Dispersed Mobile-Phase Countercurrent Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. Selection of Two-Phase System
2.3. DMCC Setup
3. Rusults
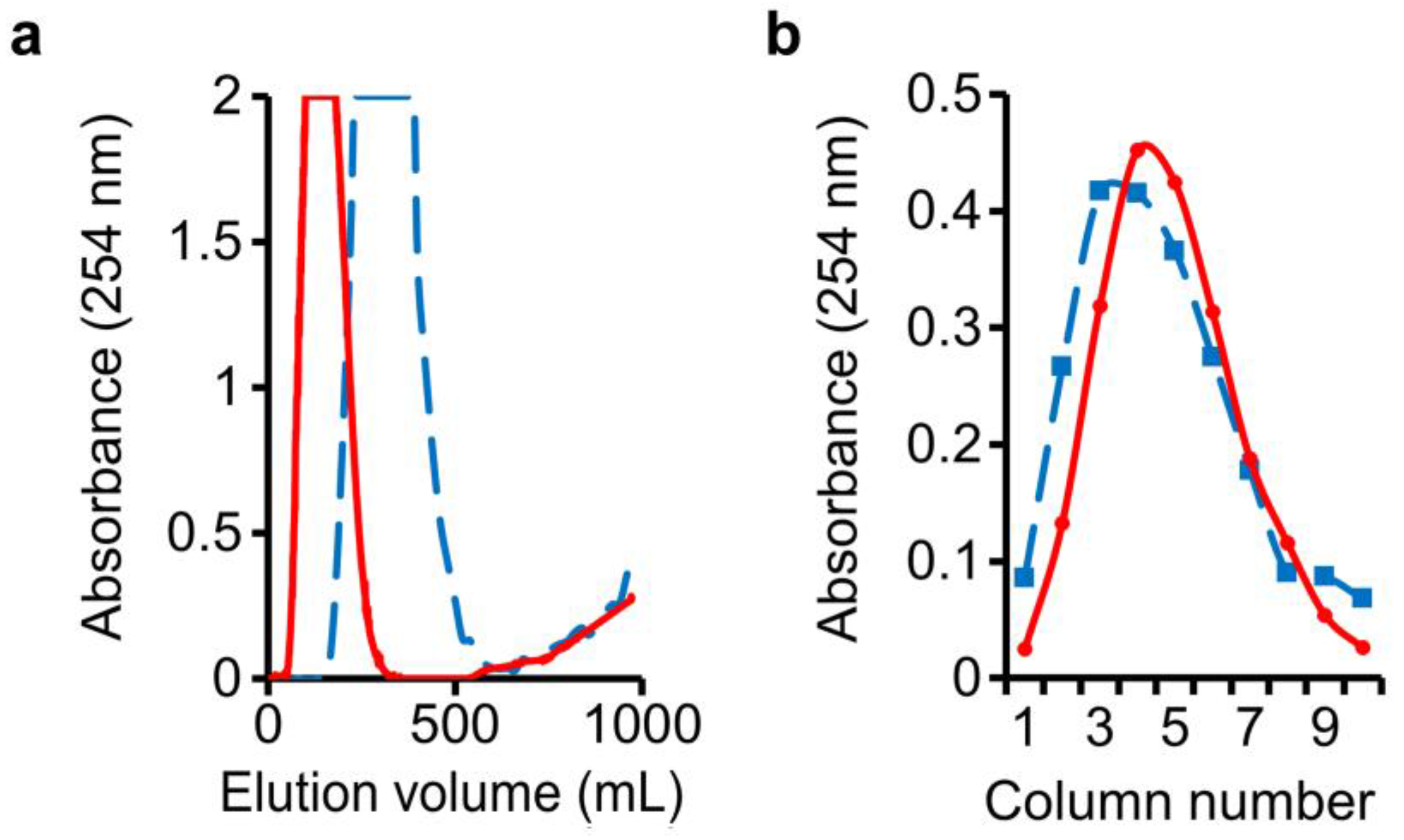
3.1. Separation of o-Cresol and Benzyl Alcohol Using an n-Butanol–NaOH(aq) Two-Solvent System
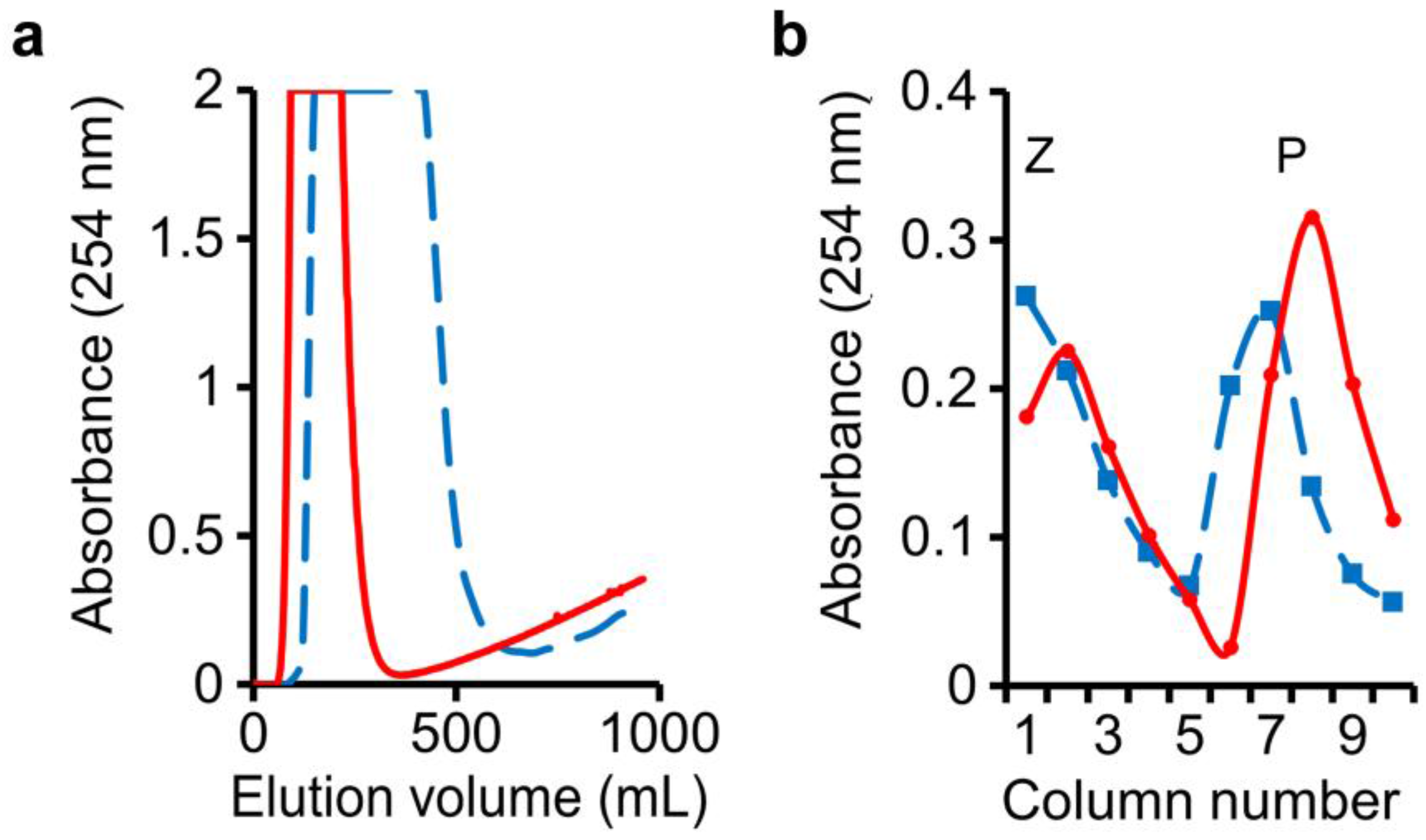
3.2. Separation of Benzoic Acid, Phenol, and Benzyl Alcohol Using n-Butanol–NaHCO3(aq) and n-Butanol–NaOH(aq) Two-Solvent Systems
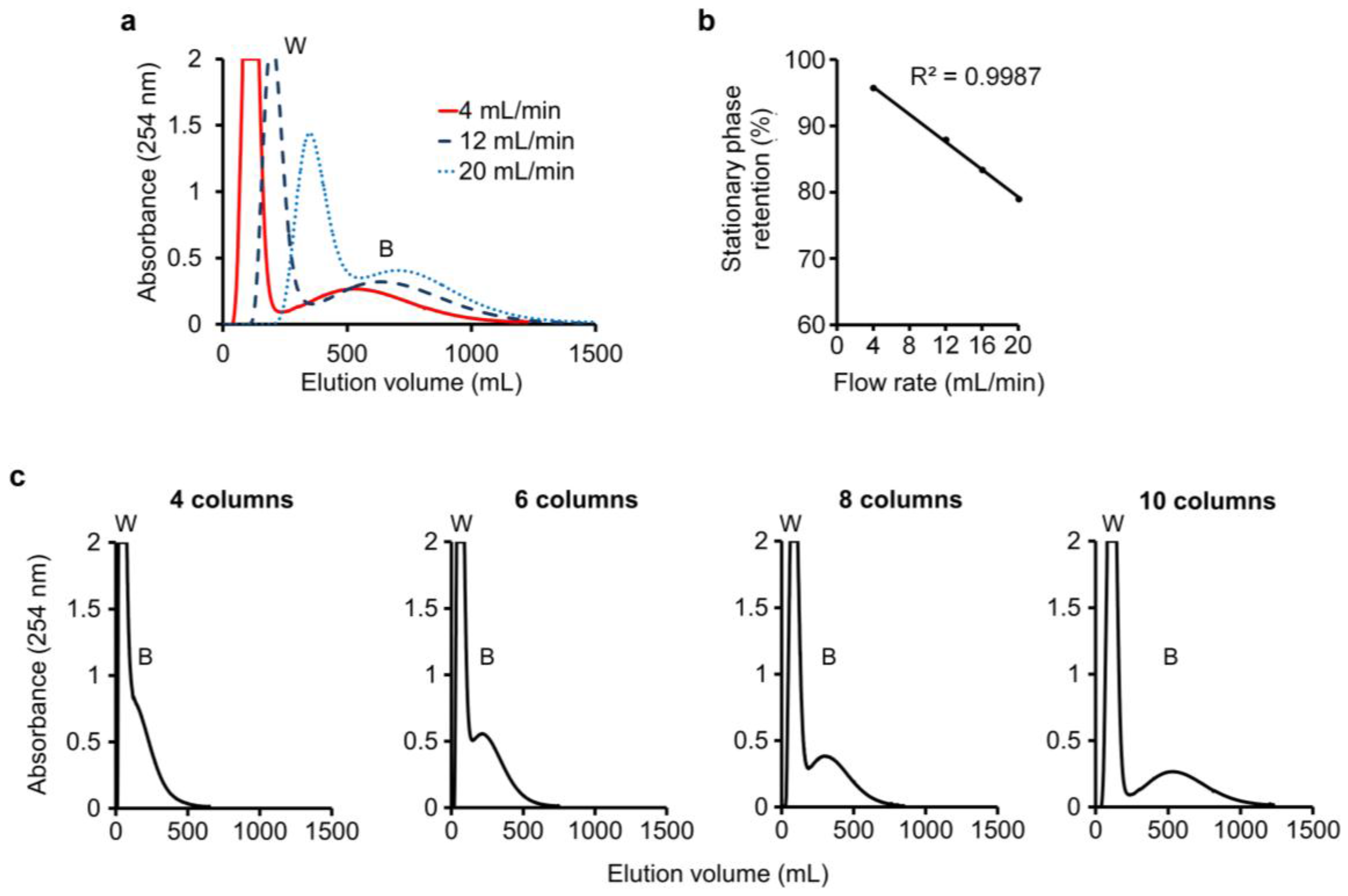
3.3. Separation of Baicalein and Wogonin Using a n-Butanol–K3PO4(aq) Two-Solvent System
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPC | Centrifugal Partition Chromatography |
| CPLC | Controlled-cycle Pulsed Liquid-liquid Chromatography |
| DCCC | Droplet Countercurrent Chromatography |
| DMCC | Dispersed Mobile-phase Countercurrent Chromatography |
| HSCCC | High Speed Countercurrent Chromatography |
| KD | Distribution coefficient |
| RNA | Ribonucleic acid |
| SOSLC | Stationary-phase Optimized Selectivity Liquid Chromatography |
References
- Craig, L. Identification of smalll amounts of organic compounds by distribution studies: II. Separation by counter-current distribution. J. Biol. Chem. 1944, 155, 535–546. [Google Scholar]
- Kimura, Y.; Kitamura, H.; Hayashi, K. A method for separating commercial colistin complex into new components: Colistins pro-A, pro-B and pro-C. J. Antibiot. (Tokyo) 1982, 35, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin a from the medicinal plant scutellaria baicalensis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Apgar, J.; Holley, R.W.; Merrill, S.H. Purification of the alanine-, valine-, histidine-, and tyrosine-acceptor ribonucleic acids from yeast. J. Biol. Chem. 1962, 237, 796–802. [Google Scholar] [PubMed]
- Holley, R.W.; Apgar, J.; Everett, G.A.; Madison, J.T.; Marquisee, M.; Merrill, S.H.; Penswick, J.R.; Zamir, A. Structure of a ribonucleic acid. Science 1965, 147, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, T.; Pisano, J.J.; Ito, Y.; Bowman, R.L. Droplet countercurrent chromatography. Science 1970, 169, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A. Countercurrent Chromatography: The Support-Free Liquid Stationary Phase; Elsevier Science Ltd.: Boston, MA, USA, 2002; Volume 38, p. 397. [Google Scholar]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent chromatography in analytical chemistry (IUPAC Technical report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef]
- Conway, W.D. Countercurrent Chromatography: Apparatus, Theory and Applications; VCH Publishers: New York, NY, USA, 1990. [Google Scholar]
- Du, Q.; Wu, P.; Ito, Y. Low-speed rotary countercurrent chromatography using a convoluted multilayer helical tube for industrial separation. Anal. Chem. 2000, 72, 3363–3365. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Conway, W.D. High-Speed Countercurrent Chromatography; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Ito, Y.; Sandlin, J.; Bowers, W.G. High-speed preparative counter-current chromatography with a coil planet centrifuge. J. Chromatogr. A 1982, 244, 247–258. [Google Scholar] [CrossRef]
- Sutherland, I.A. Recent progress on the industrial scale-up of counter-current chromatography. J. Chromatogr. A 2007, 1151, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, A.; Liu, R. Separation and purification of baicalin and wogonoside from the Chinese medicinal plant Scutellaria baicalensis georgi by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1066, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.P. Centrifugal partition chromatography. In Chromatographic Science Series; Foucault, A.P., Ed.; Marcel Dekker: New York, NY, USA, 1995; Volume 68, pp. 25–49. [Google Scholar]
- Marchal, L.; Legrand, J.; Foucault, A. Centrifugal partition chromatography: A survey of its history, and our recent advances in the field. Chem. Rec. 2003, 3, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Murayama, W.; Kobayashi, T.; Kosuge, Y.; Yano, H.; Nunogaki, Y.; Nunogaki, K. A new centrifugal counter-current chromatograph and its application. J. Chromatogr. A 1982, 239, 643–649. [Google Scholar] [CrossRef]
- Kostanyan, A.E.; Voshkin, A.A.; Kodin, N.V. Controlled-cycle pulsed liquid–liquid chromatography. A modified version of Craig’s counter-current distribution. J. Chromatogr. A 2011, 1218, 6135–6143. [Google Scholar] [CrossRef] [PubMed]
- Belter, P.A.; Speaker, S.M. Controlled-cycle operations applied to extraction processes. Ind. Eng. Chem. Process Des. Dev. 1967, 6, 36–42. [Google Scholar] [CrossRef]
- Ito, Y.; Bowman, R.L. Countercurrent chromatography. Anal. Chem. 1971, 43, 69A–75A. [Google Scholar] [CrossRef]
- Craig, L.; Otto, P. Apparatus for countercurrent distribution. Anal. Chem. 1949, 21, 500–504. [Google Scholar] [CrossRef]
- Nyiredy, S.; Szucs, Z.; Szepesy, L. Stationary phase optimized selectivity liquid chromatography: Basic possibilities of serially connected columns using the “prisma” principle. J. Chromatogr. A 2007, 1157, 122–130. [Google Scholar] [CrossRef] [PubMed]
- De Beer, M.; Lynen, F.; Chen, K.; Ferguson, P.; Hanna-Brown, M.; Sandra, P. Stationary-phase optimized selectivity liquid chromatography: Development of a linear gradient prediction algorithm. Anal. Chem. 2010, 82, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lynen, F.; Szucs, R.; Hanna-Brown, M.; Sandra, P. Gradient stationary phase optimized selectivity liquid chromatography with conventional columns. Analyst 2013, 138, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lynen, F.; De Beer, M.; Hitzel, L.; Ferguson, P.; Hanna-Brown, M.; Sandra, P. Selectivity optimization in green chromatography by gradient stationary phase optimized selectivity liquid chromatography. J. Chromatogr. A 2010, 1217, 7222–7230. [Google Scholar] [CrossRef] [PubMed]


| Solvent System | Sample | KD |
|---|---|---|
| n-butanol–1% NaOH (1:1, v/v) | cresol | 1.06 |
| benzyl alcohol | 4.78 | |
| n-butanol–0.1 M NaHCO3 (1:1, v/v) | benzyl alcohol | 4.77 |
| phenol | 4.68 | |
| benzoic acid | 0.51 | |
| n-butanol–0.1 M NaOH (1:1, v/v) | benzyl alcohol | 4.77 |
| benzoic acid | 0.49 | |
| benzyl alcohol | 0.50 | |
| n-butanol–0.1 M K3PO4 (1:1, v/v) | wogonin | 12.1 |
| baicalein | 2.56 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, T.Y.-C.; Xue, H. Dispersed Mobile-Phase Countercurrent Chromatography. Separations 2016, 3, 32. https://doi.org/10.3390/separations3040032
Ho TY-C, Xue H. Dispersed Mobile-Phase Countercurrent Chromatography. Separations. 2016; 3(4):32. https://doi.org/10.3390/separations3040032
Chicago/Turabian StyleHo, Timothy Yiu-Cheong, and Hong Xue. 2016. "Dispersed Mobile-Phase Countercurrent Chromatography" Separations 3, no. 4: 32. https://doi.org/10.3390/separations3040032





