Computational Fluid Dynamics Modeling of the Catalytic Partial Oxidation of Methane in Microchannel Reactors for Synthesis Gas Production
Abstract
:1. Introduction
2. Model Development
2.1. Reaction System
2.2. Mathematical Model
2.3. Reaction Mechanisms
2.4. Computation Scheme
2.5. Model Validation
3. Results and Discussion
3.1. Base Case
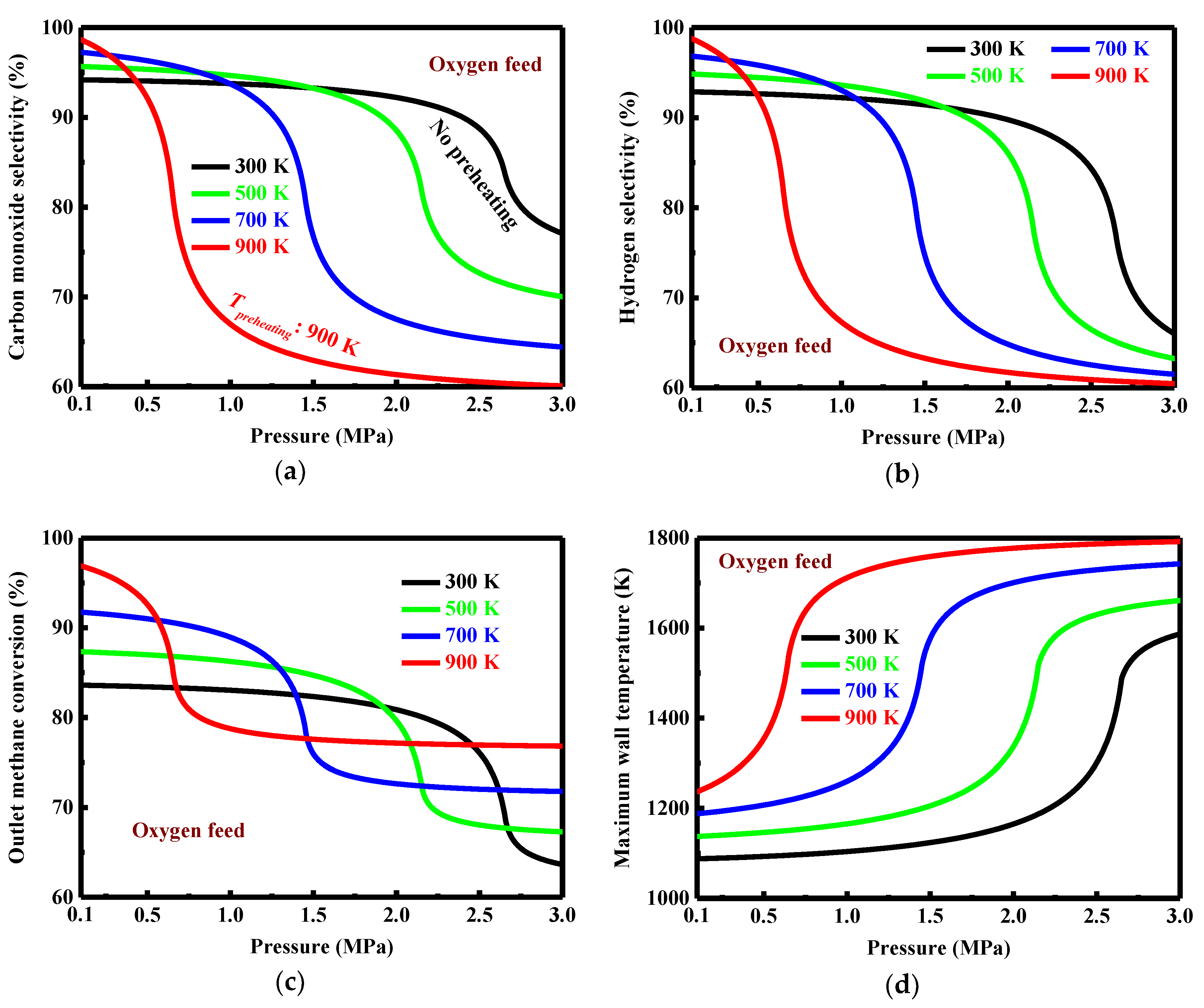
3.2. Effect of Preheating for Oxygen Feed
3.3. Effect of Preheating for Air Feed
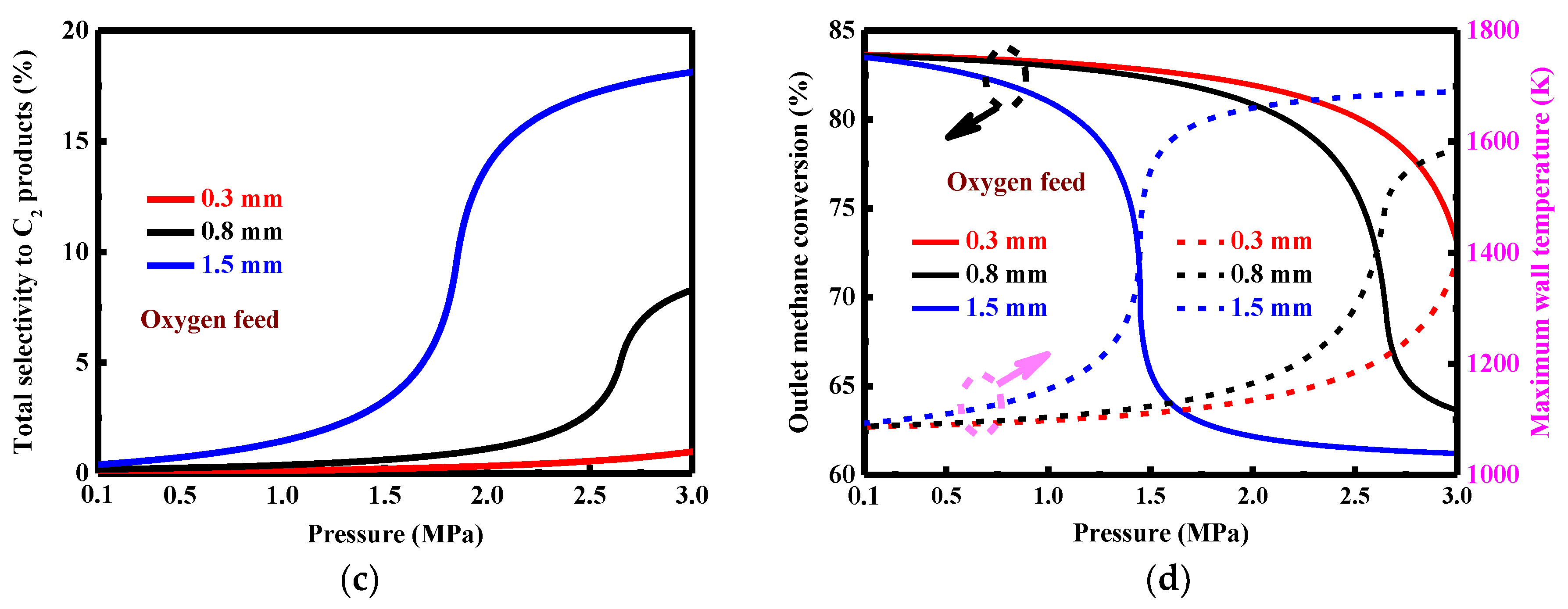
3.4. Effect of Reactor Dimension for Oxygen Feed
3.5. Effect of Reactor Dimension for Air Feed
3.6. Effect of Nitrogen Diluent
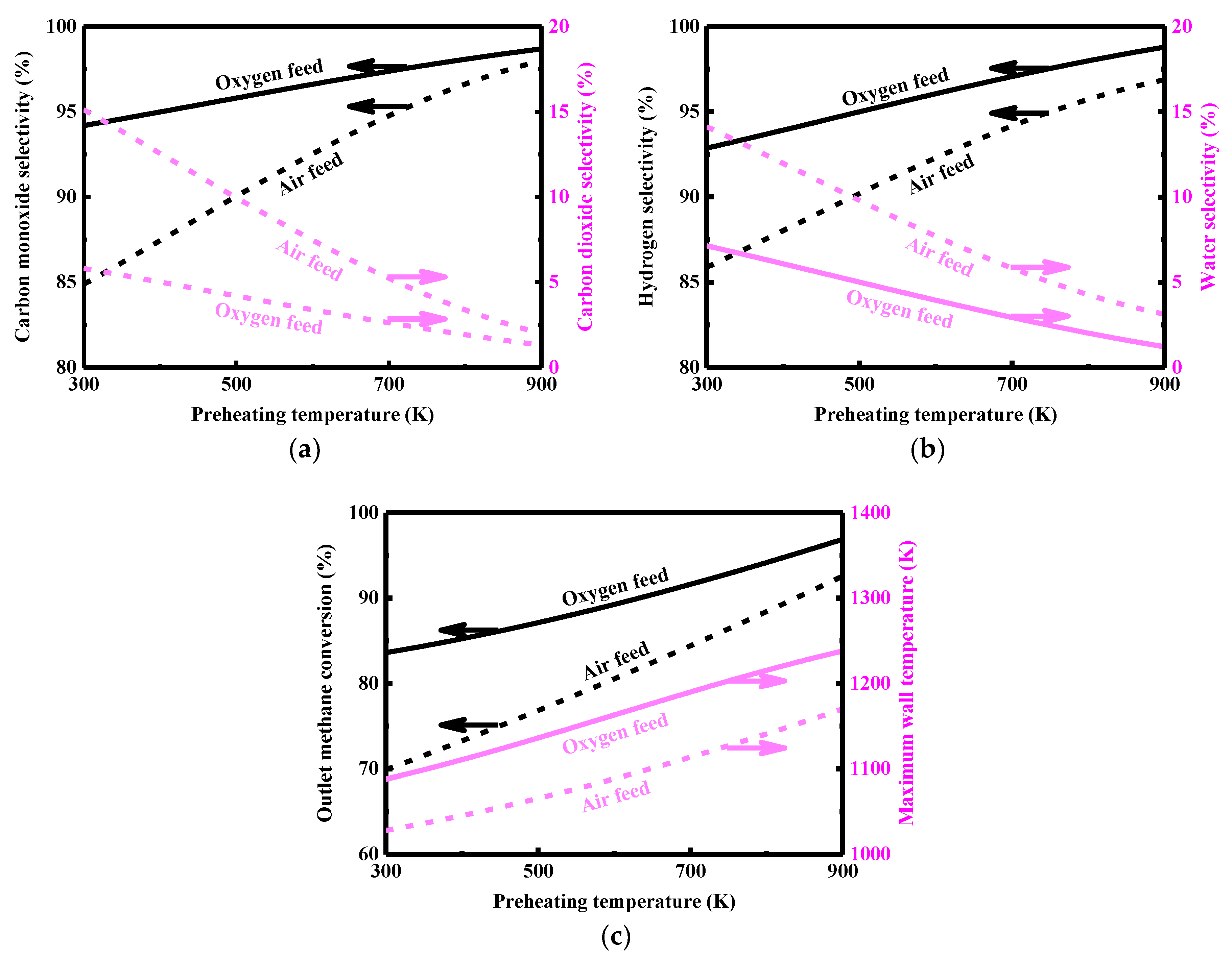
3.7. Air Feed Versus Oxygen Feed
4. Further Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | pre-exponential factor |
| A′ | surface area |
| C | concentration |
| c | specific heat capacity |
| D | diffusivity |
| DT | thermal diffusivity |
| Deff | effective diffusivity |
| Dm | mixture-averaged diffusivity |
| d | channel height |
| dpore | mean pore diameter |
| Ea | activation energy |
| Fcat/geo | catalyst/geometric surface area |
| F | view factor |
| standard molar enthalpy of reaction | |
| h | specific enthalpy |
| ho | external heat loss coefficient |
| Kg, Ks | number of gaseous species and number of surface species |
| l | length |
| m | total number of gaseous and surface species |
| p | pressure |
| q | heat flux |
| R | ideal gas constant |
| rate of appearance of a heterogeneous product | |
| s | sticking coefficient |
| T, To | absolute temperature and reference temperature |
| u, v | streamwise and transverse velocity components |
| V, | diffusion velocity and diffusion velocity vector |
| W, | relative molecular mass and relative molecular mass of the mixture |
| x | streamwise coordinate |
| Y | mass fraction |
| y | transverse coordinate |
| Greek variables | |
| γ | surface area per unit catalyst volume |
| ε | emissivity |
| δ | thickness |
| εp | catalyst porosity |
| λ | thermal conductivity |
| η | effectiveness factor |
| μ | dynamic viscosity |
| ρ | density |
| σ | Stefan-Boltzmann constant |
| ϑ | site occupancy |
| τp | catalyst tortuosity factor |
| φ | inlet molar ratio |
| rate of appearance of a homogeneous product | |
| Γ | site density |
| Θ | surface coverage |
| Φ | Thiele modulus |
| Subscripts | |
| amb | ambient |
| eff | effective |
| g | gas |
| i, k, m | species index, gaseous species index, and surface species index |
| in | inlet |
| o | outer |
| rad | radiation |
| s | solid |
| w | wall |
| x, y | streamwise and transverse components |
References
- Guo, S.; Wang, J.; Ding, C.; Duan, Q.; Ma, Q.; Zhang, K.; Liu, P. Confining Ni nanoparticles in honeycomb-like silica for coking and sintering resistant partial oxidation of methane. Int. J. Hydrogen Energy 2018, 43, 6603–6613. [Google Scholar] [CrossRef]
- Grundner, S.; Luo, W.; Sanchez-Sanchez, M.; Lercher, J.A. Synthesis of single-site copper catalysts for methane partial oxidation. Chem. Commun. 2016, 52, 2553–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, R.J.; Zhu, H.; Sukeshini, A.M.; Jackson, G.S. Solid oxide fuel cells: Operating principles, current challenges, and the role of syngas. Combust. Sci. Technol. 2008, 180, 1207–1244. [Google Scholar] [CrossRef]
- Baldinelli, A.; Barelli, L.; Bidini, G. Performance characterization and modelling of syngas-fed SOFCs (solid oxide fuel cells) varying fuel composition. Energy 2015, 90, 2070–2084. [Google Scholar] [CrossRef]
- Pramanik, S.; Ravikrishna, R.V. Numerical study of rich catalytic combustion of syngas. Int. J. Hydrogen Energy 2017, 42, 16514–16528. [Google Scholar] [CrossRef]
- Zheng, X.; Mantzaras, J.; Bombach, R. Homogeneous combustion of fuel-lean syngas mixtures over platinum at elevated pressures and preheats. Combust. Flame 2013, 160, 155–169. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Tran, A.; Pont, M.; Crose, M.; Christofides, P.D. Real-time furnace balancing of steam methane reforming furnaces. Chem. Eng. Res. Des. 2018, 134, 238–256. [Google Scholar] [CrossRef]
- Lu, N.; Gallucci, F.; Melchiori, T.; Xie, D.; Van Sint Annaland, M. Modeling of autothermal reforming of methane in a fluidized bed reactor with perovskite membranes. Chem. Eng. Process 2018, 124, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Goralski, C.T.; O’Connor, R.P.; Schmidt, L.D. Modeling homogeneous and heterogeneous chemistry in the production of syngas from methane. Chem. Eng. Sci. 2000, 55, 1357–1370. [Google Scholar] [CrossRef]
- Shelepova, E.; Vedyagin, A.; Sadykov, V.; Mezentseva, N.; Fedorova, Y.; Smorygo, O.; Klenov, O.; Mishakov, I. Theoretical and experimental study of methane partial oxidation to syngas in catalytic membrane reactor with asymmetric oxygen-permeable membrane. Catal. Today 2016, 268, 103–110. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Chen, T.; Yu, X.; Wang, J.; Wang, T. Simulations of methane partial oxidation by CFD coupled with detailed chemistry at industrial operating conditions. Chem. Eng. Sci. 2016, 142, 126–136. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Kruger, J.S.; Hermann, R.J.; Blass, S.D.; Schmidt, L.D. Spatial profiles in partial oxidation of methane and dimethyl ether in an autothermal reactor over rhodium catalysts. Appl. Catal. A 2014, 483, 97–102. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, S.-C. Characterization of catalytic partial oxidation of methane with carbon dioxide utilization and excess enthalpy recovery. Appl. Energy 2016, 162, 1141–1152. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Christian Enger, B.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Guo, D.; Wang, G.-C. Partial oxidation of methane on anatase and rutile defective TiO2 supported Rh4 cluster: A density functional theory study. J. Phys. Chem. C 2017, 121, 26308–26320. [Google Scholar] [CrossRef]
- Kraus, P.; Lindstedt, R.P. Microkinetic mechanisms for partial oxidation of methane over platinum and rhodium. J. Phys. Chem. C 2017, 121, 9442–9453. [Google Scholar] [CrossRef]
- Urasaki, K.; Kado, S.; Kiryu, A.; Imagawa, K.-i.; Tomishige, K.; Horn, R.; Korup, O.; Suehiro, Y. Synthesis gas production by catalytic partial oxidation of natural gas using ceramic foam catalyst. Catal. Today 2018, 299, 219–228. [Google Scholar] [CrossRef]
- Gil-Calvo, M.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Effect of Ni/Al molar ratio on the performance of substoichiometric NiAl2O4 spinel-based catalysts for partial oxidation of methane. Appl. Catal. B 2017, 209, 128–138. [Google Scholar] [CrossRef]
- Kumar Singha, R.; Shukla, A.; Yadav, A.; Sain, S.; Pendem, C.; Kumar Konathala, L.N.S.; Bal, R. Synthesis effects on activity and stability of Pt-CeO2 catalysts for partial oxidation of methane. Mol. Catal. 2017, 432, 131–143. [Google Scholar] [CrossRef]
- Osman, A.I.; Meudal, J.; Laffir, F.; Thompson, J.; Rooney, D. Enhanced catalytic activity of Ni on η-Al2O3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane. Appl. Catal. B 2017, 212, 68–79. [Google Scholar] [CrossRef]
- Singha, R.K.; Ghosh, S.; Acharyya, S.S.; Yadav, A.; Shukla, A.; Sasaki, T.; Venezia, A.M.; Pendem, C.; Bal, R. Partial oxidation of methane to synthesis gas over Pt nanoparticles supported on nanocrystalline CeO2 catalyst. Catal. Sci. Technol. 2016, 6, 4601–4615. [Google Scholar] [CrossRef]
- Luo, Z.; Kriz, D.A.; Miao, R.; Kuo, C.-H.; Zhong, W.; Guild, C.; He, J.; Willis, B.; Dang, Y.; Suib, S.L.; et al. TiO2 Supported gold-palladium catalyst for effective syngas production from methane partial oxidation. Appl. Catal. A 2018, 554, 54–63. [Google Scholar] [CrossRef]
- Boukha, Z.; Gil-Calvo, M.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Behaviour of Rh supported on hydroxyapatite catalysts in partial oxidation and steam reforming of methane: On the role of the speciation of the Rh particles. Appl. Catal. A 2018, 556, 191–203. [Google Scholar] [CrossRef]
- Scarabello, A.; Dalle Nogare, D.; Canu, P.; Lanza, R. Partial oxidation of methane on Rh/ZrO2 and Rh/Ce-ZrO2 on monoliths: Catalyst restructuring at reaction conditions. Appl. Catal. B 2015, 174–175, 308–322. [Google Scholar] [CrossRef]
- Figen, H.E.; Baykara, S.Z. Effect of ruthenium addition on molybdenum catalysts for syngas production via catalytic partial oxidation of methane in a monolithic reactor. Int. J. Hydrogen Energy 2018, 43, 1129–1138. [Google Scholar] [CrossRef]
- Zhu, Y.; Barat, R. Partial oxidation of methane over a ruthenium phthalocyanine catalyst. Chem. Eng. Sci. 2014, 116, 71–76. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.-Z.; Lin, J.-D.; Chen, Z.-Q.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B 2018, 231, 292–298. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Sasaki, T.; Sandupatla, A.; Deo, G.; Bal, R. Pt-CeO2 nanoporous spheres - an excellent catalyst for partial oxidation of methane: Effect of the bimodal pore structure. Catal. Sci. Technol. 2017, 7, 4720–4735. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Weng, W.-Z.; Zhang, Q.; Huang, C.-J.; Wan, H.-L. Synthesis gas production from partial oxidation of methane over highly dispersed Pd/SiO2 catalyst. Fuel 2013, 103, 1032–1038. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Singha, R.K.; Shukla, A.; Yadav, A.; Sivakumar Konathala, L.N.; Bal, R. Effect of metal-support interaction on activity and stability of Ni-CeO2 catalyst for partial oxidation of methane. Appl. Catal. B 2017, 202, 473–488. [Google Scholar] [CrossRef]
- Kaddeche, D.; Djaidja, A.; Barama, A. Partial oxidation of methane on co-precipitated Ni-Mg/Al catalysts modified with copper or iron. Int. J. Hydrogen Energy 2017, 42, 15002–15009. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ikenaga, N.; Suzuki, T.; Kobayashi, T.; Haruta, M. Partial oxidation of methane to synthesis gas over supported iridium catalysts. Appl. Catal. A 1998, 169, 281–290. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ikenaga, N.; Teng, Y.; Kobayashi, T.; Suzuki, T. Partial oxidation of methane to synthesis gas over iridium-nickel bimetallic catalysts. Appl. Catal. A 1999, 180, 183–193. [Google Scholar] [CrossRef]
- De SantanaSantos, M.; Neto, R.C.R.; Noronha, F.B.; Bargiela, P.; da Graça Carneiro da Rocha, M.; Resini, C.; Carbó-Argibay, E.; Frétya, R.; Brandão, S.T. Perovskite as catalyst precursors in the partial oxidation of methane: The effect of cobalt, nickel and pretreatment. Catal. Today 2018, 299, 229–241. [Google Scholar] [CrossRef]
- López-Ortiz, A.; González-Vargas, P.E.; Meléndez-Zaragoza, M.J.; Collins-Martínez, V. Thermodynamic analysis and process simulation of syngas production from methane using CoWO4 as oxygen carrier. Int. J. Hydrogen Energy 2017, 42, 30223–30236. [Google Scholar] [CrossRef]
- Horn, R.; Williams, K.A.; Degenstein, N.J.; Schmidt, L.D. Syngas by catalytic partial oxidation of methane on rhodium: Mechanistic conclusions from spatially resolved measurements and numerical simulations. J. Catal. 2006, 242, 92–102. [Google Scholar] [CrossRef]
- Nogare, D.D.; Degenstein, N.J.; Horn, R.; Canu, P.; Schmidt, L.D. Modeling spatially resolved data of methane catalytic partial oxidation on Rh foam catalyst at different inlet compositions and flowrates. J. Catal. 2011, 277, 134–148. [Google Scholar] [CrossRef]
- Horn, R.; Williams, K.A.; Degenstein, N.J.; Bitsch-Larsen, A.; Dalle Nogare, D.; Tupy, S.A.; Schmidt, L.D. Methane catalytic partial oxidation on autothermal Rh and Pt foam catalysts: Oxidation and reforming zones, transport effects, and approach to thermodynamic equilibrium. J. Catal. 2007, 249, 380–393. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, Y.; Pillai, M.; Kim, I.; Barnett, S.A. High-rate electrochemical partial oxidation of methane in solid oxide fuel cells. J. Power Sources 2006, 161, 460–465. [Google Scholar] [CrossRef]
- Lee, D.; Myung, J.; Tan, J.; Hyun, S.-H.; Irvine, J.T.S.; Kim, J.; Moon, J. Direct methane solid oxide fuel cells based on catalytic partial oxidation enabling complete coking tolerance of Ni-based anodes. J. Power Sources 2017, 345, 30–40. [Google Scholar] [CrossRef]
- Wang, B.; Albarracín-Suazo, S.; Pagán-Torres, Y.; Nikolla, E. Advances in methane conversion processes. Catal. Today 2017, 285, 147–158. [Google Scholar] [CrossRef]
- Taifan, W.; Baltrusaitis, J. CH4 conversion to value added products: Potential, limitations and extensions of a single step heterogeneous catalysis. Appl. Catal. B 2016, 198, 525–547. [Google Scholar] [CrossRef]
- Védrine, J.C.; Fechete, I. Heterogeneous partial oxidation catalysis on metal oxides. C. R. Chim. 2016, 19, 1203–1225. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Strekova, L.N. The interplay of catalytic and gas-phase stages at oxidative conversion of methane: A review. J. Mol. Catal. A Chem. 2017, 426, 326–342. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Du, L.; Liu, J.; Yao, J. Review of the applications of microreactors. Renew. Sustain. Energy Rev. 2015, 47, 519–539. [Google Scholar] [CrossRef]
- Geyer, K.; Codée, J.D.C.; Seeberger, P.H. Microreactors as tools for synthetic chemists—The chemists’ round-bottomed flask of the 21st century? Chem. Eur. J. 2006, 12, 8434–8442. [Google Scholar] [CrossRef] [PubMed]
- Kashid, M.N.; Kiwi-Minsker, L. Microstructured reactors for multiphase reactions: State of the art. Ind. Eng. Chem. Res. 2009, 48, 6465–6485. [Google Scholar] [CrossRef]
- Kiwi-Minsker, L.; Renken, A. Microstructured reactors for catalytic reactions. Catal. Today 2005, 110, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Kolb, G.; Hessel, V. Micro-structured reactors for gas phase reactions. Chem. Eng. Sci. 2004, 98, 1–38. [Google Scholar] [CrossRef]
- Jensen, K.F. Microreaction engineering—Is small better? Chem. Eng. Sci. 2001, 56, 293–303. [Google Scholar] [CrossRef]
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in microstructured reactors. Angew. Chem. Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, O. Modeling of the interactions between catalytic surfaces and gas-phase. Catal. Lett. 2015, 145, 272–289. [Google Scholar] [CrossRef]
- Bawornruttanaboonya, K.; Devahastin, S.; Mujumdar, A.S.; Laosiripojana, N. A computational fluid dynamic evaluation of a new microreactor design for catalytic partial oxidation of methane. Int. J. Heat Mass Transf. 2017, 115, 174–185. [Google Scholar] [CrossRef]
- Navalho, J.E.P.; Pereira, J.M.C.; Pereira, J.C.F. Multiscale modeling of methane catalytic partial oxidation: From the mesopore to the full-scale reactor operation. AIChE J. 2018, 64, 578–594. [Google Scholar] [CrossRef]
- Tonkovich, A.; Kuhlmann, D.; Rogers, A.; McDaniel, J.; Fitzgerald, S.; Arora, R.; Yuschak, T. Microchannel technology scale-up to commercial capacity. Chem. Eng. Res. Des. 2005, 83, 634–639. [Google Scholar] [CrossRef]
- Tonkovich, A.Y.; Perry, S.; Wang, Y.; Qiu, D.; LaPlante, T.; Rogers, W.A. Microchannel process technology for compact methane steam reforming. Chem. Eng. Sci. 2004, 59, 4819–4824. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018. [Google Scholar] [CrossRef]
- ANSYS Fluent User’s Guide; Release 16.0; ANSYS Inc.: Canonsburg, PA, USA, 2014.
- Kee, R.J.; Dixon-lewis, G.; Warnatz, J.; Coltrin, M.E.; Miller, J.A.; Moffat, H.K. A Fortran Computer Code Package for the Evaluation of Gas-Phase, Multicomponent Transport Properties; Report No. SAND86-8246B; Sandia National Laboratories: Livermore, CA, USA, 1998.
- Von Rickenbach, J.; Lucci, F.; Narayanan, C.; Dimopoulos Eggenschwiler, P.; Poulikakos, D. Effect of washcoat diffusion resistance in foam based catalytic reactors. Chem. Eng. J. 2015, 276, 388–397. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 978-1-119-32042-5. [Google Scholar]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Chebout, R.; Barama, S.; Barama, A.; Benaliouche, F. Hydrogen production over partial oxidation of methane using NiMgAl spinel catalysts: A kinetic approach. C. R. Chim. 2017, 20, 738–746. [Google Scholar] [CrossRef]
- Pruksawan, S.; Kitiyanan, B.; Ziff, R.M. Partial oxidation of methane on a nickel catalyst: Kinetic Monte-Carlo simulation study. Chem. Eng. Sci. 2016, 147, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Neagoe, C.; Boffito, D.C.; Ma, Z.; Trevisanut, C.; Patience, G.S. Pt on Fecralloy catalyses methane partial oxidation to syngas at high pressure. Catal. Today 2016, 270, 43–50. [Google Scholar] [CrossRef]
- Schwiedernoch, R.; Tischer, S.; Correa, C.; Deutschmann, O. Experimental and numerical study on the transient behavior of partial oxidation of methane in a catalytic monolith. Chem. Eng. Sci. 2003, 58, 633–642. [Google Scholar] [CrossRef]
- Hughes, K.J.; Turányi, T.; Clague, A.R.; Pilling, M.J. Development and testing of a comprehensive chemical mechanism for the oxidation of methane. Int. J. Chem. Kinet. 2001, 33, 513–538. [Google Scholar] [CrossRef] [Green Version]
- Turanyi, T.; Zalotai, L.; Dobe, S.; Berces, T. Effect of the uncertainty of kinetic and thermodynamic data on methane flame simulation results. Phys. Chem. Chem. Phys. 2002, 4, 2568–2578. [Google Scholar] [CrossRef]
- Aseem, A.; Harold, M.P. C2 yield enhancement during oxidative coupling of methane in a nonpermselective porous membrane reactor. Chem. Eng. Sci. 2018, 175, 199–207. [Google Scholar] [CrossRef]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Meeks, E.; Miller, J.A. CHEMKIN-III: A Fortran Chemical Kinetics Package for the Analysis of Gasphase Chemical and Plasma Kinetics; Report No. SAND96-8216; Sandia National Laboratories: Livermore, CA, USA, 1996. [CrossRef]
- Coltrin, M.E.; Kee, R.J.; Rupley, F.M.; Meeks, E. SURFACE CHEMKIN-III: A Fortran Package for Analyzing Heterogeneous Chemical Kinetics at a Solid-Surface-Gas-Phase Interface; Report No. SAND96-8217; Sandia National Laboratories: Livermore, CA, USA, 1996. [CrossRef]
- Bodke, A.S.; Bharadwaj, S.S.; Schmidt, L.D. The effect of ceramic supports on partial oxidation of hydrocarbons over noble metal coated monoliths. J. Catal. 1998, 179, 138–149. [Google Scholar] [CrossRef]
- Hunt, G.; Karimi, N.; Torabi, M. Two-dimensional analytical investigation of coupled heat and mass transfer and entropy generation in a porous, catalytic microreactor. Int. J. Heat Mass Transf. 2018, 119, 372–391. [Google Scholar] [CrossRef]
- Venvik, H.J.; Yang, J. Catalysis in microstructured reactors: Short review on small-scale syngas production and further conversion into methanol, DME and Fischer-Tropsch products. Catal. Today 2017, 285, 135–146. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.-N.; Larachi, F. Reactions, hydrodynamics and mass transfer in micro-packed beds—Overview and new mass transfer data. Chem. Eng. Process 2016, 110, 80–96. [Google Scholar] [CrossRef]










| Parameter | Variable | Value |
|---|---|---|
| Geometry | ||
| Channel length | l | 8.0 mm |
| Channel height | d | 0.8 mm |
| Solid wall | ||
| Thickness | δ | 0.8 mm |
| Thermal conductivity | λs | 16 W/(m·K) (300 K) |
| Gas phase | ||
| Inlet methane-to-oxygen molar ratio | φ | 2.0 |
| Inlet pressure | pin | 0.1 MPa |
| Inlet temperature | Tin | 300 K |
| Inlet velocity | uin | 0.8 m/s |
| Catalyst | ||
| Washcoat thickness | δcatalyst | 0.08 mm |
| Mean pore diameter | dpore | 20 nm |
| Porosity | εp | 0.5 |
| Tortuosity factor | τp | 3 |
| Catalyst/geometric surface area | Fcat/geo | 8 |
| Density of rhodium surface sites | Γ | 2.72 × 10−9 mol/cm2 |
| Other conditions | ||
| Ambient temperature | Tamb | 300 K |
| Surface emissivity | ε | 0.8 |
| External heat loss coefficient | ho | 20 W/(m2·K) |
| Reactions | A (cm, mol, s) | s | Ea (kJ/mol) |
|---|---|---|---|
| Adsorption | |||
| H2 + * + * => H * + H * | 1.0 × 10−2 | ||
| O2 + * + * => O * + O * | 1.0 × 10−2 | ||
| CH4 + * => CH4 * | 8.0 × 10−3 | ||
| H2O + * => H2O * | 1.0 × 10−1 | ||
| CO2 + * => CO2 * | 1.0 × 10−5 | ||
| CO + * => CO * | 5.0 × 10−1 | ||
| Desorption | |||
| H * + H * => * + * + H2 | 3.0 × 1021 | 77.8 | |
| O * + O * => * + * + O2 | 1.3 × 1022 | 355.2–280ΘO* | |
| H2O * => H2O + * | 3.0 × 1013 | 45.0 | |
| CO * => CO + * | 3.5 × 1013 | 133.4–15ΘCO* | |
| CO2 * => CO2 + * | 1.0 × 1013 | 21.7 | |
| CH4 * => CH4 + * | 1.0 × 1013 | 25.1 | |
| Surface reactions | |||
| H * + O * => OH * + * | 5.0 × 1022 | 83.7 | |
| OH * + * => H * + O * | 3.0 × 1020 | 37.7 | |
| H * + OH * => H2O * + * | 3.0 × 1020 | 33.5 | |
| H2O * + * => H * + OH * | 5.0 × 1022 | 104.7 | |
| OH * + OH * => H2O * + O * | 3.0 × 1021 | 100.8 | |
| H2O * + O * => OH * + OH * | 3.0 × 1021 | 171.8 | |
| C * + O * => CO * + * | 3.0 × 1022 | 97.9 | |
| CO * + * => C * + O * | 2.5 × 1021 | 169.0 | |
| CO * + O * => CO2 * + * | 1.4 × 1020 | 121.6 | |
| CO2 * + * => CO * + O * | 3.0 × 1021 | 115.3 | |
| CH4 * + * => CH3 * + H * | 3.7 × 1021 | 61.0 | |
| CH3 * + H * => CH4 * + * | 3.7 × 1021 | 51.0 | |
| CH3 * + * => CH2 * + H * | 3.7 × 1024 | 103.0 | |
| CH2 * + H * => CH3 * + * | 3.7 × 1021 | 44.0 | |
| CH2 * + * => CH * + H * | 3.7 × 1024 | 100.0 | |
| CH * + H * => CH2 * + * | 3.7 × 1021 | 68.0 | |
| CH * + * => C * + H * | 3.7 × 1021 | 21.0 | |
| C * + H * => CH * + * | 3.7 × 1021 | 172.8 | |
| CH4 * + O * => CH3 * + OH * | 1.7 × 1024 | 80.3 | |
| CH3 * + OH * => CH4 * + O * | 3.7 × 1021 | 24.3 | |
| CH3 * + O * => CH2 * + OH * | 3.7 × 1024 | 120.3 | |
| CH2 * + OH * => CH3 * + O * | 3.7 × 1021 | 15.1 | |
| CH2 * + O * => CH * + OH * | 3.7 × 1024 | 158.4 | |
| CH * + OH * => CH2 * + O * | 3.7 × 1021 | 36.8 | |
| CH * + O * => C * + OH * | 3.7 × 1021 | 30.1 | |
| C * + OH * => CH * + O * | 3.7 × 1021 | 145.5 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Song, W.; Xu, D. Computational Fluid Dynamics Modeling of the Catalytic Partial Oxidation of Methane in Microchannel Reactors for Synthesis Gas Production. Processes 2018, 6, 83. https://doi.org/10.3390/pr6070083
Chen J, Song W, Xu D. Computational Fluid Dynamics Modeling of the Catalytic Partial Oxidation of Methane in Microchannel Reactors for Synthesis Gas Production. Processes. 2018; 6(7):83. https://doi.org/10.3390/pr6070083
Chicago/Turabian StyleChen, Junjie, Wenya Song, and Deguang Xu. 2018. "Computational Fluid Dynamics Modeling of the Catalytic Partial Oxidation of Methane in Microchannel Reactors for Synthesis Gas Production" Processes 6, no. 7: 83. https://doi.org/10.3390/pr6070083