Determination of the Three Main Components of the Grapevine Moth Pest Pheromone in Grape-Related Samples by Headspace-Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. HS-GC-MS Analysis
2.3. Analytical Procedure
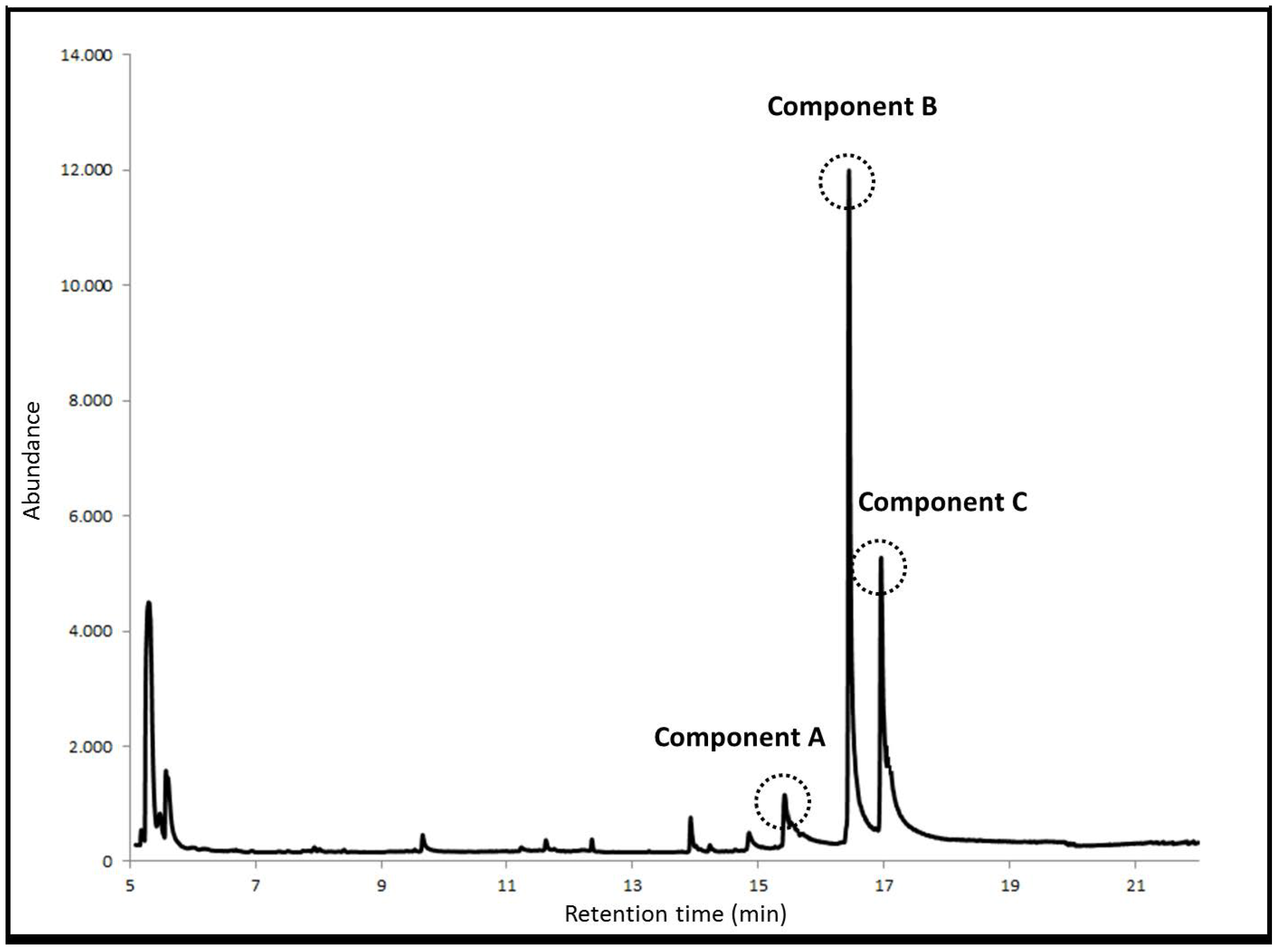
3. Results and Discussion
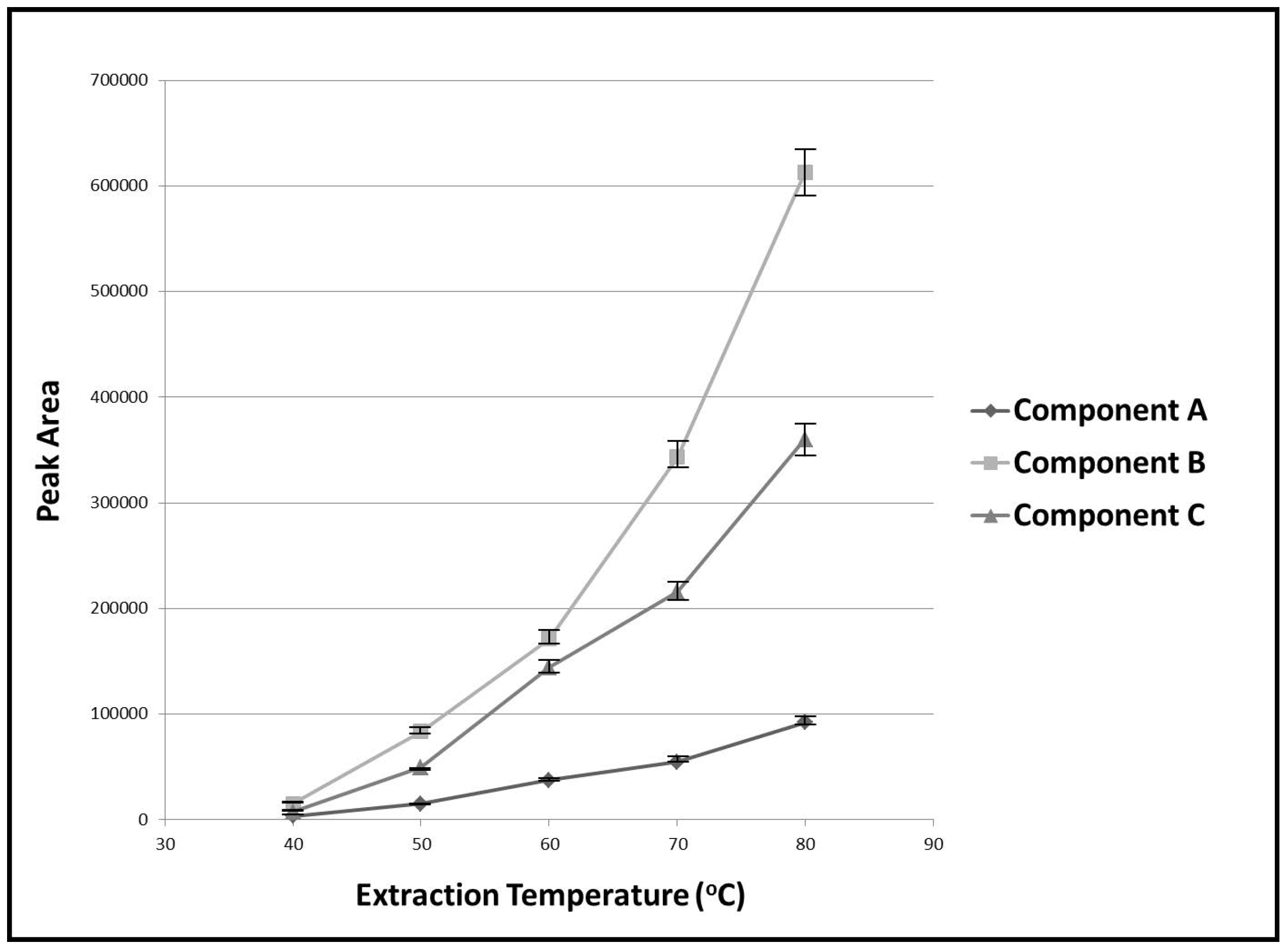
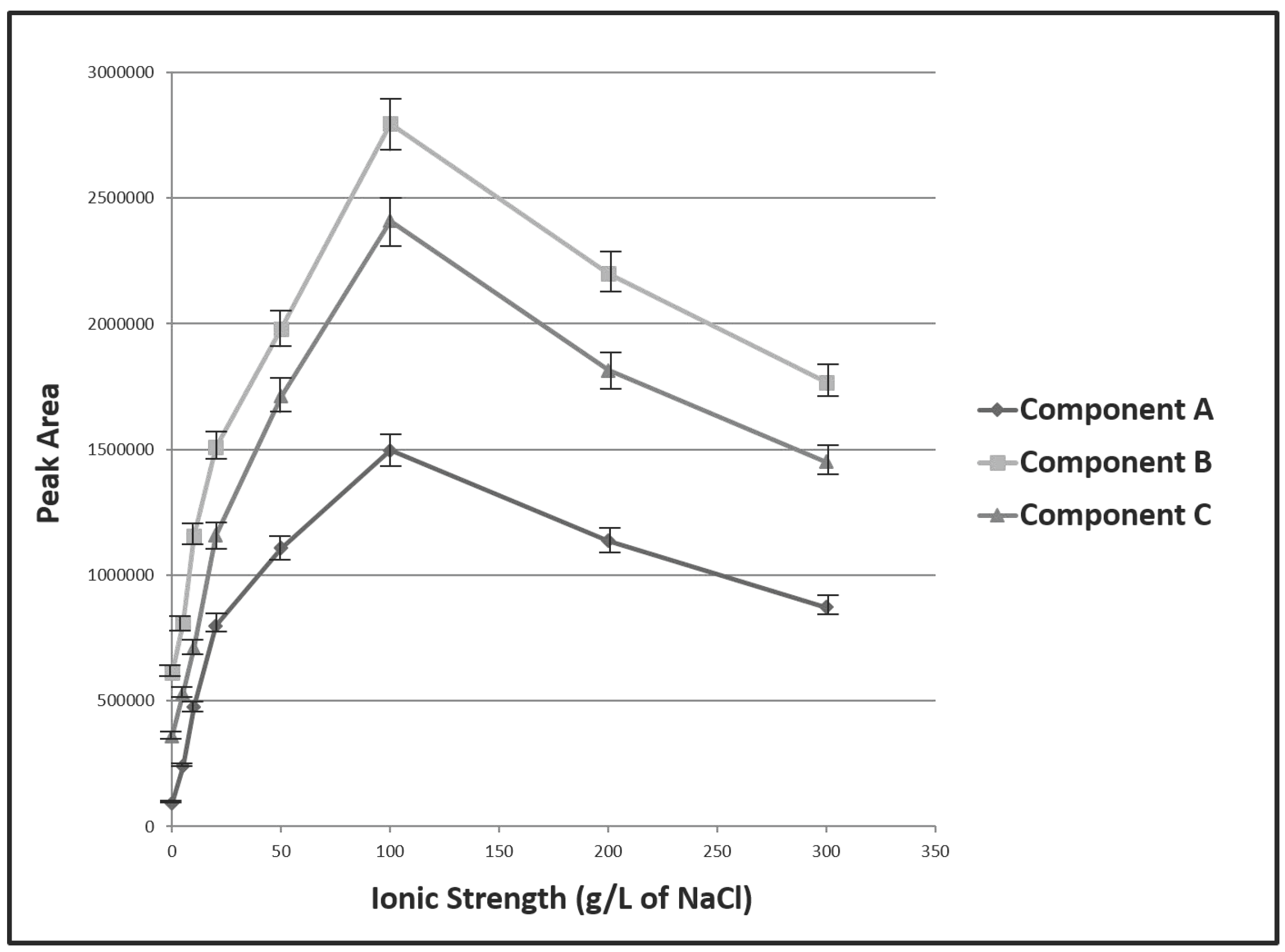
3.1. Optimization of Experimental Variables
3.2. Analytical Figures of Merit
3.3. Analysis of Grape Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Savopoulou-Soultani, M.; Stavridis, D.G.; Tzanakakes, M.E. Development and reproduction on Lobesia botrana on vine and olive inflorescences. Entomol. Hell. 1990, 8, 29–35. [Google Scholar]
- Vacas, S.; Alfaro, C.; Zarzo, M.; Navarro-Llopis, V.; Primo, J. Effect of sex pheromone emission on the attraction of Lobesia botrana. Etomol. Exp. Appl. 2011, 139, 250–257. [Google Scholar] [CrossRef]
- Caffarelli, V.; Vita, G. Heat accumulation for timing grapevine moth control measures. Bull. SROP 1988, 11, 24–26. [Google Scholar]
- Oliva, J.; Navarro, S.; Navarro, G.; Camara, M.A.; Baarba, A. Integrated control of grape berry moth (Lobesia botrana), powdery mildew (Uncinula necátor), downy ildew (Plasmopara vitícola) and grapevine sour rot (Acetobacter spp.). Crop Prot. 1999, 18, 581–587. [Google Scholar] [CrossRef]
- Boselli, M.; Scannavini, M. Lotta alla tignoletta della vite in Emilia-Romagna. Inf. Agrar. 2001, 19, 97–100. [Google Scholar]
- Pimentel, D. Environmental and Economic Cost of the Application of Pesticides Primarily in the United States. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A., Eds.; Springer: Amsterdam, The Netherlands, 2009; pp. 89–111. [Google Scholar]
- McNeil, J.N.; Cotnoir, P.A.; Leroux, T.; Laprade, R.; Schwartz, J.L. A Canadian national survey on the public perception of biological control. Biocontrol 2010, 55, 445–454. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.M.; Rial-Otero, R.; Cancho-Grande, B.; Gonzalez-Barreiro, C.; Simal-Gandara, J. A review on the fate of pesticides during the processes within the food-production chain. Crit. Rev. Food Sci. 2011, 51, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact in pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, W.L.; Kochansky, J.; Cardé, R.T.; Arn, H.; Rauscher, S. Sex attractant of the grape vine moth, Lobesia botrana. Mitt. Schweiz. Entomol. Ges. 1973, 46, 71–73. [Google Scholar]
- Buser, H.R.; Rauscher, S.; Arn, H. Sex pheromone of Lobesia botrana: (E,Z)-7,9-Dodecadienyl acetate in the female grape vine moth. Z. Naturforschung C 1974, 29, 781–783. [Google Scholar]
- Arn, H.; Rauscher, S.; Guerin, P.; Buser, H.R. Sex pheromone blends of three tortricid pests in European vineyards. Agric. Ecosyst. Environ. 1988, 21, 111–117. [Google Scholar] [CrossRef]
- Gordon, D.; Zahavi, T.; Anshelevich, L.; Harel, M.; Ovadia, S.; Dunkelblum, E.; Harari, A.R. Mating disruption of Lobesia botrana (Lepidoptera: Tortricidae): Effect of pheromone formulations and concentration. J. Econ. Entomol. 2005, 98, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Baker, C.F.; Cooney, T. A rapid, sensitive, and selective method for quantitation of lamprey migratory pheromones in river water. J. Chem. Ecol. 2011, 31, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Galvan, T.L.; Kells, S.; Hutchison, W.D. Determination of 3-Alkyl-2-methoxypyrazines in Lady Beetle-Infested Wine by Solid-Phase Microextraction Headspace Sampling. J. Agric. Food Chem. 2008, 56, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Cudjoe, E.; Wiederkehr, T.B.; Brindle, I.D. Headspace gas chromatography-mass spectrometry: A fast approach to the identification and determination of 2-akyl-3-methoxyperazinepheromones in ladybugs. Analyst 2005, 130, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Risticebic, S.; Pawliszyn, J. Simultaneous sampling and analysis of indoor air infested with Cimex lectularius L. (Hemiptera: Cimicidae) by solid phase microextraction, thin film microextraction and needle trap device. Anal. Chim. Acta 2012, 716, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Determination of Tuta absoluta pheromones in water and tomato samples by headspace-gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Integrated sampling and analysis unit for the determination of sexual pheromones in environmental air using fabric phase sorptive extraction and headspace-gas chromatography-mass spectrometry. J. Chromatogr. A 2017, 1488, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Cärdenas, S.; Valcárcel, M.; Lucena, R. Green detection of the olive fruit fly pest by the direct determination of its sexual pheromone. Anal. Methods 2015, 7, 7228–7233. [Google Scholar] [CrossRef]
| Variable | Initial Value | Interval Studied | Optimal Value |
|---|---|---|---|
| Extraction temperature (°C) | 40–80 | 80 | |
| Headspace injected volume (mL) | 1 | 0.5–2.5 | 2.5 |
| Ionic strength (g/Kg of NaCl) | 0 | 0–300 | 100 |
| Sample amount (g) | 2.5 | 0.5–7 | 6 |
| Extraction time (min) | 15 | 2–140 | 30 |
| Analyte | LOD a | LOQ b | Linear Range c | RSD d |
|---|---|---|---|---|
| Component A | 0.42 | 1.39 | LOQ-1000 | 8.5 |
| Component B | 0.06 | 0.21 | LOQ-1000 | 7.2 |
| Component C | 0.17 | 0.55 | LOQ-1000 | 7.9 |
| Analyte | White Wine Grape a (Moscatel) | Red Wine Grape a (Carbenet Sauvignon) | White Must a | Red Must a |
|---|---|---|---|---|
| Component A | 5.2 ± 0.4 | 4.5 ± 0.4 | Not Detected | Not Detected |
| Component B | 64 ± 5 | 82 ± 6 | 94 ± 7 | 122 ± 9 |
| Component C | 21 ± 2 | 29 ± 2 | Not Detected | Not Detected |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcudia-León, M.D.C.; Sánchez-Parra, M.; Lucena, R.; Cárdenas, S. Determination of the Three Main Components of the Grapevine Moth Pest Pheromone in Grape-Related Samples by Headspace-Gas Chromatography-Mass Spectrometry. Separations 2017, 4, 31. https://doi.org/10.3390/separations4040031
Alcudia-León MDC, Sánchez-Parra M, Lucena R, Cárdenas S. Determination of the Three Main Components of the Grapevine Moth Pest Pheromone in Grape-Related Samples by Headspace-Gas Chromatography-Mass Spectrometry. Separations. 2017; 4(4):31. https://doi.org/10.3390/separations4040031
Chicago/Turabian StyleAlcudia-León, María Del Carmen, Mónica Sánchez-Parra, Rafael Lucena, and Soledad Cárdenas. 2017. "Determination of the Three Main Components of the Grapevine Moth Pest Pheromone in Grape-Related Samples by Headspace-Gas Chromatography-Mass Spectrometry" Separations 4, no. 4: 31. https://doi.org/10.3390/separations4040031





