Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard and Sample Preparation
2.3. CE Apparatus
2.4. Electrophoretic Conditions
3. Results and Discussion
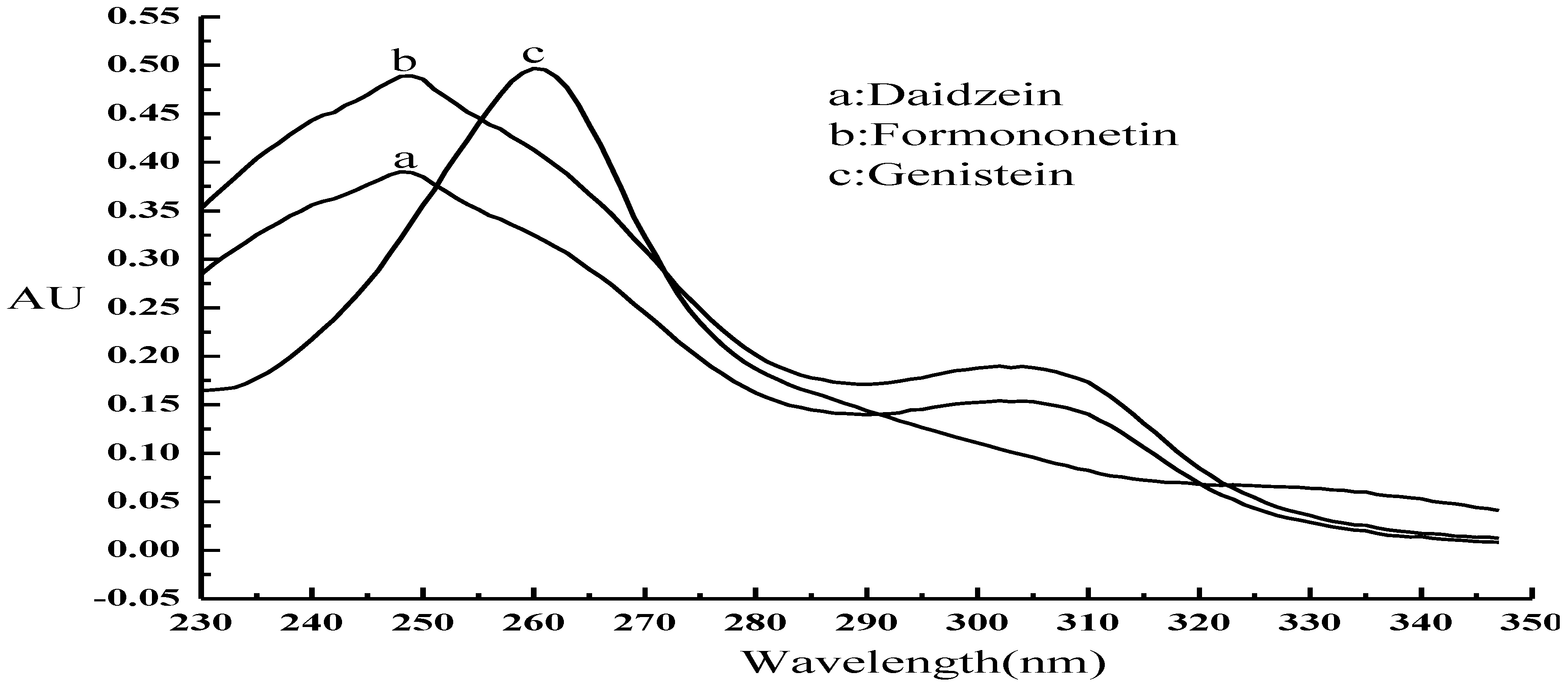
3.1. Optimization of the CE Method
3.2. Validation of the CE Method
3.2.1. Linearity, Limit of Detection and Limit of Quantification
3.2.2. Study of Precision and Accuracy
3.3. Optimization of the Extraction Conditions and Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breithofer, A.; Graumann, K.; Scicchitano, M.S.; Karathanasis, S.K.; Butt, T.R.; Jungbauer, A. Regulation of human estrogen receptor by phytoestrogens in yeast and human cells. J. Steroid Biochem. Mol. Biol. 1998, 67, 421–429. [Google Scholar] [CrossRef]
- Mueller, S.O. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J. Chromatogr. B 2002, 777, 155–165. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Dell’aquila, C.; Low, Y.L.; Kussmaul, M.; Bingham, S.A. Extraction and quantification of phytoestrogens in foods using automated solid-phase extraction and LC/MS/MS. Anal. Chem. 2007, 79, 9234–9239. [Google Scholar] [CrossRef] [PubMed]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, M.; Allan, S.; Jama, J.D. Effects of estrogen plus progestin on risk of fracture and bone mineral density. J. Am. Med. Assoc. 2002, 288, 872–881. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Chen, L.T.; Chiang, C.J.; Wu, L.C.; Chiang, Y.F.; Chen, P.Y.; Sun, J.S.; Wang, C.C. Isoflavones prevent bone loss following ovariectomy in youth adult rats. J. Orthop. Surg. Res. 2008, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Siow, R.C.; Li, F.Y.; Rowlands, D.J.; de Winter, P.; Mann, G.E. Cardiovascular targets for estrogens and phytoestrogens: Transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic. Biol. Med. 2007, 42, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Reinli, K.; Block, G. Phytoestrogen content of foods—A compendium of literature values. Nutr. Cancer 1996, 26, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar] [CrossRef]
- De Kleijn, M.J.; van der Schouw, Y.T.; Wilson, P.W.; Adlercreutz, H.; Mazur, W.; Grobbee, D.E.; Jacques, P.F. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: The Framingham Study (1–4). J. Nutr. 2001, 131, 1826–1832. [Google Scholar] [PubMed]
- Van Elswijk, D.A.; Schobel, U.P.; Lansky, E.P.; Irth, H.; van der Greef, J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry 2004, 65, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Fröhlich, R.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Estrogens and congeners from Spent Hops (Humulus lupulus). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, E.R.; Stauber, R.E.; Van Thiel, D.H.; Campbell, I.M.; Gavaler, J.S. Assessment of the estrogenic activity of phytoestrogens isolated from bourbon and beer. Alcohol. Clin. Exp. 1993, 17, 1207–1209. [Google Scholar] [CrossRef]
- Albert-Puleo, M. Fennel and anise as estrogenic agents. J. Ethnopharmacol. 1980, 2, 337–344. [Google Scholar] [CrossRef]
- Dewell, A. Effects of Phytoestrogens on Lipids in Hypercholestrolemic Postmenopausal Women. Master’s Thesis, San Jose State University, San Jose, CA, USA, December 2001. [Google Scholar]
- Yanaka, K.; Takebayashi, J.; Matsumoto, T.; Ishimi, Y. Determination of 15 isoflavone isomers in soy foods and supplements by high-performance liquid chromatography. J. Agric. Food. Chem. 2012, 60, 4012–4016. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Hämäläinen, E.; Gorbach, S.; Goldin, B. Dietary phyto-oestrogens and the menopause in Japan. Lancet 1992, 339, 1233–1234. [Google Scholar] [CrossRef]
- CBS News. National Coffee Day: Do You Know What’s in Your Joe? Available online: http://www.cbsnews.com (accessed on 29 September 2016).
- Vacek, J.; Klejdus, B.; Lojkova, L.; Kubán, V. Current trends in isolation, separation, determination and identification of isoflavones: A review. J. Sep. Sci. 2008, 31, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.; Wähälä, K.; Rasku, S.; Makkonen, A.; Hase, T.; Adlercreutz, H. Lignans and isoflavonoid polyphenols in tea and coffee. J. Med. Food 1999, 2, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC–MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Alves, R.C.; Almeida, I.M.C.; Casal, S.; Oliveira, M.B.P.P. Method development and validation for isoflavones quantification in coffee. Food Chem. 2010, 122, 914–919. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Nurmi, T.; Adlercreutz, H. A simplified HPLC method for total isoflavones in soy products. Food Chem. 2004, 87, 297–305. [Google Scholar] [CrossRef]
- Apers, S.; Naessens, T.; Van Den Steen, K.; Cuyckens, F.; Claeys, M.; Pieters, L.; Vlietinck, A. Fast high-performance liquid chromatography method for quality control of soy extracts. J. Chromatogr. B 2004, 1038, 107–112. [Google Scholar] [CrossRef]
- Ha, H.; Lee, Y.S.; Lee, J.H.; Choi, H.; Kim, C. High performance liquid chromatographic analysis of isoflavones in medicinal herbs. Arch. Pharm. Res. 2006, 29, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Cherdshewasart, W.; Subtang, S.; Dahlan, W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J. Pharm. Biomed. Anal. 2007, 43, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Dias, P.M.B.; Morais, C.B.; Fröehlich, P.E.; Dall’Agnol, M.; Zuanazzi, J.A. LC determination of four isoflavone aglycones in red clover (Trifolium pratense L.). Chromatographia 2008, 67, 125–129. [Google Scholar] [CrossRef]
- Matsumoto, D.; Kotani, A.; Hakamata, H.; Takahashi, K.; Kusu, F. Column switching high-performance liquid chromatography with two channels electrochemical detection for high-sensitive determination of isoflavones. J. Chromatogr. A 2010, 1217, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wu, Q.; Sciarappa, W.J.; Simon, J.E. Chromatographic fingerprints and quantitative analysis of isoflavones in tofu-type soybeans. Food Chem. 2012, 130, 1003–1009. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of beverages, nuts, seeds, and oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Arabshahi, A.; Moore, D., II; Greendale, G.A.; Wyss, J.M.; Barnes, S. Simultaneous determination of 11 phytoestrogens in human serum using a 2 min liquid chromatography/tandem mass spectrometry method. J. Chromatogr. B 2010, 878, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Guan, S.H.; Yang, M.; Feng, R.H.; Wang, Y.; Zhang, Y.B.; Guo, D.A. Simultaneous determination of 24 constituents in Cortex Lycii using high-performance liquid chromatography–triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2013, 77, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aramendia, M.A.; Garcia, I.; Lafont, F.; Marinas, J.M. Determination of isoflavones using capillary electrophoresis in combination with electrospray mass spectrometry. J. Chromatogr. A 1995, 707, 327–333. [Google Scholar] [CrossRef]
- García-Villalba, R.; León, C.; Dinelli, G.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Garcia-Cañas, V.; Cifuentes, A. Transgenic vs. conventional soybean: A comparative metabolomic study using capillary electrophoresis-time of flight-mass spectrometry. J. Chromatogr. A 2008, 1195, 164–173. [Google Scholar] [CrossRef] [PubMed]
- HaiBo, W.; Dachuan, L.; Yongming, L.; Haiying, W. Study on extracting and purifying isoflavones from soybean by hydrolysing. Food Sci. 2003, 24, 98–101. [Google Scholar]
- Grynkiewicz, G.; Ksycinska, H.; Ramza, J.; Zagrodzka, J. Chromatographic quantification of isoflavones (why and how). Acta Chromatogr. 2005, 15, 31–64. [Google Scholar]
- Müllner, C.; Sontag, G. Determination of some phytoestrogens in soybeans and their processed products with HPLC and coulometric electrode array detection. Fresenius J. Anal. Chem. 1999, 364, 261–265. [Google Scholar] [CrossRef]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of food consumed in Canada, including isoflavones, lignans and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef] [PubMed]


| Compound | Intra-Day (%) | Inter-Day (%) | ||
|---|---|---|---|---|
| Migration Time | Peak Area | Migration Time | Peak Area | |
| Formononetin | 0.34 | 4.60 | 0.55 | 5.31 |
| Daidzein | 0.62 | 3.45 | 1.23 | 4.51 |
| Genistein | 1.03 | 4.29 | 1.52 | 5.01 |
| Compound | Concentration Added (µg/mL) | Recovery (%, n = 3) | RSD (%) |
|---|---|---|---|
| Formononetin | 0.5 | 105.20 | 1.76 |
| 1.0 | 95.53 | ||
| 2.0 | 97.44 | ||
| Daidzein | 0.5 | 92.31 | 2.11 |
| 1.0 | 103.34 | ||
| 2.0 | 100.48 | ||
| Genistein | 0.5 | 103.58 | 3.74 |
| 1.0 | 90.87 | ||
| 2.0 | 97.66 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, F.; Tang, L.L.; Chen, X.X.; Liu, H.T. Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis. Separations 2017, 4, 1. https://doi.org/10.3390/separations4010001
Luan F, Tang LL, Chen XX, Liu HT. Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis. Separations. 2017; 4(1):1. https://doi.org/10.3390/separations4010001
Chicago/Turabian StyleLuan, Feng, Li Li Tang, Xuan Xuan Chen, and Hui Tao Liu. 2017. "Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis" Separations 4, no. 1: 1. https://doi.org/10.3390/separations4010001





