Comparison in Partition Efficiency of Protein Separation between Four Different Tubing Modifications in Spiral High-Speed Countercurrent Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. HSCCC Apparatus
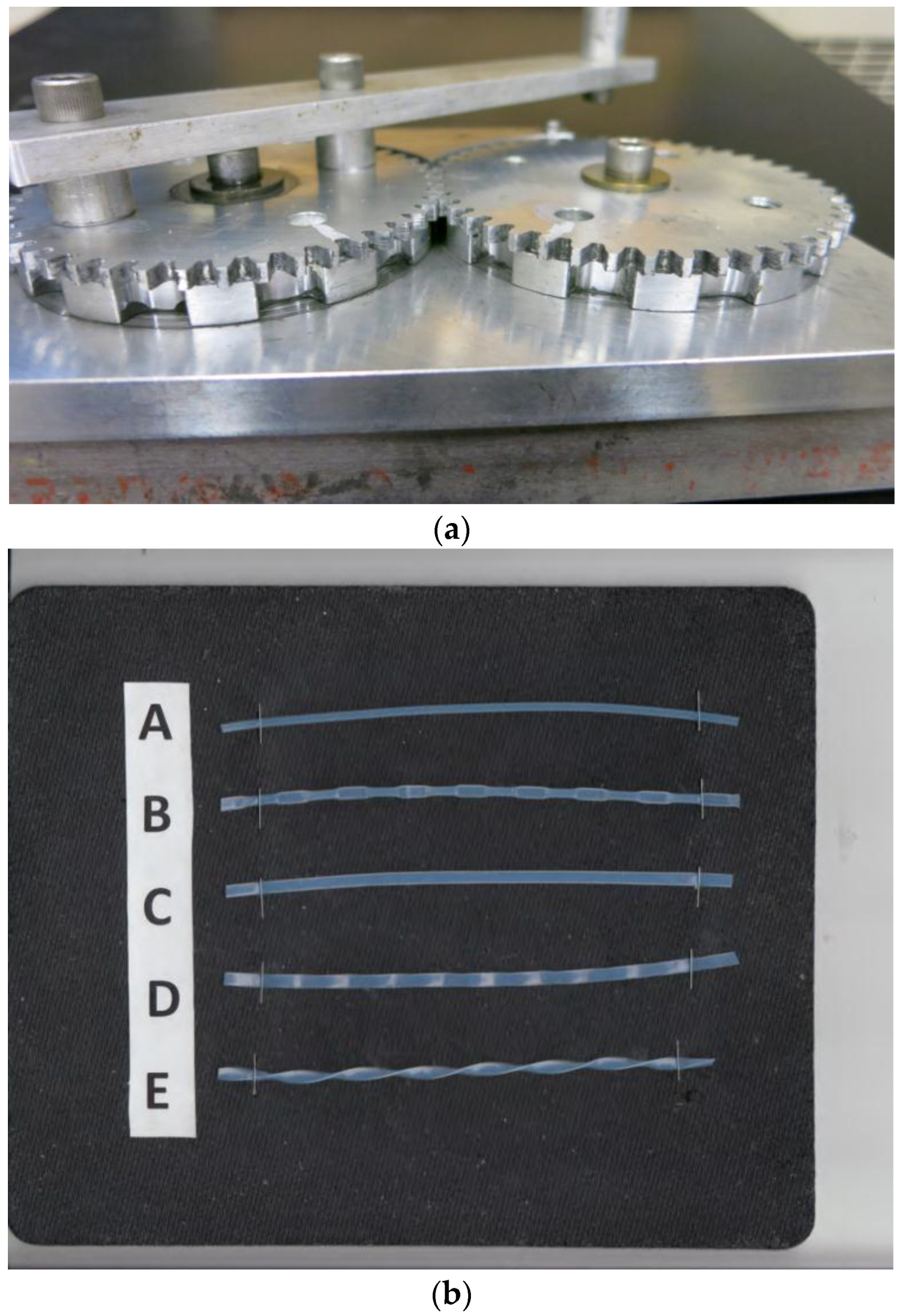
2.2. Design of the Tubing Modifier
2.3. Reagents
2.4. Preparation of Two-Phase Solvent System and Sample Solution
2.5. HSCCC Separation Procedure
2.6. Analysis of HSCCC Fractions
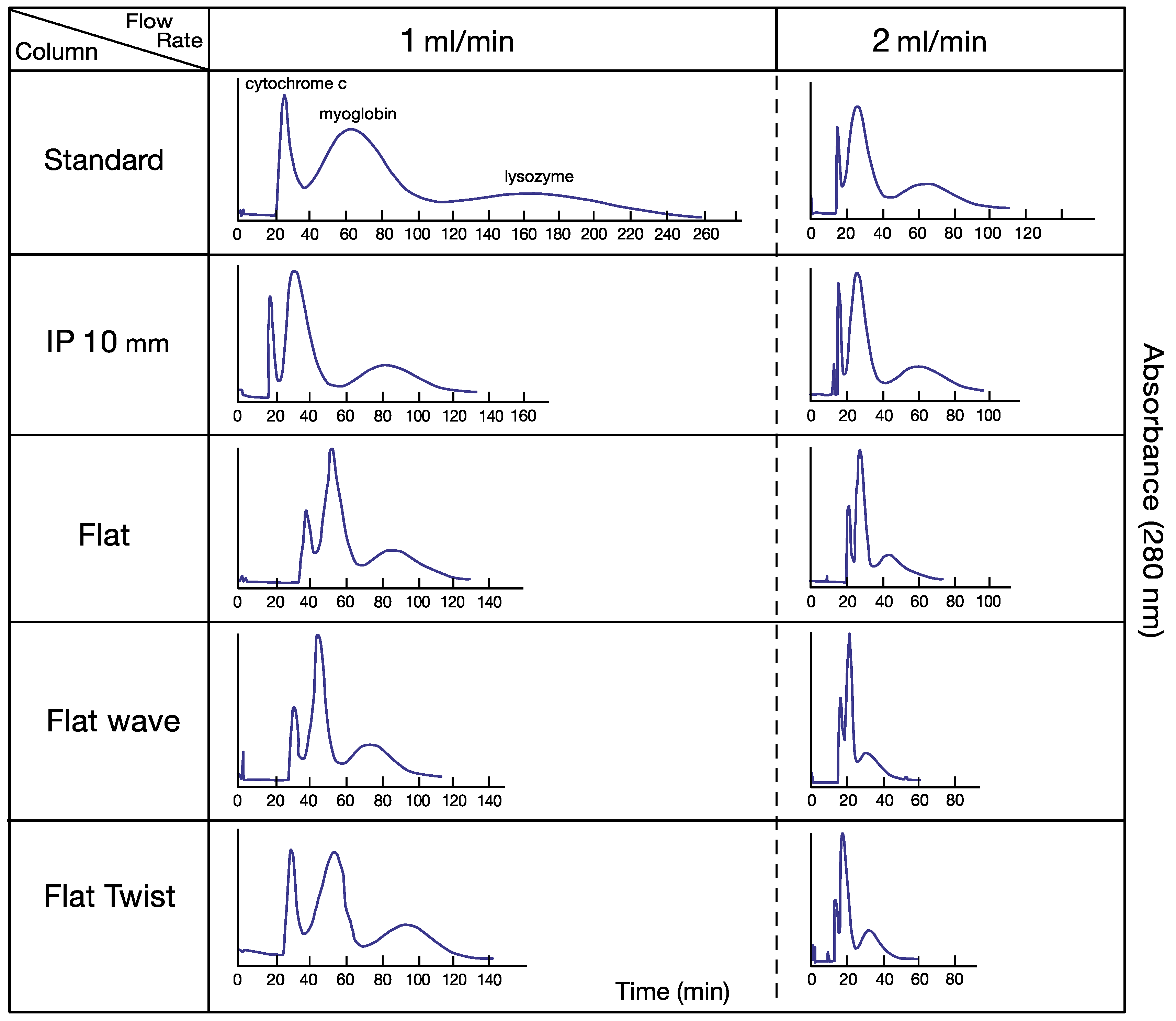
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Ito, Y.; Bowman, R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Science 1970, 167, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Mandava, N.B.; Ito, Y. Countercurrent Chromatography: Theory and Practice; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Conway, W.D. Countercurrent Chromatography: Apparatus, Theory & Applications; VCH Publishers, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Berthod, A. Countercurrent Chromatography: The Support-Free Liquid Stationary Phase; Wilson & Wilsons, Elsevier: New York, NY, USA, 2002. [Google Scholar]
- Menet, J.-M.; Thiébaut, D. Countercurrent Chromatography, Chromatographic Science Series; Marcel Dekker: New York, NY, USA, 1999; Volume 82. [Google Scholar]
- Ito, Y.; Conway, W.D. (Eds.) High-Speed Countercurrent Chromatography; Wiley-Interscience: New York, NY, USA, 1996.
- Ito, Y. High-speed countercurrent chromatography. Crit. Rev. Anal. Chem. 1986, 17, 65–143. [Google Scholar] [CrossRef]
- Ito, Y. High-speed countercurrent chromatography. Nature 1987, 326, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.Å. Partition of Cell Particles and Macromolecules; Wiley-Interscience, John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Ito, Y.; Yang, F.-Q.; Fitze, P.E.; Powell, J.; Ide, D. Improved spiral disk assembly for high-speed countercurrent chromatography. J. Chromatogr. A 2003, 1017, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Spiral column design for separation of proteins by high-speed countercurrent chromatography. Chem. Eng. Process. 2010, 49, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Clary, R.; Powell, J.; Knight, M.; Finn, T.M. Spiral tube support for high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Clary, R.; Sharpnack, F.; Metger, H.; Powell, J. Mixer-settler counter-current chromatography with multiple spiral disk assembly. J. Chromatogr. A 2007, 1172, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Clary, R.; Powell, J.; Knight, M.; Finn, T.M. Spiral tube assembly for high-speed countercurrent chromatography: Choice of elution modes for four typical two-phase solvent systems. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2013–2029. [Google Scholar] [CrossRef]
- Tong, S.-Q.; Ito, Y.; Ma, Y. Enantioseparation of dl-tryptophan by spiral tube assembly counter-current chromatography and evaluation of mass transfer rate for enantiomers. J. Chromatogr. A 2014, 374, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Englert, M.; Vetter, W. Tubing modifications for countercurrent chromatography (CCC): Stationary phase retention and separation efficiency. Anal. Chim. Acta 2015, 884, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ma, X.-F.; Clary, R. A simple tool for tubing modification to improve the spiral high-speed counter-current chromatography. Curr. Chromatogr. 2016, 3, 129–135. [Google Scholar] [CrossRef]
| 1 mL/min | 2 mL/min | |||||
|---|---|---|---|---|---|---|
| Column | Nnorm | Rsnorm | Sf | Nnorm | Rsnorm | Sf |
| Standard | 108/11/19 | 1.05/0.90 | 78% | 455/35/27 | 1.09/1.15 | 63% |
| IP 10 mm | 460/46/39 | 1.55/1.27 | 65% | 294/45/43 | 1.17/1.21 | 56% |
| Flat | 266/120/37 | 1.21/0.92 | 40% | 777/189/45 | 0.92/1.02 | 38% |
| Flat-wave | 644/176/77 | 1.36/1.35 | 38% | 340/72/40 | 0.86/0.75 | 26% |
| Flat-twist | 404/96/80 | 1.82/1.43 | 40% | 718/111/72 | 0.97/1.02 | 38% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, Y.; Clary, R. Comparison in Partition Efficiency of Protein Separation between Four Different Tubing Modifications in Spiral High-Speed Countercurrent Chromatography. Separations 2016, 3, 31. https://doi.org/10.3390/separations3040031
Ito Y, Clary R. Comparison in Partition Efficiency of Protein Separation between Four Different Tubing Modifications in Spiral High-Speed Countercurrent Chromatography. Separations. 2016; 3(4):31. https://doi.org/10.3390/separations3040031
Chicago/Turabian StyleIto, Yoichiro, and Robert Clary. 2016. "Comparison in Partition Efficiency of Protein Separation between Four Different Tubing Modifications in Spiral High-Speed Countercurrent Chromatography" Separations 3, no. 4: 31. https://doi.org/10.3390/separations3040031





