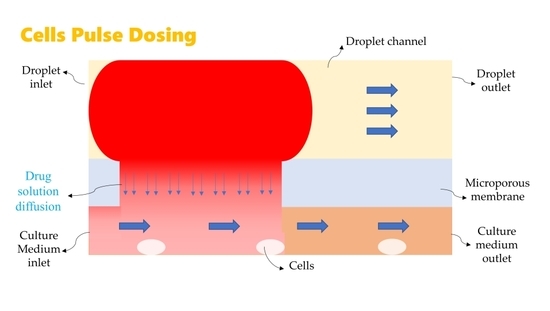
1. Introduction
Microfluidic chip can achieve the basic function of macroscopic laboratory by manipulating the flow at the micro scale. The use of microfluidic chip for chemical analysis, drug screening and transportation, cell culture and other functions by means of microfluidic control is regarded as the miniaturization and integration of macroscopic laboratories [
1,
2,
3,
4], which has advantages as follows: (1) controllable liquid flow; (2) rare consumption of samples and reagents; (3) analysis process automation; (4) significant increased analysis efficiency [
5]. In the aspect of cell culture, the microfluidic chip cell laboratory has micron-level and relatively closed three-dimensional cell culture, sorting, cracking and other operating units, which plays a unique role in cell research [
6,
7]. For cell dosing, the latest fourth-generation targeted dosing system can concentrate the drug to the target cell or the target tissue, which accumulates the drug in a small range to realize the improvement of therapeutic effect yet reducing the drug side-effect [
8,
9]. Therefore, many researchers have been focused on the application of microfluidic chip in cell dosing system. The first invented microchip based on electrochemical dissolution microcapsule experimentally demonstrated the release of drug [
10]. A multilayer polymer drug handling facility was designed for oral dosing of treatment and provided a variety of unidirectional release treatments, the concentration of drug into the intestinal epithelial cells was more than 10 times that of the conventional method [
11]. Three-dimensional tissue platform based on a microfluidic chip can be used to diagnose and quantify cellular heterogeneity [
12]. Integrated microfluidic chip platform can simulate the microenvironment of the blood-brain barrier and glioma in vivo and conduct drug penetration experiments [
13].
The conventional cell dosing methods on a chip often inject drug solution into microfluidic chip directly or use two-phase fluid in the microchannel through the semi-permeable membrane to realize drug diffusion [
14,
15,
16,
17,
18]. The drug was wasted a lot and the precision was low for the former and it was difficult to control drug distribution for the latter. So, the jet microfluidic was used to dose cell and the control precision was improved, both the consumption of drug and the dosing time were reduced [
19,
20,
21,
22,
23]. But the dosing pressure towards to cell was quite tough, which may impair cell severely and the dosing time was difficult to control. For overcoming these dosing problems, the microporous membrane is used to support drug microdroplets flowing and buffer the diffusion, the microdroplets velocity is controlled to adjust dosing time. Considering that polydimethylsiloxane (PDMS) has good biocompatibility, high transparence and is permeable to water and CO
2 and polycarbonate (PCTE) etching membrane also has good biocompatibility, high transparence and impact resistance, customizable micropore diameter and porosity, a novel three-layer sandwich structure of PDMS/PCTE microporous membrane/PDMS chip was designed, which supports microdroplets flow in the microchannel upon membrane, the drug carried in microdroplets could diffuse through microporous membrane into cells trapped at micro-traps. Precise pulse dosing to cells could be achieved by controlling the flow of microdroplets and culture solution. Researching the drug transfer process in this novel device is necessary for supporting the control method for cell pulse dosing. It is known that the mass transfer between deionized water droplets and continuous phases octanol can be observed that there is a vortex on the surface of the microdroplets [
24] and the solute transfer at the instantaneous two-phase interface is proportional to the square of the time [
25]. The drug diffusion to cells through microporous membrane from microdroplet surround by oil is influenced by these factors, especially the mechanism of mass transfer through membrane in fluid. The hydrophilic nanoporous membrane does not exhibit any pore wetting because of the dramatically increased shear viscous force of water with respect to the hydrophilic pore wall [
26]. The surface tension of water decreases with the decreasing of film thickness on the surface of aqueous nano-films [
27]. More investigations about drug diffusion through membrane could be expanded and more deeply discussed based on these achievements.
In current study, we studied the convective diffusion process of microdroplet cell dosing in the three-layer sandwich structure of PDMS/PCTE microporous membrane/PDMS chip. The flow velocities of microdroplets and culture medium were investigated for the convenience of studying and controlling the cells dosing pulse time ignoring other factors influence. Although the microdroplets size or surface chemical complexity and so forth also influence the diffusive dosing process. But it is not convenient to control the pulse time in drug experiments by change the microdroplets size or surface chemical character. The mathematical model was established to solve the convective diffusion process and the process of drug transfer to micro-traps was simulated. The drug concentration, convective diffusion time, pressure field and velocity field around micro-traps were analyzed. The applicability of microdroplet micropore diffusion for cell dosing was further promoted, which contributes more controllable instant pulse dosing time and flux to cells while less impact to cells.
3. Results and Discussion
3.1. Area-Weighted Rhodamine Mass Fraction on Cell Surfaces
The advantages of microdroplet dosing to cell on a chip is tiny waste of drug, controllable pulse dosing and less impact to cell. So, the area-weighted mass fraction on cell surfaces that describes the rhodamine molecules mass reaching cell surfaces, the method for controlling pulse time and the impact on cells especially about cell surfaces pressure are significant parameters in this microfluidic system. More rhodamine mass fraction on cell surfaces, more mild pressure impact on cell surfaces and less pulse dosing time would contribute better cell pulse dosing effect.
The rhodamine mass fraction profiles at Cell 1–7 cell surfaces except at Cell 3 and Cell 6 cells are shown as
Figure 4, because Cell 3 and Cell 6 cells are symmetric along the plane at middle of micro-traps perpendicular to Y axis with Cell 4 and Cell 5 cells. The diffusion effect is uniform and the maximum rhodamine mass fraction is invariable when fluid velocities in droplet channel and culture solution channel are same. The maximum rhodamine mass fraction on Cell 1–7 cell surfaces is 0.38 and all cells have attained it. It is obvious that the maximum rhodamine mass frication on cell surfaces is independent with velocity change when microdroplet velocity is equal to culture solution velocity.
As the rhodamine molecules last Brownian motion, they would collide with other molecules. The rhodamine molecules would keep a certain speed and would move a distance after the collision at certain moments causing self-diffusion [
30]. The multi-component inter-diffusion happens when concentration gradient exists in mixture, rhodamine molecules diffuse to culture solution while culture solution diffuses to droplet. But in this dosing system, there are convective diffusion and capillary force particularly influencing the rhodamine transport process besides self-diffusion and inter-diffusion. These factors play different important roles as the microdroplet and culture solution inlet velocity change.
More drug delivery efficiency could be realized by controlling the relative velocity of microdroplet to culture solution. The maximum mass fraction at cell surfaces when the relative velocity of microdroplet to culture solution changes from 0–0.09 m/s is shown as
Figure 5. As culture solution velocity is 0.01 m/s, the maximum mass fraction at cell surfaces improved with the increase of the relative velocity of microdroplet and it almost all exceeds 0.5 when relative velocity is 0.03 m/s but the maximum mass fraction decreases gradually when relative velocity exceeds 0.03 m/s.
The trend of convective diffusion of rhodamine from microdroplet to culture solution was aroused by the flow of high velocity fluid to low velocity fluid zone and was enhanced gradually when the relative velocity of microdroplet increased. However, large difference between fluid velocities also promotes impacts of oil on membrane and microdroplet, a little two phases mixing caused by a part of oil break the surface tension between water phase and oil phase. The vortex between microdroplet and culture solution caused by the relative velocity was aggravated, so the mass diffusion was hindered [
31]. Thus, the rhodamine convective diffusion is weakened when the vortex and mix effect are stronger as microdroplets flow velocity increases.
3.2. Pulse Time
Controllable and instant pulse drug delivery is the most advantage of this microfluidic chip. The time is assumed as effective dosing time when the rhodamine solution mass fraction at cell surfaces is larger than 0.01. The drug delivery pulse time changing with microdroplet and culture solution inlet velocity is shown as
Figure 6. The pulse time decreases rapidly as all fluids inlet velocity increases to 0.03 m/s but then decreases slowly when culture solution inlet velocity is over 0.03 m/s. The pulse time decreases rapidly when the relative velocity of microdroplet to culture solution varies from 0.01 m/s to 0.06 m/s but decreases slightly under other relative velocities.
The inter-diffusion effect is predominant when the concentration gradient is pretty large and culture solution inlet velocity is relatively slow, so culture solution takes more time to dilute and carry rhodamine to outlet direction. The rhodamine mass fraction distribution in micro-traps at 2 ms is shown as
Figure 7. The rhodamine mass fraction diffuses from microdroplet to culture solution through microporous membrane once the microdroplet attains membrane zone and it dramatically decreased from inlet to outlet at 4 ms when both microdroplet and culture solution velocity are 0.03 m/s. However, the rhodamine mass fraction is quite uniform in 7 micro-traps just at 2 ms when the relative velocity of microdroplet to culture solution is 0.06 m/s.
Inter-diffusion velocity along X direction is nearly equal to culture solution velocity when little rhodamine diffuses to inlet direction and it is around 0.03 m/s. Thus, the time of rhodamine staying at cell surfaces almost only depends on culture solution inlet velocity, because inter-diffusion velocity is far less than culture solution velocity, the inter-diffusion direction was changed mainly towards outlet. The rhodamine was carried away by culture solution rapidly once they diffused to cell surfaces. Moreover, the microdroplet is extruded to micro-traps zone through porous zone when relative velocity is larger than 0.01 m/s. As a result, the mass fraction of rhodamine on micro-traps grows more rapidly than the situation when the microdroplet velocity is the same as culture solution velocity and the convective diffusion effect is much enhanced when the relative velocity increases. But the time of microdroplet extruded into culture solution decreases slightly when the relative velocity of microdroplet to culture solution is larger than 0.06 m/s, the fluid velocity located under microdroplet zone could not be improved significantly.
3.3. Pressure Field
The fluid pressure on cell surfaces is directly related with cell culture and dosing analysis, high pressure on cell surfaces should be avoided in this system. Other than that, the fluid pressure around microdroplet also influences the rhodamine diffusion efficiency and the impact to cells. The microdroplet was affected by capillary force and especially fluid pressure, its shape is changed to trapezoid from slug as shown in
Figure 8. The contact angle and area between microdroplet and culture solution are expanded when microdroplet velocity and culture solution velocity increase and the microdroplet is extruded to culture solution more rapidly when relative velocity enlarges.
From the pressure change around microdroplet with microdroplet and culture solution velocities shown as
Figure 9. The pressure increase on the inlet side is 1200 Pa when the microdroplet and culture solution velocities increase from 0.05 m/s to 0.1 m/s; meanwhile, the pressure on the outlet side only increases by 200 Pa. From
Figure 8 and
Figure 9 it can be concluded that the pressure around the microdroplet is mainly responsible for the much larger contact angle and area on the outlet side than those on inlet side. Because the pressure difference between the two ends of the droplet will increase when the relative velocity of the droplet increases, regardless of whether the medium velocity increases or not. The interface between microdroplet and oil close to inlet suffers from much severer fluid pressure, which pushes microdroplet to flow and maintain original shape. However, the interface between microdroplet and oil towards outlet side not only lacks sufficient pressure support but also is dragged by faster culture solution below the membrane. The pressure difference between the two ends of the droplet is mainly affected by the oil phase velocity. The contact angle of between microdroplets and microporous membrane that close the outlet increases with the increase of the flow velocity of the culture medium, so that the contact area between the droplet and the medium also increases.
The capillary force at microporous membrane zone plays an important role in rhodamine diffusion when microdroplet flow velocity is equal to culture solution flow velocity. The capillary force drags the microdroplet, arousing a tendency to flow into culture solution. What’s more, the microporous membrane surface is quite rough in microscale, which causing droplet spreading and absorption phenomenon [
32]. As a result, the contact area microdroplet between culture solution is relatively extended, so rhodamine inter-diffusion effect is improved and the number of cells dosing at a moment is increased too.
In addition, microdroplet would be extruded to culture solution channel through porous membrane gradually because of relative velocity and it is extruded more rapidly as the relative velocity becomes larger. The cell surfaces pressure caused by fluids flow field change may influence cell culture environment, culture solution velocity and the relative velocity of microdroplet and should be focused. The cell surfaces pressure influenced by culture velocity when the relative velocity of microdroplet is 0 and as shown in
Figure 10a, the cell surfaces pressure influenced by the relative velocity of microdroplet when culture velocity is 0.01 m/s as shown in
Figure 10b. All the cell surfaces pressure increases especially for Cell 1–4 when the flow velocity of microdroplet and culture solution increase at equal rates. All the cell surfaces pressure increased generally when microdroplet and culture velocity or the relative velocity of microdroplet enlarged but it is only Cell 4 cell surface pressure always keeps growing tendency.
Cells located at micro-traps close to the culture solution inlet suffer more dramatic fluid impact force when microdroplet velocity increases, while the cells far from the inlet suffer only slight impact. The microdroplet velocity does not influence cells because the laminar situation, the convective diffusion between microdroplet and culture solution is tiny. Because the microdroplet diffusion process always covers Cell 4 cell, it causes more pressure as it diffuses through microporous membrane faster. The cells behind Cell 4 would not suffer too much from microdroplet diffusion pressure because of most microdroplets diffused to culture solution and buffered located around Cell 1–4 cells zone. In general, this microfluidic system could further reduce the drug consumption in cells dosing experiments and promote experiments efficiency and observability. The impairment of cells caused by fluid pressure would be reduced a lot compared to jet dosing and the dosing efficiency would be improved, as well as an instant controllable dosing time is realized [
19,
20,
21,
22,
23].
3.4. Combined Influence on Cell Dosing
The cell pulse dosing should be carried out in condition of low pressure, as high as possible drug mass fraction on cell surfaces and controllable pulse time. From above results and discussion, the change of rhodamine mass fraction with relative velocity and pressure is shown in
Figure 11. The diffusion process of rhodamine to cells attains best effect when the relative velocity of microdroplet to culture solution is 0.03 m/s and cells suffer more pressure when relative velocity increases.
Convective velocity of Rhodamine molecules to micro-traps increases when the relative velocity of microdroplet enhanced. The pressure around the microdroplets would not only extrude microdroplets into culture medium but also increase the friction between the microdroplets and the wall surface or the surface of the microporous membrane. And the vortex between the microdroplets and the microporous membrane would be enhanced because of high fluid pressure, which may hinder the diffusion process. In the meanwhile, cells would be impaired in high fluid pressure environment should be considered. As a result, high efficiency cell pulse dosing could be achieved at the relative velocity of microdroplet is 0.03 m/s when culture solution velocity is 0.01 m/s and shorter pulse time could be achieved by increasing microdroplet and culture solution velocity at the same time or increasing the relative velocity of microdroplet to culture solution.
4. Conclusions
In this paper, we analyzed the convective diffusion process of microdroplet cell dosing in the three-layer sandwich structure of PDMS/PCTE microporous membrane/PDMS chip, which mainly focused on the drug delivery mechanism, pulse time, mass fraction on cell surfaces, the effect of the change on microdroplet velocity and culture solution velocity. We found that cells close to culture solution suffer more fluid pressure impact when culture solution inlet velocity or the relative velocity of microdroplet to culture solution increased, otherwise cells are affected slightly. The mechanism of the changes of cell dosing pulse time with microdroplet and culture solution velocity is obtained. Inter-diffusion and convective diffusion play different degrees of importance during drug delivery through microporous membrane to cells. In condition of the relative velocity of microdroplet to culture solution is 0, the cell dosing pulse time decreases notably until flow velocity increases to 0.03 m/s, while it decreased slowly when flow velocity is faster than 0.03 m/s. In condition of constant culture solution velocity, the cell dosing pulse time decreases notably until the relative velocity of microdroplet increases to 0.06 m/s, while it decreases slowly when flow velocity is faster than 0.06 m/s. The pulse time could be controlled by changing microdroplet and culture solution velocities. For better cell dosing efficiency, the rhodamine mass fraction on cell surfaces attains the maximum value when the relative velocity of microdroplet to culture solution is 0.03 m/s. And the cells dosing pulse time or drug concentration could be controlled by changing the flow velocity of microdroplets and culture medium, which is convenient for studying the pulse time influence. Compared to traditional dosing in living body or jet dosing in microfluidic chip, this microdroplet cell dosing chip could achieve more controllable pulse dosing and observe the instant drug effects on cells. The knowledge obtained in this work may provide useful information for microfluidic chip applied to cell culture and dosing.