1. Introduction
The term “sulfonamide” derives from para-amino-benzene-sulfonamide (sulfanilamide) and it is also known as streptocid. The structure is similar to para-aminobenzoic acid (PABA), which is demanded by microorganisms (such as bacteria) for dihydrofolic and folic acid synthesis. SAs (SAs) or alternative sulfa drugs have been known since the middle of the twentieth century and were proved to have antibacterial properties in 1935, thus they are some of the oldest antimicrobial drugs. Nowadays, SAs are widely used as antibiotics in veterinary medicine either in single formulation or synergistically with other antibiotics (such as tetracyclines (TCs), SAs, quinolones (Qs), fluoroquinolones (FQs) and trimethoprim (TMP). Their use aims to protect the animals from infectious diseases. In addition, their administration as additives to animal feed can promote growth that results in the rise of the productivity of livestock, despite the fact that the use of antibiotics is prohibited in various places around the world. For example, the concentration of SAs in meat produced in Denmark was on average 4.82 mg/kg (pork), 17.2 mg/kg (cattle), 0.033 mg/kg (broilers) and 58.5 mg/kg (fish). Human health can be influenced by the consumption of meat and dairy products (such as eggs, milk and cheese) which contain amounts of SAs. The use of SAs as additives is forbidden, aiming to ensure safety in human health. Furthermore, SAs are widely used as antibacterial drugs because they are of low cost and active against a broad spectrum of microorganisms. SAs can act, despite their bacteriostatic and chemotherapeutic activity, against infections caused by gram-positive and gram-negative bacteria and protozoa. As it is observed, every human is a passive consumer of sulfa-drugs, which are obtained from the treatment of diseases in animals. The systematic and long-term intake of SAs through the food could be characterized as dangerous and in some cases toxic, appearing in the form of allergic reactions, suppression of enzyme activity and alteration of the intestinal microflora [
1,
2].
It should be noted that only 40 of the 10,000 sulfanilamide derivatives that have been synthesized are used in medical and veterinary practice. SAs have similarities in their structure, as all of them carry the same molecule with the addition of diverse radicals at the R position [
2].
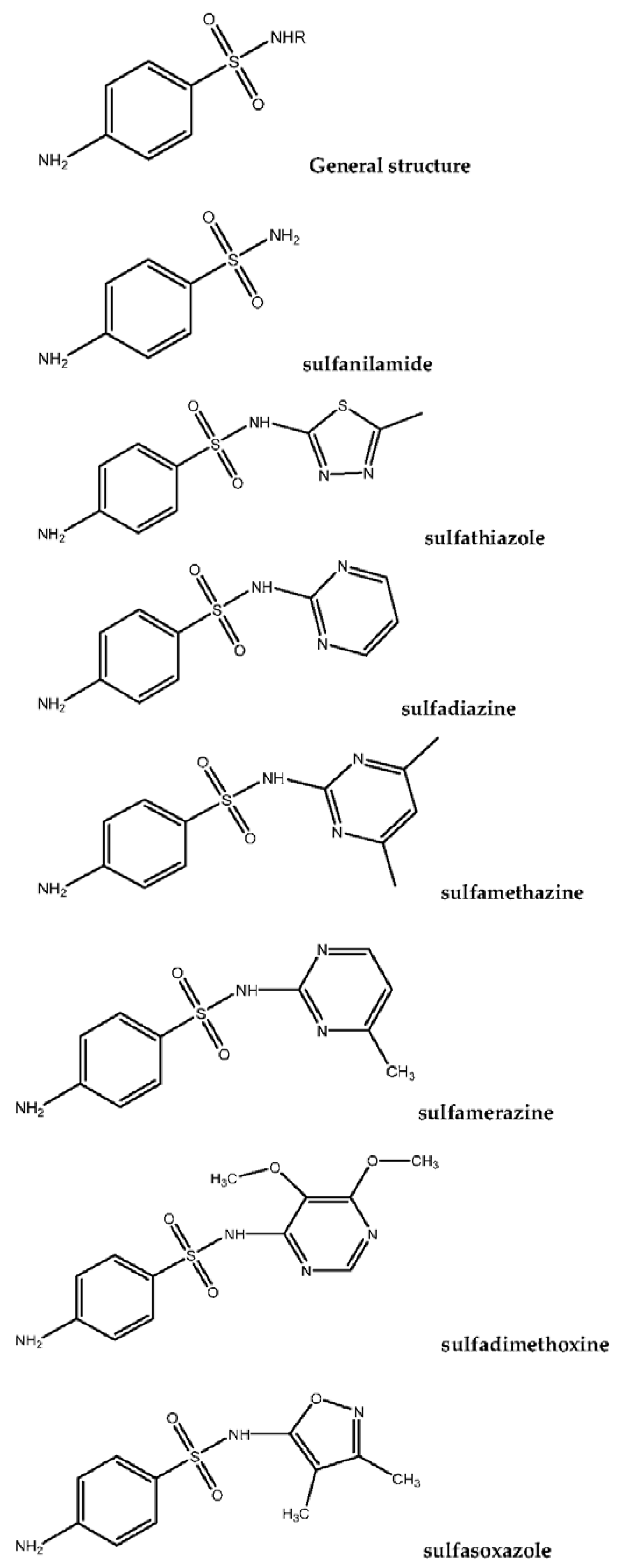
The structures of some of the most commonly used SAs that are mentioned in this review are presented in
Figure 1.
Instructions for the withdrawal period have to be followed in order not be found as residues in milk, eggs, meat, tissues and other livestock products after drug administration [
1,
3].
On the other hand, milk is one of the most widely consumed foods and it is a rich source of protein and calcium, especially in children’s growth. With regards to its analysis, milk is considered as a complex matrix, which contains water, proteins, lactose, fats, minerals and vitamins. According to the European Union (EU), the combined total residues of all substances within the sulfonamide group should not exceed 100 μg/kg [
4]. This is the maximum residue level (MRL) which should be in force for all target tissues (muscle, fat, liver and kidney) and for milk coming from bovine, ovine and caprine. The same MRLs were established in the USA and Canada [
3]. Also, the regulation of
Codex Alimentarius for the MRLs of the above drugs is referred to sulfadimidine and is set as 100 μg/kg for all target tissues, except for milk that is set to 25 μg/L [
5].
Several methods have been developed and validated for the determination of SAs in different matrices such as environmental, biological or food by applying various techniques including photometry, spectrophotometry, gas chromatography (GC), gas chromatography tandem mass spectrometry (GC-MS/MS), capillary electrophoresis-ultraviolet detection (CE-UV), and high performance liquid chromatography (HPLC) coupled with ultraviolet (UV) detection, fluorescence detection (FD) and mass spectrometry (MS) [
6]. All of the developed methods require various sample preparation procedures. Sample preparation is the most demanding step of the analysis in comparison with the other two steps, such as sampling or measurement. At this critical step, the analytes need to be successfully isolated and the sample should reach a capable form for analysis. Sample preparation is crucial to produce accurate results. Thus, this step requires special attention, and it is a time-consuming procedure as well. There are many well-established sample preparation techniques and this field is very interesting for researchers. Therefore, novel techniques are introduced and used in different matrices. In recent years, the trend for every novel technique is to be environmentally friendly according to the principles of Green Analytical Chemistry (GAC) [
7].
During recent decades, several extraction techniques have been used for sample pre-treatment. For the analysis of solid samples, the most applied techniques are Soxhlet (SOX) and pressurized solvent extraction techniques (e.g., supercritical fluid extraction (SFE), accelerated solvent extraction (ASE) and subcritical water extraction (SWE)), and the well-known liquid–liquid extraction (LLE) for the analysis of liquid samples. However most of the conventional techniques (SOX, SPE and LLE) seem to have significant drawbacks. They are time-consuming and complicated, consume large amounts of sample and organic solvents and are difficult to be automated.
In 1990, a novel technique, known as solid phase microextraction (SPME), was introduced by Pawliszyn and co-workers. SPME uses a fused silica fiber, which is coated with a sorbent (the fiber is incorporated in a chromatographic syringe) to extract the target analytes which subsequently are directly transferred into GC or HPLC. The technique has significant advantages. It is fast, simple, solvent free and it is compatible with analyte separation and detection by a chromatographic system (directly in gas chromatography, or via an interface in high-performance liquid chromatography) [
8]. Due to the plethora of advantages, the SPME was extensively used for sample preparation of environmental and food samples. The environmental samples include water, air, soil and sediments, whereas food applications are based on fruits, vegetables, fats, oils, wine, meat and dairy products [
9].
However, the use of SPME fibers involves some drawbacks such as:
their maximum operating temperatures are in the range between 240–280 °C
they are not stable with the organic solvents due to swelling
they break easily
the possibility of stripping of coatings due to analyst’s handling errors.
A modification of SPME was introduced in 1999 by Baltussen. This novel technique is called stir bar sorptive extraction (SBSE) and uses a stir bar consisting of a magnet covered with glass which in turn is coated by a layer (typically 0.5–1 mm) of sorptive material (usually polydimethylsiloxane- PDMS) for the extraction. Furthermore, microextraction by packed sorbent (MEPS) was developed and introduced as a further miniaturization version of SPE. In MEPS, SPE’s conventional polymeric cartridge was replaced by a stainless steel, miniaturized version termed the barrel insert and needle (BIN), which could hold any of a great number of sorbents, such as those used in SPE [
7,
10].
In the meantime, liquid phase microextraction (LPME) was introduced in order to overcome significant drawbacks of liquid phase extraction modes. In LPME, the amount of solvents is smaller in comparison with LLE; only some μL are required, whereas LLE consumes hundreds of mL. It is simple and cheap, as well as adaptable with capillary electrophoresis (CE), HPLC and GC.
Three modes of LPME can be applied. These include single drop microextraction (SDME), hollow fiber liquid-phase microextraction (HF-LPME) and dispersive liquid–liquid microextraction (DLLME). Extraction occurs into a small amount (usually 1–100 μL) of organic solvent (acceptor phase) from an aqueous matrix containing the analytes (donor phase) [
11].
The essential feature of the above extraction techniques is the elimination of large amounts of organic solvents due to the fact that organic solvents are toxic and hazardous for the environment and human health. This complies with the principles of GAC, following the trend of using solvent-less or better described as solvent-free extraction methods.
The introduction of new sorbent materials in sample preparation is also of significant importance and has been widely investigated in order to prepare materials with higher adsorption capacity and selectivity, as well as to expand the availability of cheaper, more easily synthesized sorbents. The combination of microextraction techniques with the new sorbent materials is also base to the GAC demands [
12,
13].
The purpose of this review was to focus on the most recently developed microextraction techniques for the determination of SAs in milk.
2. The Demand of Microextraction Techniques
As already mentioned, sample preparation is the most demanding step in any analytical workflow. The main purpose of sample preparation is to transfer the analytes of interest from a complex matrix to a compatible medium for further determination by an analytical technique. In addition, sample preparation often includes procedures such as clean up, analyte enrichment and derivatization. So, it is clear that this step is time consuming and usually requires the use of large organic solvent volumes and the waste of reagents and consumables. For these reasons, the trend is the introduction of more “green” and micro-techniques in sample preparation.
The idea of sustainable ecological development was introduced in 1987 in a report of the World Commission on Environment and Development. The term green chemistry was mentioned by P. Anastas in 1991 at the US Environmental Protection Agency (EPA). As a result, in 1993, the comprehensive US Green Chemistry Program was established, which included cooperation among many governmental agencies and research institutions. While Anastas and co-workers were elaborating the ideas of green chemistry, the first paradigms of green analytical chemistry were introduced. GAC, introduced in 1999, became a whole part of chemical nomenclature, and numerous reviews and original studies have been published in this topic. The principles of green chemistry and by extension of GAC are presented in
Table 1 [
14].
Concerning green analytical methods, the goals to be achieved include:
elimination or reduction of the use of chemical substances
(such as solvents, reagents, preservatives, additives for pH adjustment)
elimination of energy consumption
proper management of analytical waste
increased safety for the operator
The demands of GAC are automatization, no derivatization, and no sample treatment in the step of sample preparation. The latter is not possible in most cases, so sample preparation by a microextraction technique is the next best choice. Microextraction techniques arose as a development of conventional extraction techniques. The term microextraction means that all modes of these techniques require small volumes of extraction, which becomes under described conditions [
13,
14].
With a quick review of the literature, it is obvious that various methods have been developed for the determination of the SAs in several food matrices. The most often applied techniques for the determination of SAs in milk are HPLC coupled with different detectors, such as ultraviolet [
15], diode-array [
16], or mass spectroscopy [
17].
A significant number of contributions can be found in literature with regards to sample preparation of milk for the determination of SAs. These include either traditional techniques or modern ones.
Solid phase extraction is widely used either in the classical approach or in an alternative way, based on the use of modern adsorbent materials. The use of the commercial SPE presents some disadvantages:
Although there is a wide range of chemistries, many choices for manipulating solvent and pH conditions, optimization is time consuming. In many cases, several steps are required.
The cost per sample is higher than that of simple liquid–liquid extraction (LLE).
Novel microexraction techniques were introduced to overcome these drawbacks. The new techniques require less time and labor than the multi-step procedures of SPE. These include SPME, SBSE, magnetic solid phase microextraction (MSPE), and other greener approaches. For example, a new in-tube solid-phase microextraction technique was introduced by Wen Y. et al. in 2005. The aim for this sample preparation technique is the determination of five SA residues in milk with HPLC-UV. The on-line in-tube SPME used poly-(methacrylic acid-ethylene glycol dimethacrylate) monolithic capillary as extraction media. The method is easily applied and environmentally friendly, following the demands of GAC [
18].
3. Microextraction Techniques for the Determination of SAs in Milk
Some green microextraction techniques that have been developed and applied to determine SAs in milk will be presented in the next few paragraphs. The reported techniques are summarized in
Table 2.
3.1. Magnetic Solid Phase Extraction
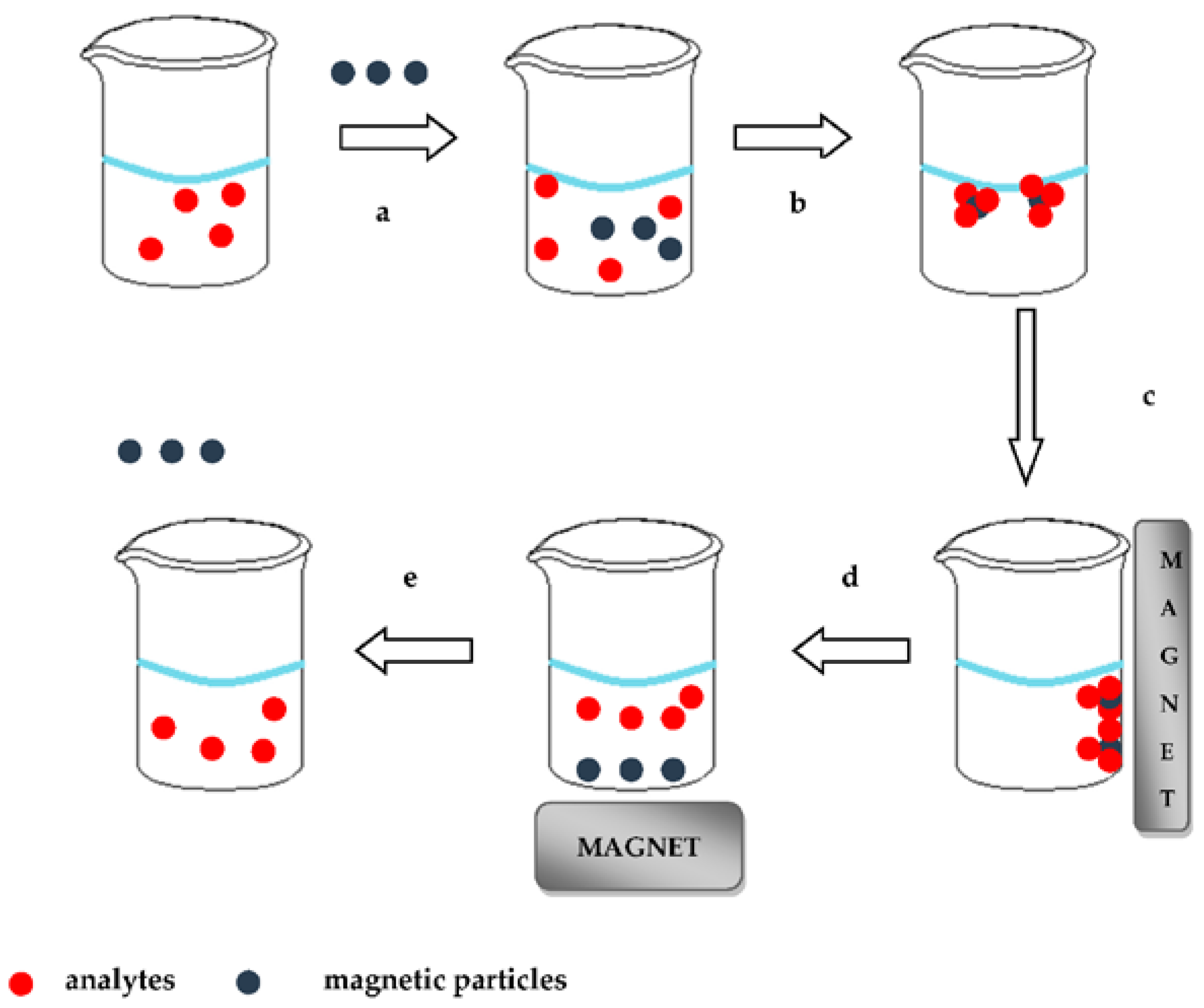
MSPE uses magnetic particles (MPs) as absorbents and in recent years has attracted the interest of analytical scientists. Magnetic separation was introduced by Robinson and co-workers in 1993, although the term MSPE was introduced for the first time in analytical chemistry by Safarikova and Safarik in 1999. The MSPE includes immersion of the MPs to the sample solution. The magnetic core of these MPs is coated with silica or alumina oxides according to the sol–gel technique. After adsorption of analytes on the surface of the MPs, separation of the latter from aqueous solution is achieved by applying an external magnetic field. Target analytes are subsequently desorbed by a suitable eluent and determined by a suitable analytical technique. The procedure of the MSPE technique is illustrated in
Figure 2.
In comparison to the commercial SPE, the application of MSPE simplifies the sample preparation procedure because no pre-packed columns are used. Moreover, separation can be faster due to fewer necessary steps [
10,
16].
Micromagnetic particles, nanomagnetic particles and magnetic molecular imprinted polymers are used as absorbents in MSPE. The application of magnetic solid phase extraction reduces the total time of analysis and provides simultaneous isolation and enrichment of the analytes. In addition, MSPE reduces the use of organic solvents and thus the accumulation of toxic and dangerous wastes, which is acceptable by the principles of GAC [
10].
In 2010, Gao Q. et al. [
27] reported a MSPE technique which achieved the extraction of eleven SAs from milk. A magnetite/silica/poly (methacrylic acid-coethylene glycol dimethacrylate) (Fe
3O
4/SiO
2/P(MAA-
co-EGDMA)) sorbent material was synthesized and characterized by elemental analysis, electron microscopy, X-Ray diffraction and Fourier-transformed infrared spectroscopy. A quantity of 50 mg of the sorbent material was inserted into a vial, and the magnetic particles were preconditioning with methanol and water. Then, the sample was added to the vial, and vortexed for 30 s. Subsequently, the sorbent with adsorbed SAs was separated rapidly from the solution under a strong external magnetic field. After the removal of supernatant solution, SAs were eluted from the magnetic composite by 1 mL of acetone containing 5% ammonium hydroxide solution (
v/
v) with the assistance of vortex mixing for 30 s. An external magnet was used for the separation of magnetic composite from the solution. The sample solution was injected into a LC-MS/MS system for further analysis [
27].
A method applying MSPE of SAs from milk samples was developed by Li Y. and his team in 2015, using a graphene-based magnetic nanocomposite (CoFe
2O
4-graphene, CoFe
2O
4-G). The target analytes included sulfamerazine, sulfamethizole, sulfadoxine, sulfamethoxazole and sulfisoxazole. After the extraction of the absorbent, the analytes were determined by HPLC. In order to achieve the optimal extraction efficiency, several parameters were investigated, such as sample pH, extraction time, the amount of the magnetic material (CoFe
2O
4-G) and elution solvent. As it is mentioned in this work, pH is an important parameter in the MSPE technique because it could affect the speciation of SAs. Therefore, after an optimization study in the range of pH values from 2.0 to 12.0, it was found that higher extraction efficiency was achieved at pH 4.0. The optimum time of the extraction was selected at 20 min. A quantity of 15.0 mg of the CoFe
2O
4-G was selected as optimal. The key parameter of a successful extraction with high recovery was the elution solvent. Several solvents were investigated and finally a volume of 0.5 mL of MeOH solution containing 5% (
v/
v) acetic acid was selected. Among the advantages of this research is the fact that the CoFe
2O
4-G nanocomposite could be re-used after washing with acetone and ultrapure water successively. Moreover, the MSPE procedure presents easy operation, sensitivity and efficiency [
19].
Ibarra I. and co-workers in 2014 published a method for the simultaneous determination of nine SAs in milk samples with HPLC, according to the EU MRLs, using a magnetic solid phase extraction consisting of a silica-based magnetic absorbent (Fe
3O
4-SiO
2-phenyl modified). The advantages were that the developed method was faster than traditional preparation techniques such as SPE and also demanded minimum sample pretreatment and reduced volumes of organic solvents, thus being cost effective. Moreover, the analytical results provide sensitivity and accuracy [
16].
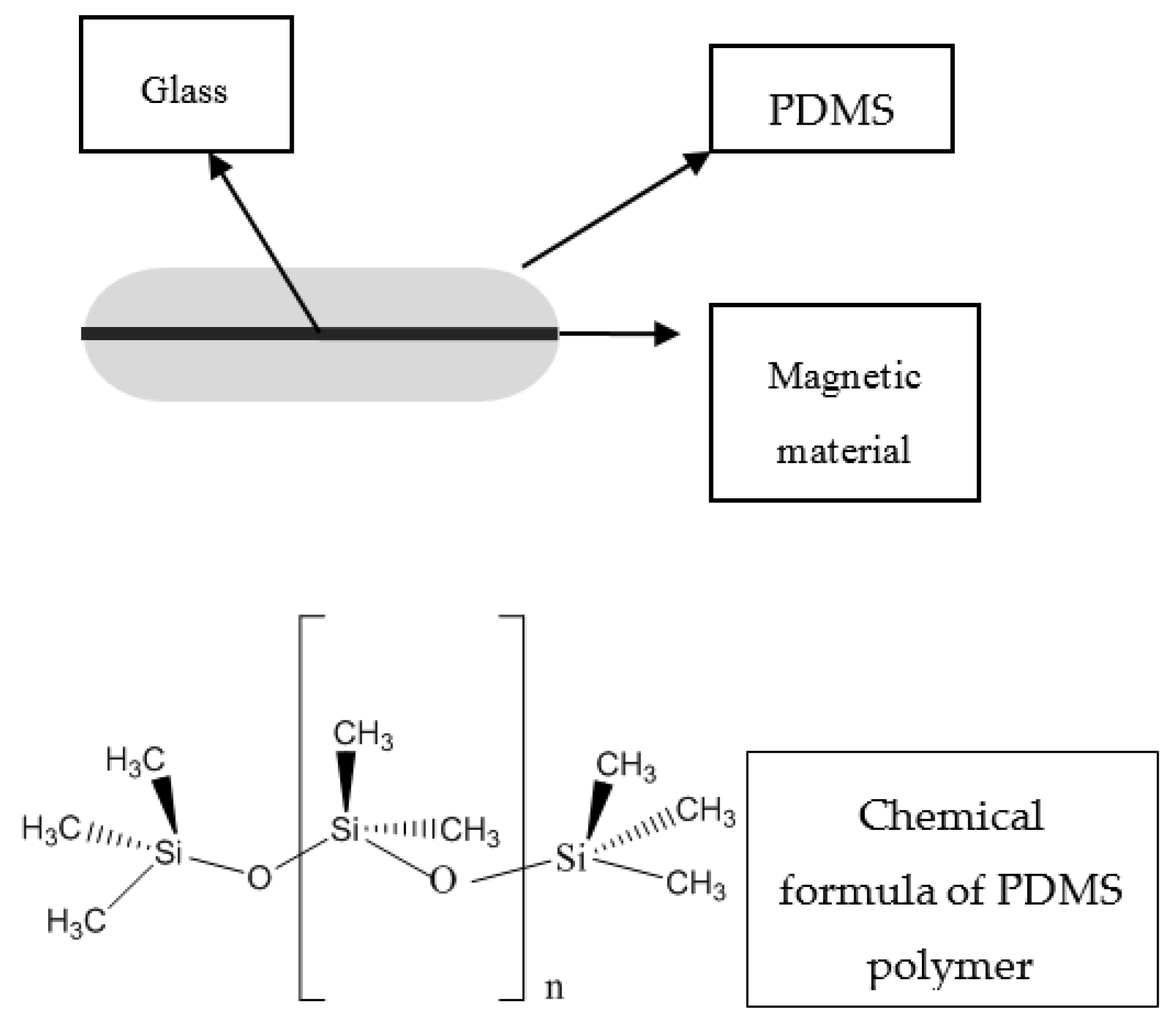
3.2. Stir Bar Sorptive Extraction
SBSE is another novel technique which fulfills the requirements of GAC, and was introduced by Baltussen and co-workers in 1999. This technique uses a polymer coated bar in which the sorptive material is usually the polydimethylsiloxane (PDMS), as shown in
Figure 3. The bar is inserted in the vial containing the sample, and equilibrium of analyte concentration between sorbent and sample matrix is reached by stirring. When the extraction is completed, the bar is removed and transferred to a clean vial where the target compounds are desorbed thermally or in liquid mode. Analysis can be subsequently performed by liquid or gas chromatography using various detection techniques such as ultraviolet, mass spectrometry or fluorescence, etc. [
10,
28,
29].
SBSE takes place in two distinctive procedures: sorption/extraction and desorption. During sorption, the polymer-coated stir bar comes in contact with analytes, either by immersion or in the headspace (HS). HS mode is more preferable for volatile compounds. After the extraction step, the stir bar is removed, rinsed with distilled water, wiped with a paper tissue and submitted to desorption procedure. This can be accomplished either thermally (thermal desorption (TD)) or by means of a suitable solvent system (liquid desorption (LD)). TD is typically followed by Gas Chromatographic analysis, while LD is followed by HPLC, CE or GC. TD is usually used for thermally stable volatile or semi-volatile compounds, whereas LD is preferred for semi-volatile, non-volatile and thermo-labile compounds.
Besides PDMS, which is the most commonly-used SBSE sorbent, other materials that are either commercially available or developed in the lab can be used. Some of them include:
polyurethane foams
silicone materials
poly(ethylene glycol)-modified silicone
poly(dimethylsiloxane)/polypyrrole
poly(phthalazine ether sulfone ketone)
polyvinyl alcohol
polyacrylate
carbon nanotube-poly(dimethylsiloxane) (CNT-PDMS)
alkyl-diol-silica (ADS) restricted access materials
molecularly imprinted polymers (MIPs)
sorbents obtained with sol–gel techniques
monolithic materials, or
cyclodextrin.
SBSE is a promising sample preparation technique, showing reliability in terms of enrichment capacity, outstanding performance, great sensitivity and selectivity for ultra-trace extraction of non-polar to medium-polar organic compounds from complex matrices with a great variety of applications [
28,
29].
The above-mentioned technique has been already used for the determination of SAs in milk samples.
A method of C
18-SBSE HPLC-MS/MS was proposed for the determination of six SAs (sulfadiazine, sulfamerazine, sulfamethazine, sulfamethizole, sulfamethoxazole and sulfadimethoxine) in milk and milk powder samples by Yu C. and Hu B. Adhesion method was used for the preparation of C
18 silica coated stir bar, using two types of adhesive glue, PDMS sol and epoxy glue. The results showed that the C
18-coated stir bar prepared by PDMS sol as adhesive glue is more robust than the one prepared by epoxy glue when liquid desorption was used, with regards to lifetime and organic solvent compatibility. Several parameters, such as extraction and desorption time, ionic strength, sample pH and stirring speed were investigated in order to achieve the optimized performance. Optimum parameters lead to a sensitive, accurate, rapid, and inexpensive method, which can be used as an alternative for trace SAs analysis in milk and milk powder samples [
17].
Huang X. and co-workers suggested a simple, rapid, and sensitive method for the determination of five SAs in milk. The proposed method was applied by SBSE coupling to HPLC with diode array detection. Stir bar used poly (vinylimidazole-divinylbenzene) monolithic (VIDB) as a polymer-coated material. To achieve optimum performance with the application of SBSE-VIDV, several parameters, including extraction and desorption time, desorption solvent, ionic strength and pH value of sample matrix, were investigated. The preparation of milk samples was simple and the manufactured SBSE-VIDB showed higher selectivity to SAs than commercial SBSE-PDMS [
20].
3.3. QuEChERS Approach
A popular procedure, recently developed, called QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe), has been widely applied. The advantages of QuEChERS include simplicity, with short steps, and effective cleaning for complex samples, by avoiding a great number of steps. The methodology includes two basic steps: (1) extraction based on partitioning via salting-out extraction involving the equilibrium between an aqueous and an organic layer; and (2) dispersive SPE that involves further clean-up using combinations of MgSO
4 and different sorbents, such as C
18 or primary and secondary amine (PSA), to remove interference [
30].
Qin Y. et al. have developed a liquid chromatographic method tandem mass spectrometry for the determination of SAs, Tilmicosin and Avermectins residues in animal matrices including milk and using QuEChERS as the sample preparation procedure. In comparison with traditional techniques such as LLE, this approach is less time-consuming and environmentally friendly, as the use of solvents is limited [
31].
In addition, a quick method was developed for the simultaneous screening and quantification of 90 veterinary drugs in milk (including SAs) by Zhang Y. and co-workers in 2015. The determination was achieved by ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS). A modified QuEChERS method was applied and proved to be fast and easy with good repeatability. The proposed method is accurate and gives reliable results for drug concentrations below or above the MRL [
32].
3.4. Dispersive Liquid–Liquid Microextraction
Assadi et al. was the first to mention DLLME as an alternative LLE technique that uses l μL volume of extraction solvent, along with a few mL of dispersive solvents. In this technique, a cloudy solution is formed when an appropriate mixture of extraction and dispersive solvents is injected into an aqueous sample. The extraction solvent is dispersed into the bulk aqueous solution. Centrifugation is applied prior to the determination of the analytes in the lower phase (in the bottom of a conical tube) by conventional analytical techniques. The main advantages of DLLME are simplicity of operation, quickness, low cost, great recovery, high enrichment factor and very short extraction time (usually a few seconds) [
11].
An ultrasound-assisted ionic liquid/ionic liquid-dispersive liquid–liquid microextraction (UA-IL/IL-DLLME) HPLC method was developed by Gao S. et al. [
33] The method was proposed for the extraction, separation and determination of six SAs in infant formula milk powder samples. Hydrophobic ionic liquids were used for extraction, while hydrophilic ionic liquids were used as dispersive solvents. The formation of cloudy solutions, which was achieved by fine drops of [C
6MIM][PF
6], facilitated the extraction procedure. The above method was applied to the analysis of infant formula milk powder samples and the recoveries of the analytes ranged from 90.4% to 114.8% [
33].
DLLME and QuEChERS were applied for the determination of nine SAs in milk. The quantification was achieved by HPLC with fluorescence detection. This study was proposed by Arroyo-Manzanares N. et al. in 2014. [
21] The DLLME and QuEChERS have been proposed as extraction modes due to the fact that they are environmentally friendly, use a reduced amount of organic solvents and are in agreement with the new trends of GAC. For the optimization, several parameters such as extraction and disperser solvent (for DLLME) and value of pH were investigated. The developed methods were accurate and were successfully applied to the extraction and determination of SAs in milk samples [
21].
3.5. The Molecularly Imprinted Polymers Approach
MIPs play an important role in the field of extraction. There are numerous applications using MIPs as absorbents for a wide variety of substances in complex matrices applying different extraction or microextraction techniques. Among these, there are some applications with regards to the extraction and determination of SAs in milk.
MIPs are artificial materials synthesized by polymerization of functional and cross-linking monomers in the presence of the target molecule (template). According to this procedure, MIPs transfer the molecular imprint of the template and could recognize it, with high selectivity among other molecules which are similar to the template. Due to the fact that they present high selectivity and other advantages such as easy preparation, chemical and thermal stability and low manufacturing cost, MIPs have been suggested and used in many applications [
10].
Su S. and Zhang M. developed a novel method for the determination of sulfamethazine (SMZ) in milk. A new polymer material was synthesized, and the MIP layer was grafted on the silica surface. For its preparation, the authors used sulfamethazine (SMZ) as the template, methacrylic acid (MAA) as the functional monomer and ethylene dimethacrylate (EDMA) as cross-linker. As it is mentioned, during synthesis, the initiator-transfer in combination with the agent-terminator (called iniferter) were immobilized on a silica surface using chemical reagents, which shows good availability. The imprinting polymerization was initialized by the silica-supported iniferter under the UV radiation. Two chromatographic columns were investigated, which included a MIP-silica (3h-MIP-Sil) and its non-imprinted polymer-grafted silica (NIP-silica) as the stationary phases. The results show that the MIP-silica column has better selectivity to the template molecule in comparison with the NIP-silica phase. Furthermore, with the use of this stationary phase, better column efficiency and low backpressure were observed. Finally, the constructed SMZ-MIP-silica was applied for the determination of SMZ in milk samples [
22].
A novel restricted access-molecularly imprinted material (RA-MIP) with selectivity for SAs was synthesized using the initiator-transfer agent-terminator method, a “living”/controlled radical polymerization technique by Xu W. et al. Two layers with different functions on the silica support, grafted, were used for the preparation of the material. To perform a “grafting from” polymerization, iniferter was immobilized on the surface of silica, followed by grafting of the internal sulfamethazine imprinted polymer and the external poly(glycidyl methacrylate) [poly(GMA)]. The hydrophilic structures were formed on the external layer of the material by the hydrolysis of the linear poly(GMA) for protein removal. The RA-MIP-SG was used as a pre-column for the determination of SAs in milk. The manufactured material has the properties of a MIP and a RAM material and can be used in selective extraction and sample clean-up in SAs analysis in milk. Thus, a simple direct-injection HPLC method was established using the RAM-MIP grafted silica for sample online pretreatment [
23].
The above methods promise minimization of the analytical steps with the use of MIP either as a stationary phase or as pre-column material. Thus, the methods are approaching the principles of GAC.
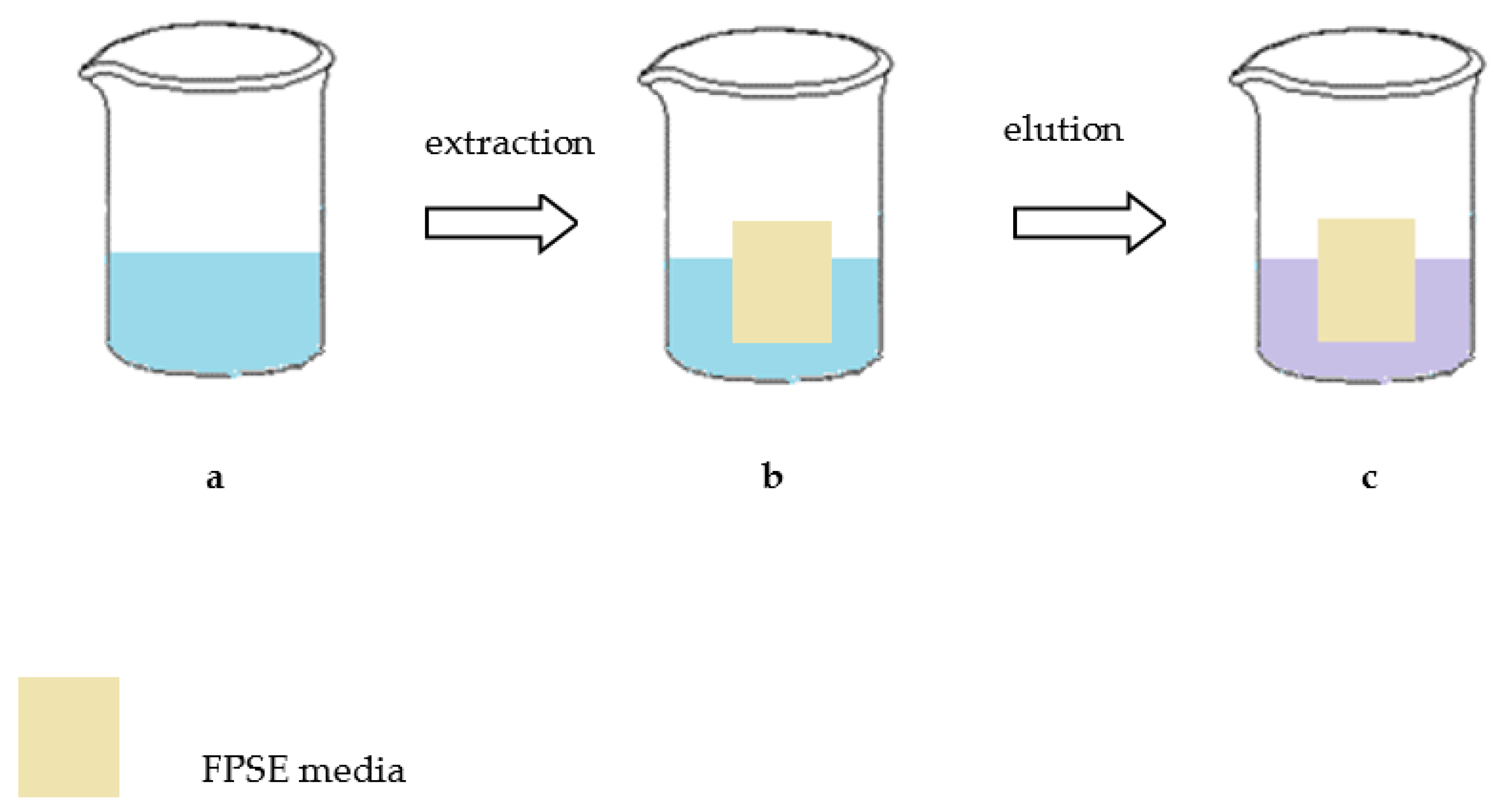
3.6. Fabric Solid Phase Extracton
Fabric solid phase extraction (FPSE) is a novel microextraction technique introduced by Kabir and Furton in 2014. It can be used as a solvent-free or solvent-minimized technique in various applications. FPSE includes the advantages of sol–gel technology as well as the rich surface chemistry of cellulose fabric substrates. The result is a manufactured robust microextraction device with high sample capacity, quick extraction equilibrium, and very high solvent and chemical stability. The ability of FPSE to extract (without any sample preparation) target analytes directly from a raw sample matrix containing particulates, biomasses, and debris is outstanding. In comparison with other applications for the determination of SAs in milk, it is obvious that FPSE is a simple, quick and green sample preparation procedure. In the application of FPSE, many unnecessary steps such as filtration, protein precipitation, solvent evaporation and sample reconstitution are omitted, making total time of sample preparation as short as possible. Also, the consumption of organic solvents is reduced comparatively with the other developed techniques. Summing it up, the above technique covers the requirements of GAC. The main steps of the FPSE approach are presented in
Figure 4 [
24].
FPSE was successfully applied for the extraction of three SAs (sulfamethazine, sulfisoxazole and sulfadimethoxine) in milk by Samanidou V. and co-workers, using a highly polar sol–gel poly(ethylene glycol) (sol–gel PEG) coated FPSE medium, followed by analysis using HPLC with UV detection. The optimization of fabric solid phase extraction conditions, which include the diluents of conditioning, sample loading conditions, elution solvent, elution time and sonication, showed that the sol–gel PEG material yielded the higher recovery of the three examined SAs. The FPSE procedure is applied at four steps. The FPSE medium was immersed in a mixture of 1 mL CH
3OH and 1 mL ACN for 5 min and then rinsed with 2 mL of deionized water. Subsequently, a quantity of 1 g milk spiked with 0.5 mL of standard solution was transferred in a new, clean glass vial in which the FPSE media was added with the magnetic stir bar. Magnetic stirring was performed for 30 min. Finally, the FPSE media was inserted in a clean vial with 250 μL MeOH for 8 min and then in another clean vial with 250 μL ACN for 5 min. The sample was injected to HPLC after filtration. The FPSE media could be reused up to 30 times. In conclusion, the above method is convenient, reliable, and fast and it can be easily applied in any food testing laboratory [
24].
3.7. Miniaturized Graphene-Based Pipette Tip Extraction
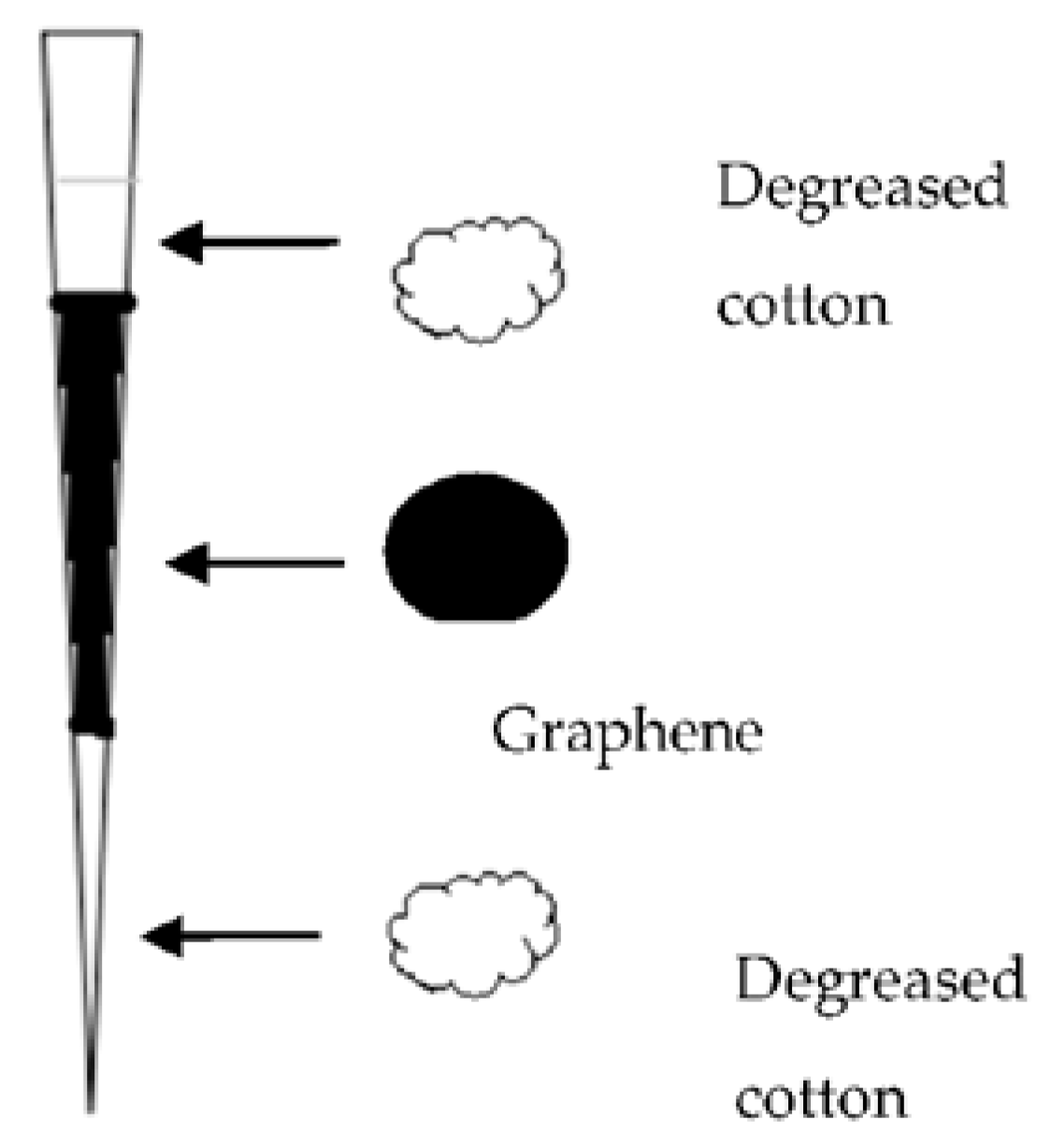
A miniaturized graphene-based pipette tip extraction (M-G-PTE) method coupled with liquid chromatography-ultraviolet detection was developed for the determination of four SAs (sulfadimidine, sulfachloropyridazine, sulfamonomethoxine, and sulfachloropyrazine) in bovine milk by Yan H. et al. [
25] In comparison with other adsorbents such as C
18, HLB, SCX, PCX, and multi-walled carbon nanotubes, the manufactured M-G-PTE device showed better recovery and selectivity for SAs. Several parameters were investigated for the optimization of the pretreatment, such as the amount of grapheme in M-G-PTE cartridge, the type and volume of washing, and elution sorbents. The procedure of the extraction is very interesting. Two dried and clean pipette tips (100 μL and 1.0 mL) were used for assembling the pipette tip cartridge. Then, 3 mg of the graphene adsorbent was packed in the smaller tip using degreased cotton at both ends to stabilize the adsorbent. The configuration of the pipette that was used is presented in
Figure 5. The tip of the larger pipette was cut and was connected with the packed tip. The sorbent was conditioned successively with 1.0 mL methanol and 1.0 mL water. Then, 2.0 mL of the sample was loaded, washed with 1.0 mL water and eluted with 0.5 mL 5% ammonia-methanol. The eluent was evaporated to dryness at 40 °C under vacuum and redissolved in 100 μL of mobile phase prior to HPLC analysis. The proposed method, beyond being simple and economic, also uses reduced amounts of organic solvents [
25].
3.8. Ionic Liquid Based Microextraction
Ionic liquids (ILs) can be used instead of organic solvents as a more green approach. ILs have unique physicochemical properties, such as negligible vapor pressure, miscibility with water and organic solvents, good solubility for organic and inorganic compounds, and high thermal stability. Therefore, they can promise a greener extraction than the commonly used organic solvents such as chlorobenzene, chloroform, and carbon tetrachloride, which were typically highly toxic and not environmentally friendly. The ILs have been already used in DLLME as extraction solvents with good results.
Xu X. et al. [
26] developed an ionic liquid-based microwave-assisted dispersive liquid–liquid microextraction (IL-based MADLLME) followed by HPLC-FD for the determination of six SAs in various liquid samples including milk. The aim of this work was to simplify the analytical step, to reduce the consumption of toxic solvents and improve the sensitivity. Extraction, derivatization and preconcentration were carried out by adding methanol (disperser), fluorescamine solution (derivatization reagent) and ionic liquid (extraction solvent) into the sample. Several experimental parameters, such as the type and volume of extraction solvent, the type and volume of disperser, amount of derivatization reagent, microwave power, microwave irradiation time, pH of sample solution, and ionic strength were investigated and optimized [
26].
3.9. Dispersive Micro-Solid Phase Extraction
Dispersive micro-solid phase extraction is a miniaturized technique which is based on the dispersion of sorbents (micro- or nano-) in the sample solutions. The isolation/extraction step includes centrifugation, filtration or using an external magnetic media (for the removal of magnetic sorbents). The operation of dispersive micro-SPE is similar to the classical SPE approach. The main difference is that a small amount of sorbent (in a range of μg to mg) is added to the sample solution without conditioning. The phenomenon of dispersion minimizes the extraction time, as the analytes provide better and rapid interaction with the sorbent. Several commercial or in-house made types of sorbents have been applied in dispersive extraction, such as functionalized silica, multi-walled carbon nanotubes, graphene, graphene oxide, modified magnetic NPs, polymers, MIPs, silica, graphene, surfactants, carbon nanotubes, ionic liquids and metal/metal oxides [
34].
A metal-organic framework/graphite oxide (MIL-101(Cr)@GO) was synthesized (using the hydrothermal method) and introduced by Jia X. and co-workers in 2017. This novel material was applied as sorbent in dispersive micro-solid phase extraction, for the determination of SAs in milk samples. Several parameters were investigated for the extraction, such as type of sorbents, the effect of pH, the amount of MIL-101(Cr)@GO, ionic strength, adsorption time, desorption solvent and desorption time. A UPLC-MS/MS method was developed and validated for the determination of analytes. The proposed method is characterized by advantages such as easy and quick modification, minimized use of organic solvents and stability of the sorbent [
35].
Finally, another dispersive micro solid-phase extraction approach coupled with liquid chromatography-high resolution mass spectrometry (LC-HRMS) was introduced for the analysis of 24 SAs in milk by Hu S. et al. [
36]. In this study, a commercially available polymer cation exchange PCX powder sorbent was used. The extraction efficiency was evaluated by several parameters, including the pH of sample solution, the amount of PCX, the desorption solvent, and volume. This rapid, selective and environmentally green method was validated under the optimized conditions [
36].