016 | Zeolite Fau | 0.96 | 99.9 | Zhou et al. [27] |
| 2017 | Zeolite NaA-X | 3.6 | 95 | Malekpour et al. [28] |
| 2013 | Pure silica | 6.8 | 98 | Elma et al. [29] |
| 2013 | Ordered hybrid SiO2 | 2.9 | 99.9 | Chua et al. [30] |
| 2016 | * RTP pure silica pH4 sol-gel | 2.5 | 99.5 | Wang et al. [31] |
| 2017 | * RTP pure silica pH1 sol-gel | 1.9 | 99.5 | Wang et al. [32] |
| 2009 | C6 silica | 1.4 | 92 | Wijaya et al. [33] |
| 2011 | PEG-PPG | 4.9 | 97 | Ladewig et al. [34] |
| 2015 | Silica P123 | 2.3 | 99.5 | Elma et al. [35] |
| 2017 | Carbonised template | 8.3 | 99.4 | Yang et al. [36] |
| 2018 | Carbonised template | 2.5 | 99.8 | Yang et al. [37] |
| 2012 | cobalt oxide silica | 1.5 | 99.9 | Lin et al. [38] |
| 2015 | cobalt oxide silica | 4.6 | 99.7 | Elma et al. [39] |
| 2016 | nickel oxide silica | 2.5 | 97 | Darmwan et al. [40] |
| 2015 | Titania | 3 | >99 | Yacou et al. [41] |
| 2016 | carbon alumina | 9.4 | 99.9 | Song et al. [42] |
| 2017 | carbon alumina | 9.2 | 99.9 | Song et al. [43] |
| Substrate | Supplier | Substrate Material, Pore Size and Dimensions |
|---|---|---|
| S-C | Ceramic Fabricators | α-Al2O3, dp = ~100 nm Dimensions: OD 9 mm ID 6 mm |
| S-T | TAMI | TiO2 substrate and top layer, dp = ~140 nm Dimensions: OD 10 mm, ID 6 mm |
| S-P | Pall | α-Al2O3, dp = ~600 nm Dimensions: OD 10 mm ID 6.5 mm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Motuzas, J.; Wang, D.K.; Birkett, G.; Smart, S.; Diniz da Costa, J.C. Substrate Effect on Carbon/Ceramic Mixed Matrix Membrane Prepared by a Vacuum-Assisted Method for Desalination. Processes 2018, 6, 47. https://doi.org/10.3390/pr6050047
Song Y, Motuzas J, Wang DK, Birkett G, Smart S, Diniz da Costa JC. Substrate Effect on Carbon/Ceramic Mixed Matrix Membrane Prepared by a Vacuum-Assisted Method for Desalination. Processes. 2018; 6(5):47. https://doi.org/10.3390/pr6050047
Chicago/Turabian StyleSong, Yingjun, Julius Motuzas, David K. Wang, Greg Birkett, Simon Smart, and João C. Diniz da Costa. 2018. "Substrate Effect on Carbon/Ceramic Mixed Matrix Membrane Prepared by a Vacuum-Assisted Method for Desalination" Processes 6, no. 5: 47. https://doi.org/10.3390/pr6050047