Determination of the Biocide Econea® in Artificial Seawater by Solid Phase Extraction and High Performance Liquid Chromatography Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Sample Preparation: Solid Phase Extraction
2.4. Artificial Seawater Preparation
2.5. Sampling under Controlled Release Conditions
2.6. Synthesis of BCCPCA
2.7. Synthesis of Internal Standard: Methyl Ester of BCCPCA
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Woods Hole Oceanographic Institution United States (1952). “Chapter 2: Ship Resistance” in Marine Fouling and Its Prevention. Massachusetts Navy Department Bureau of Ships (United States Naval Institute). Available online: http://hdl.handle.net/1912/191 (accessed on 1 July 2017).
- Omae, I. Organotin Antifouling Paints and their Alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Lovell, J.B. Insecticidal and Synergistic Miticidal Compositions. U.S. Patent 5,187,184, 16 February 1993. [Google Scholar]
- JANNSEN PMP. Antifouling Downloads. Available online: http://www.janssenpmp.com/antifouling.html (accessed on 17 November 2017).
- Kramer, J.P.; Vos, M. Light-and Bright-Coloured Antifouling Paints. WO Patent 1998012269 A1, 26 March 1998. [Google Scholar]
- Paints and Varnishes—Determination of Release Rate of Biocides from Antifouling Paints—Determination of Econea Release Rate by Quantitation of Its Degradation Product in the Extract; ISO 15181-6; International Standards Organisation: Geneva, Switzerland, 2008.
- Bassarab, P.; Williams, D.; Dean, J.R.; Ludkin, E.; Perry, J.J. Determination of quaternary ammonium compounds in seawater samples by solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.B.; Schönenberger, R.; Barroso, C.M.; Suter, M.J.-F. LC-MS/MS determination of tralopyril in water samples. Chemosphere 2016, 145, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Hellio, C.; Yebra, D.M. Advances in Marine Antifouling Coatings and Technologies; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Standard Practice for the Preparation of Substitute Ocean Water; ASTMD1141; ASTM International: West Conshohocken, PA, USA, 2013.
- Aranaz, I.; Carrasco, S.; Tardajos, M.G.; Elvira, C.; Reinecke, H.; López, D.; Gallardo, A.; Daniel, L. Singular thermosensitivity of polymethyl methacrylate/poly-N-isopropylacrylamide conetworks prepared by a facile synthetic route. Polym. Chem. 2011, 2, 709–713. [Google Scholar] [CrossRef]
- Cao, G.-P.; Zhu, Z.-N.; Zhang, M.-H.; Yuan, W.-K. Kinetics of butylacrylate polymerization in a starved feed reactor. J. Appl. Polym. Sci. 2004, 93, 1519–1525. [Google Scholar] [CrossRef]
- Paints and Varnishes—Determination of Release Rate of Biocides from Antifouling Paints—General Method for Extraction of Biocides; ISO 15181-1; International Standards Organisation: Geneva, Switzerland, 2007.
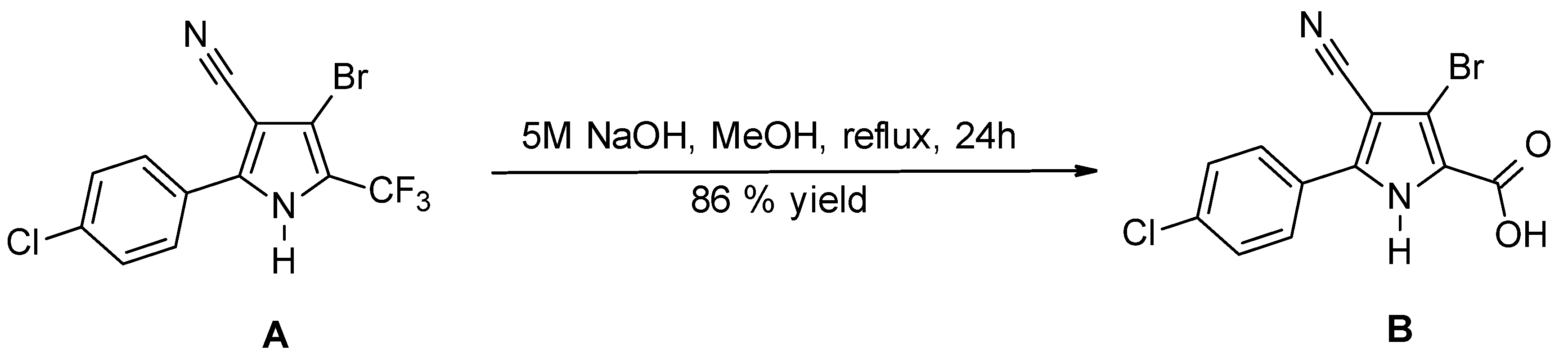


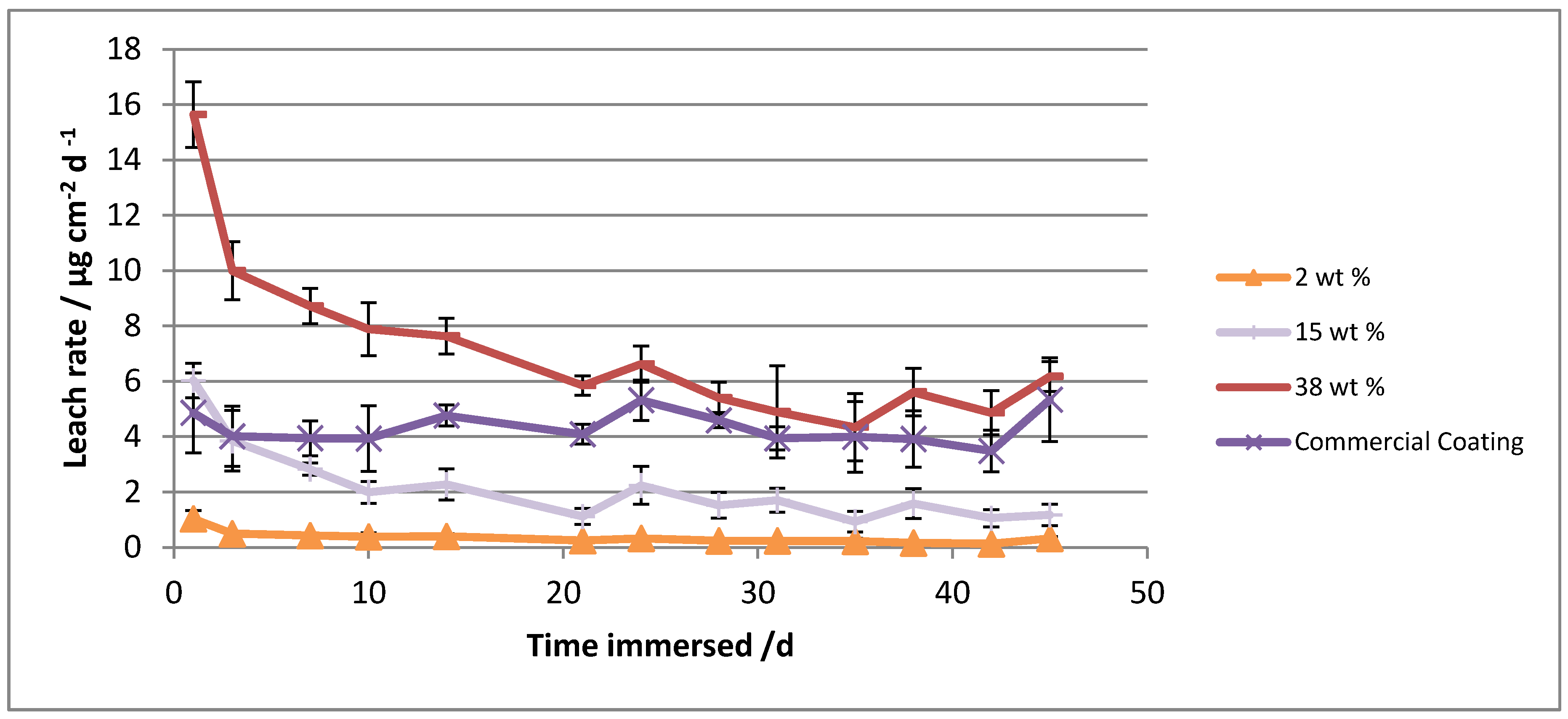
| Coating | Sampling Regime from Panels | Average Leach Rate/μg cm−2 d−1 | |
|---|---|---|---|
| Volume of Sample/mL | Volume of Eluant/mL | ||
| 2 wt % Econea® | 100 | 10 | 0.4 ± 0.1 |
| 15 wt % Econea® | 100 | 10 | 2.4 ± 0.2 |
| 38 wt % Econea® | 10 | 10 | 7.9 ± 0.7 |
| Commercial coating | 10 | 10 | 4.3 ± 0.6 |
| Compound | Calibration Range (μg/L) | Number of Data Points | Equation (y = mx + c) | Correlation coefficient, R2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|---|
| Econea® | 0.5–100 | 6 | y = 0.0193x + 0.0269 | 0.9994 | 0.05 | 0.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Downs, R.A.; Dean, J.R.; Downer, A.; Perry, J.J. Determination of the Biocide Econea® in Artificial Seawater by Solid Phase Extraction and High Performance Liquid Chromatography Mass Spectrometry. Separations 2017, 4, 34. https://doi.org/10.3390/separations4040034
Downs RA, Dean JR, Downer A, Perry JJ. Determination of the Biocide Econea® in Artificial Seawater by Solid Phase Extraction and High Performance Liquid Chromatography Mass Spectrometry. Separations. 2017; 4(4):34. https://doi.org/10.3390/separations4040034
Chicago/Turabian StyleDowns, Robert A., John R. Dean, Adrian Downer, and Justin J. Perry. 2017. "Determination of the Biocide Econea® in Artificial Seawater by Solid Phase Extraction and High Performance Liquid Chromatography Mass Spectrometry" Separations 4, no. 4: 34. https://doi.org/10.3390/separations4040034





