Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation
Abstract
:1. Introduction
2. Sorbent-Based Sorptive Microextraction Techniques
2.1. Fiber-Based Solid-Phase Microextraction, Capillary Solid-Phase Microextraction, and Related Techniques
- -
- non-bonded phases: stable with some water-miscible organic solvents, although some swelling may occur when used with non-polar solvents,
- -
- bonded phases: stable with all organic solvents, except for some non-polar solvents,
- -
- partially cross-linked phases: stable in most water-miscible organic solvents and some polar solvents,
- -
- highly cross-linked phases: similar to the partially cross-linked phases, except that some bonding to the core may occur.
2.2. Stir Bar Sorptive Extraction (SBSE)
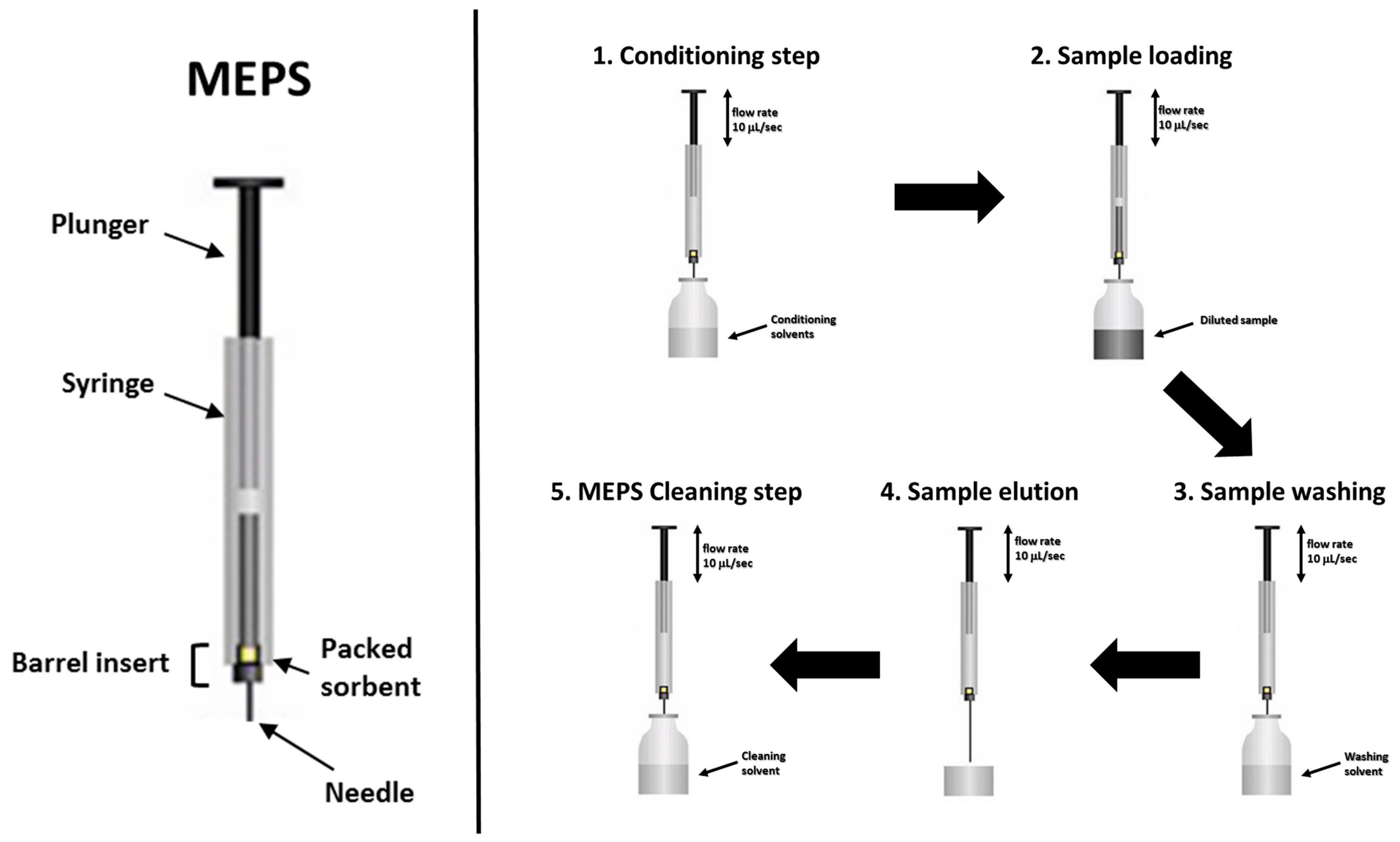
2.3. Micro Extraction by Packed Sorbent Procedures (MEPS)
- -
- Silica-based sorbents SIL (unmodified silica),
- -
- C2(ethyl),
- -
- C8 (octyl),
- -
- C18 (octadecyl);
- -
- Mixed-mode C8 and ion exchange (SCX),
- -
- Mixed-mode M1 (80% C8 and 20% SCX with sulfonic acid bonded silica);
- -
- Polystyrene-divinylbenzene (PS-DVB),
- -
- Porous graphitic carbon,
- -
- Molecular imprinted polymers (MIPs) based on different templates,
- -
- -
- Monoclonal antibodies (mAbs) for immunoaffinity sorbents production.
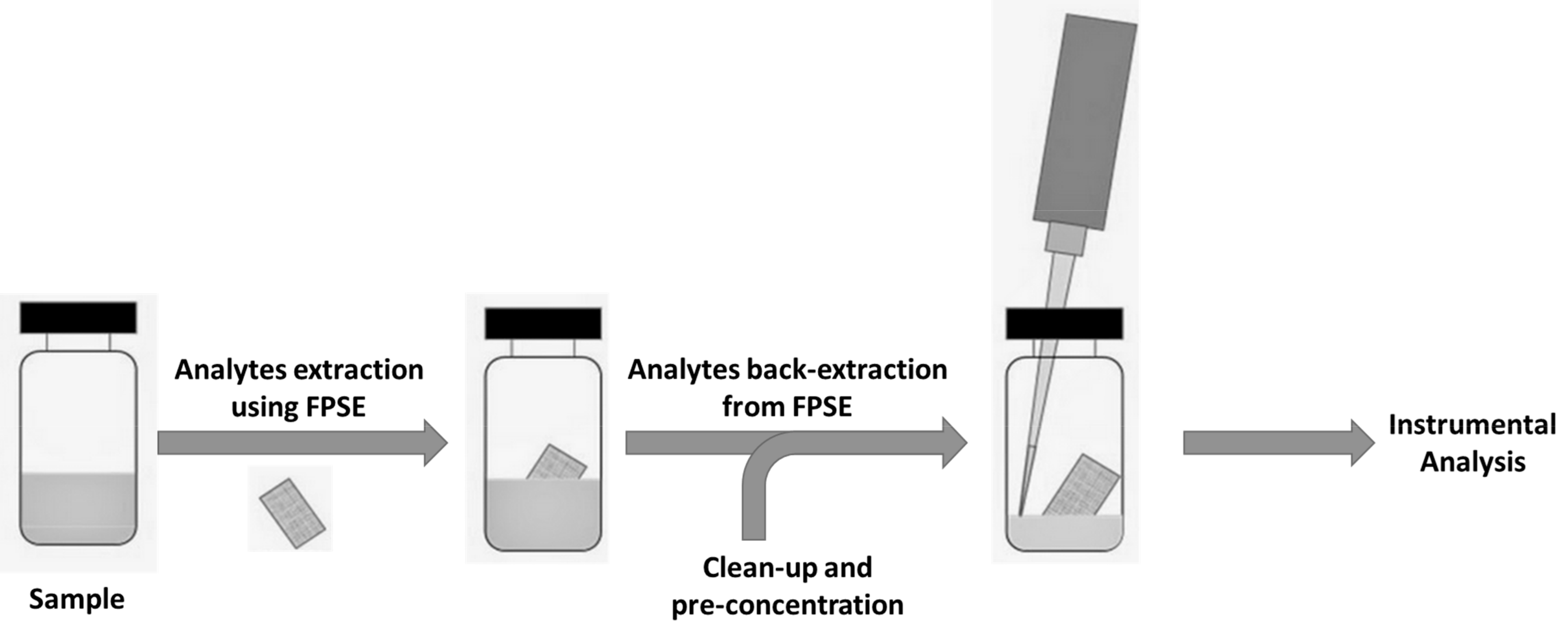
2.4. Fabric Phase Sorptive Extraction Procedures (FPSE)
2.5. Magnetic Nanoparticle Extraction
3. Solvent-Based Microextraction Techniques
3.1. Liquid-Liquid Micro Extraction (LPME)
3.2. Dispersive Liquid-Liquid Microextraction (DLLME)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kabir, A.; Furton, K.J. Sample preparation in Food Analysis: Practices, Problems and Future Outlook. In Analytical Chemistry: Developments, Applications and Challenges in Food Analysis; Locatelli, M., Celia, C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 23–54. ISBN 978-1-53612-267-1. [Google Scholar]
- Locatelli, M.; Cifelli, R.; Vitalei, S.; Santini, P.; De Luca, E.; Bellagamba, G.; Celia, C.; Carradori, S.; Di Marzio, L.; Mollica, A. Method validation and hyphenated techniques: Recent trends and future perspectives. In Analytical Chemistry: Developments, Applications and Challenges in Food Analysis; Locatelli, M., Celia, C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 1–22. ISBN 978-1-53612-267-1. [Google Scholar]
- Locatelli, M.; Sciascia, F.; Cifelli, R.; Malatesta, L.; Bruni, P.; Croce, F. Analytical methods for the endocrine disruptor compounds determination in environmental water samples. J. Chromatogr. A 2016, 1434, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Viñas, P.; Šandrejová, J.; Andruch, V. Ten years of dispersive liquid-liquid microextraction and derived techniques. Appl. Spectrosc. Rev. 2017, 52, 267–415. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H. Recent development and applications of dispersive liquid-liquid microextraction. J. Chromatogr. A 2013, 1295, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, X.; Hu, S.; Bai, X.; Gu, D. Utilization of dispersive liquid-liquid microextraction coupled with HPC-UV as a sensitive and efficient method for the extraction and determination of oleanolic acid and ursolic acid in Chinese medicinal herbs. Am. J. Anal. Chem. 2012, 3, 675–682. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Wang, H.; Han, F.; Jing, S.; Yuan, C.; Guo, A.; Zhang, Y.; Xu, Z. Dispersive liquid-liquid microextraction method for HPLC determination of phenolic compounds in wine. Food Anal. Methods 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Fariña, L.; Boido, E.; Carrau, F.; Dellacassa, E. Determination of volatile phenols in red wines by dispersive liquid-liquid microextraction and gas chromatography-mass spectrometry detection. J. Chromatogr. A 2007, 1157, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of deep eutectic solvents in analytical chemistry. A review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Tang, B.; Bi, W.; Tian, M.; Row, K.H. Application of ionic liquid for extraction and separation of bioactive compounds from plants. J. Chromatogr. B 2012, 904, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Khezeli, T.; Daneshfar, A.; Sahraei, R. A green ultrasonic-assisted liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 2016, 150, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Rodrigues, M.; Fortuna, A.; Falcão, A.; Queiroz, J. A critical review of microextraction by packed sorbent as a sample preparation approach in drug bioanalysis. Bioanalysis 2013, 5, 1409–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Yang, L.; Zhang, W.; Lin, J.; Li, C. Determination of eight quinolones in milk using immunoaffinity microextraction in a packed syringe and liquid chromatography with fluorescence detection. J. Chromatogr. B 2017, 1064, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Oliveira e Silva, H.; de Pinho, P.G.; Machado, B.P.; Hogg, T.; Marques, J.; Câmara, J.S.; Albuquerque, F.; Silva Ferreira, A.C. Impact of forced-aging process on madeira wine flavor. J. Agric. Food Chem. 2008, 56, 11989–11996. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Caldeira, M.; Rodrigues, F.; Camara, J.S. Volatile flavour constituent patterns of terras madeirenses red wines extracted by dynamic headspace solid-phase microextraction. J. Sep. Sci. 2008, 31, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.; Marques, J.; Alves, A.; Ferreira, A.S. Heterocyclic acetals in madeira wines. Anal. Bioanal. Chem. 2003, 375, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Cavaco, C.; Perestrelo, R.; Pereira, J.; Câmara, J.S. Microextraction by Packed Sorbent (MEPS) and Solid-Phase Microextraction (SPME) as Sample Preparation Procedures for the Metabolomic Profiling of Urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Abdulra’uf, L.B.; Hammed, W.A.; Tan, G.H. SPME Fibers for the Analysis of Pesticide Residues in Fruits and Vegetables: A Review. Crit. Rev. Anal. Chem. 2012, 42, 152–161. [Google Scholar] [CrossRef]
- Hou, X.D.; Wang, L.C.; Guo, Y. Recent Developments in Solid-phase Microextraction Coatings for Environmental and Biological Analysis. Chem. Lett. 2017, 46, 1444–1455. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Zhou, L.D.; Chen, H.; Wang, C.Z.; Xia, Z.N.; Yuan, C.S. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TrAC 2016, 80, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Chong, S.L.; Malik, A. Sol-gel column technology for single-step deactivation, coating, and stationary-phase immobilization in high-resolution capillary gas chromatography. Anal. Chem. 1997, 69, 4566–4576. [Google Scholar] [CrossRef]
- Amiri, A. Solid-phase microextraction-based sol–gel technique. TrAC 2016, 75, 57–74. [Google Scholar] [CrossRef]
- Bagheri, H.; Piri-Moghadam, H.; Naderi, M. Towards greater mechanical, thermal and chemical stability in solid-phase microextraction. TrAC 2012, 34, 126–139. [Google Scholar] [CrossRef]
- Dietz, C.; Sanz, J.; Camara, C. Recent developments in solid-phase microextraction coatings and related techniques. J. Chromatogr. A 2006, 1103, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol-gel microextraction phases for solvent-free sample preparation in analytical chemistry. TrAC 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Malik, A. Advances in sol-gel based columns for capillary electrochromatography: Sol-gel open-tubular columns. Electrophoresis 2002, 23, 3973–3992. [Google Scholar] [CrossRef] [PubMed]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Gilart, N.; Marce, R.M.; Borrull, F.; Fontanals, N. New coatings for stir-bar sorptive extraction of polar emerging organic contaminants. TrAC 2014, 54, 11–23. [Google Scholar] [CrossRef]
- Cardenas, S.; Lucena, R. Recent Advances in Extraction and Stirring Integrated Techniques. Separations 2017, 4, 6. [Google Scholar] [CrossRef]
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2014, 7, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, B.B.; Hu, B. Recent developments in stir bar sorptive extraction. Anal. Bioanal. Chem. 2014, 406, 2001–2026. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernandez, L.A.; Etxebarria, N.; Zuloaga, O. Stir-bar sorptive extraction: A view on method optimisation, novel applications, limitations and potential solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef] [PubMed]
- Amlashi, N.E.; Hadjmohammadi, M.R. Sol-gel coating of poly(ethylene glycol)-grafted multiwalled carbon nanotubes for stir bar sorptive extraction and its application to the analysis of polycyclic aromatic hydrocarbons in water. J. Sep. Sci. 2016, 39, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Mao, X.; He, M.; Chen, B.; Hu, B. Development of novel sol-gel coatings by chemically bonded ionic liquids for stir bar sorptive extraction-application for the determination of NSAIDS in real samples. Anal. Bioanal. Chem. 2014, 406, 7261–7273. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Mao, X.J.; He, M.; Chen, B.B.; Hu, B. Stir bar sorptive extraction combined with high performance liquid chromatography-ultraviolet/inductively coupled plasma mass spectrometry for analysis of thyroxine in urine samples. J. Chromatogr. A 2013, 1318, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Duy, S.V.; Fayad, P.B.; Barbeau, B.; Prevost, M.; Sauve, S. Using a novel sol-gel stir bar sorptive extraction method for the analysis of steroid hormones in water by laser diode thermal desorption/atmospheric chemical ionization tandem mass spectrometry. Talanta 2012, 101, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.M.; Wang, H.M.; Guan, Y.F. Preparation of stir bars for sorptive extraction using sol-gel technology. J. Chromatogr. A 2004, 1045, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Said, R.; Abdel-Rehim, M. Sorbent, device, matrix and application in microextraction by packed sorbent (MEPS): A review. J. Chromatogr. B 2017, 1043, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Iskierko, Z.; Sharma, P.S.; Prochowicz, D.; Fronc, K.; D’Souza, F.; Toczydłowska, D.; Stefaniak, F.; Noworyta, K. Molecularly Imprinted Polymer (MIP) Film with Improved Surface Area Developed by Using Metal-Organic Framework (MOF) for Sensitive Lipocalin (NGAL) Determination. ACS Appl. Mater. Interfaces 2016, 8, 19860–19865. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Fang, G.; Wang, S. A novel core-shell molecularly imprinted polymer based on metal-organic frameworks as a matrix. Chem. Commun. 2011, 47, 10118–10120. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Ribeiro, A.R.; Maia, A.S.; Gonçalves, V.M.; Tiritan, M.E. New trends in sample preparation techniques for environmental analysis. Crit. Rev. Anal. Chem. 2014, 44, 142–185. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Ahmed, H.; Wahbi, A.-A.; Amini, A.; Colmsjö, A.; Abdel-Rehim, M. Determination of metoprolol enantiomers in human plasma and saliva samples utilizing microextraction by packed sorbent and liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2016, 30, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ferrone, V.; Cifelli, R.; Barbacane, R.C.; Carlucci, G. MicroExtraction by Packed Sorbent and HPLC determination of seven non-steroidal anti-inflammatory drugs in human plasma and urine. J. Chromatogr. A 2014, 1367, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Ciavarella, M.T.; Paolino, D.; Celia, C.; Fiscarelli, E.; Ricciotti, G.; Pompilio, A.; Di Bonaventura, G.; Grande, R.; Zengin, G.; et al. Determination of Ciprofloxacin and Levofloxacin in Human Sputum Collected from Cystic Fibrosis Patients using Microextraction by Packed Sorbent-High Performance Liquid Chromatography PhotoDiode Array Detector. J. Chromatogr. A 2015, 1419, 58–66. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, V.; Tessari, F.; Bellagamba, G.; De Luca, E.; Cifelli, R.; Celia, C.; Primavera, R.; Di Francesco, M.; Paolino, D.; Di Marzio, L.; et al. MicroExtraction by Packed Sorbent and HPLC-PDA quantification of multiple anti-inflammatory drugs and fluoroquinolones in human plasma and urine. J. Enzyme Inhib. Med. Chem. 2016, 31, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Campestre, C.; Locatelli, M.; Guglielmi, P.; De Luca, E.; Bellagamba, G.; Menta, S.; Zengin, G.; Celia, C.; Di Marzio, L.; Carradori, S. Analysis of imidazoles and triazoles in biological samples after MicroExtraction by Packed Sorbent. J. Enzyme Inhib. Med. Chem. 2017, 32, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Fernández, P.; Regenjo, M.; Fernández, A.M.; Carro, A.M.; Lorenzo, R.A. A fast bioanalytical method based on microextraction by packed sorbent and UPLC-MS/MS for determining new psychoactive substances in oral fluid. Talanta 2017, 174, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, E.; Bahrami, A.; Afkhami, A.; Shahna, F.G. Determination of urinary trans,trans-muconic acid using molecularly imprinted polymer in microextraction by packed sorbent followed by liquid chromatography with ultraviolet detection. J. Chromatogr. B 2017, 1061–1062, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.N.; Santos-Neto, A.J.; Lancas, F.M. Development and optimization of a fast method for the determination of statins in human plasma using microextraction by packed sorbent (MEPS) followed by ultra high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Anal. Methods 2017, 9, 3039–3048. [Google Scholar] [CrossRef]
- Fernández, P.; González, M.; Regenjo, M.; Ares, A.M.; Fernández, A.M.; Lorenzo, R.A.; Carro, A.M. Analysis of drugs of abuse in human plasma using microextraction by packed sorbents and ultra-high-performance liquid chromatography. J. Chromatogr. A 2017, 1485, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Gonçalves, A.; Margalho, C.; Barroso, M.; Gallardo, E. Rapid analysis of cocaine and metabolites in urine using microextraction in packed sorbent and GC/MS. Anal. Bioanal. Chem. 2017, 409, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurementusing a MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moral, M.P.; Tena, M.T. Use of microextraction bypacked sorbents following selective pressurised liquid extraction for thedetermination of brominated diphenyl ethers in sewage sludge by gaschromatography-mass spectrometry. J. Chromatogr. A 2014, 1364, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.M.G.; Pinto, C.G.; Pavón, J.L.P.; Cordero, B.M. In situ derivatization combined to automatedmicroextraction by packed sorbents for the determination of chlorophenolsin soil samples by gas chromatography mass spectrometry. J. Chromatogr. A 2014, 1359, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Salami, F.H.; Queiroz, M.E.C. Microextraction inpacked sorbent for analysis of sulfonamides in poultry litter wastewatersamples by liquid chromatography and spectrophotometric detection. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2377–2388. [Google Scholar] [CrossRef]
- Amiri, A.; Ghaemi, F. Microextraction in packed syringe by using a three-dimensional carbon nanotube/carbon nanofiber-graphene nanostructure coupled to dispersive liquid-liquid microextraction for the determination of phthalate esters in water samples. Microchim. Acta 2017, 184, 3851–3858. [Google Scholar] [CrossRef]
- Fumes, B.H.; Lanças, F.M. Use of graphene supported on aminopropyl silica for microextraction of parabens from water samples. J. Chromatogr. A 2017, 1487, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent US 20140274660 A1, 18 September 2014. [Google Scholar]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A Fabric Phase Sorptive Extraction-High Performance Liquid Chromatography-Photo Diode Array Detection Method for the Determination of Twelve Azole Antimicrobial Drug Residues in Human Plasma and Urine. J. Chromatogr. B 2017, 1040, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; D’Ovidio, C.; Grossi, R.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for triple therapy in the treatment of inflammatory bowel diseases. Anal. Chim. Acta 2017. submitted. [Google Scholar]
- Locatelli, M.; Kabir, A.; Tinari, N.; Macerola, D.; Tartaglia, A.; Furton, K.G. A Fabric Phase Sorptive Extraction-High Performance Liquid Chromatography-Photo Diode Array Detection Method for the Determination of three antitumoral Drugs. Sci. Rep. 2017. submitted. [Google Scholar]
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2016, 30, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Ciofi, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Del Bubba, M.; Kabir, A.; Furton, K.G. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1437, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Galanopoulos, L.-D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Úbeda, S.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction as a reliable tool for rapid screening and detection of freshness markers in oranges. J. Chromatogr. A 2017, 1500, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Filippou, O.; Marinou, E.; Kabir, A.; Furton, K.G. Sol-gel-graphene-based fabric-phase sorptive extraction for cow and human breast milk sample cleanup for screening bisphenol A and residual dental restorative material before analysis by HPLC with diode array detection. J. Sep. Sci. 2017, 40, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- García-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marcé, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Heena; Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Determination of cobalt(II), nickel(II) and palladium(II) Ions via fabric phase sorptive extraction in combination with high-performance liquid chromatography-UV detection. Sep. Sci. Technol. 2017, 52, 81–90. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Integrated sampling and analysis unit for the determination of sexual pheromones in environmental air using fabric phase sorptive extraction and headspace-gas chromatography–mass spectrometry. J. Chromatogr. A 2017, 1488, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, H.I. Applications of magnetic nanoparticles for the selective extraction of trace species from a complex matrix. In Analytical Chemistry: Developments, Applications and Challenges in Food Analysis; Locatelli, M., Celia, C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2017; pp. 55–76. ISBN 978-1-53612-267-1. [Google Scholar]
- Hernández-Hernández, A.A.; Álvarez-Romero, G.A.; Contreras-López, E.; Aguilar-Arteaga, K.; Castañeda-Ovando, A. Food Analysis by Microextraction Methods Based on the Use of Magnetic Nanoparticles as Supports: Recent Advances. Food Anal. Methods 2017, 10, 2974–2993. [Google Scholar] [CrossRef]
- Vasconcelos, I.; Fernandes, C. Magnetic solid phase extraction for determination of drugs in biological matrices. TrAC 2017, 89, 41–52. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. TrAC 2017, 90, 114–134. [Google Scholar] [CrossRef]
- Ríos, Á.; Zougagh, M. Recent advances in magnetic nanomaterials for improving analytical processes. TrAC 2017, 84, 72–83. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Owczarek, K.; Namieśnik, J. Modern solutions in the field of microextraction using liquid as a medium of extraction. TrAC 2016, 85, 46–64. [Google Scholar] [CrossRef]
- Sharifi, V.; Abbasi, A.; Nosrati, A. Application of hollow fiber liquid phase microextraction and dispersive liquid-liquid microextraction techniques in analytical toxicology. J. Food Drug Anal. 2016, 24, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Soylak, M. Latest trends, green aspects, and innovations in liquid-phase-based microextraction techniques: A review. Turkish J. Chem. 2016, 40, 868–893. [Google Scholar] [CrossRef]
- Diuzheva, A.; Carradori, S.; Andruch, V.; Locatelli, M.; De Luca, E.; Tiecco, M.; Germani, R.; Menghini, L.; Nocentini, A.; Gratteri, P.; et al. Use of innovative (micro)extraction techniques to characterize harpagophytum procumbens root and its commercial food supplements. Phytochem. Anal. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Hu, B. Comparison of hollow fiber liquid phase microextraction and dispersive liquid-liquid microextraction for the determination of organosulfur pesticides in environmental and beverage samples by gas chromatography with flame photometric detection. J. Chromatogr. A 2008, 1193, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M. Recent advances in microextraction by packed sorbent for bioanalysis. J. Chromatogr. A 2010, 1217, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M. New trend in sample preparation: On-line microextraction in packed syringe for liquid and gas chromatography applications: I. Determination of local anaesthetics in human plasma samples using gas chromatography-mass spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef]
| Field | Analyte | MEPS | Matrix | Sample Volume | LOQ (LOD) | Reference |
|---|---|---|---|---|---|---|
| Biological | NSAIDs | C18 | Plasma Urine | 100 μL | 0.10 μg/mL (0.03 μg/mL) | [44] |
| Fluoroquinolones | C18 | Sputum | 200 μL | 0.05 μg/mL (0.017 μg/mL) | [45] | |
| NSAIDs and Fluoroquinolones | C18 | Plasma Urine | 200 μL | 0.10 μg/mL (0.03 μg/mL) | [46] | |
| Imidazoles and Triazoles | C18 | Plasma Urine | 200 μL | 0.02 μg/mL (0.007 μg/mL) | [47] | |
| New psychoactive substances | mixed-mode C8/SCX | Oral fluid | 300 μL | 0.5 ng/mL (n.r.) | [48] | |
| Trans,trans-muconic acid | MIP-MEPS | Urine | 100 μL | 0.05 μg/mL (0.015 μg/mL) | [49] | |
| Statins | C18 | Plasma | 100 μL | 10–20 ng/mL (n.r.) | [50] | |
| Drugs of abuse | C8/SCX | Plasma | 300 μL | 0.01 μg/mL (0.005 μg/mL) | [51] | |
| Cocaine and metabolites | Mixed mode M1 | Urine | 200 μL | 25 ng/mL (n.r.) | [52] | |
| Food and Food Supplements | Melatonin and other antioxidants | C8 | Foodstuffs | 100 μL | 0.05 ng/mL (0.02 ng/mL) | [53] |
| Environmental | Brominated diphenyl ethers | C18 | Sewage sludge | 15 mL reduced to 1 mL | n.r. (3 pg/mL) | [54] |
| Chlorophenols | C18 | Soil samples | 1 mL | 0.353 μg/kg (0.118 μg/kg) | [55] | |
| Sulfonamides | C8 | Wastewater | n.r. | 5 ng/mL (n.r.) | [56] | |
| Phtalate esters | graphene and CNT/CNF–G nanostructures | Water | 10 mL reduced to dry | 0.02 ng/mL (0.004 ng/mL) | [57] | |
| Parabens | graphene supported on aminopropyl silica | Water | 1 mL | 0.2 μg/mL (n.r.) | [58] |
| Field | Analyte | FPSE | Matrix | Sample Volume | LOQ (LOD) | Reference |
|---|---|---|---|---|---|---|
| Biological | Imidazoles and Triazoles | sol-gel Carbowax® 20 M | Plasma Urine | 500 μL | 0.10 μg/mL (0.03 μg/mL) | [61] |
| Ciprofloxacin Sulfasalazine Cortisone | sol-gel Carbowax® 20 M | Whole blood Plasma Urine | 100 μL 500 μL 500 μL | 0.05 μg/mL (0.015 μg/mL) 0.25 μg/mL (0.10 μg/mL) 0.10 μg/mL (0.03 μg/mL) | [62] | |
| Anastrozole Letrozole Exemestane | sol-gel PEG-PPG-PEG | Whole blood Plasma Urine | 200 μL 500 μL 1 mL | 0.1 μg/mL (0.03 μg/mL) 0.025 μg/mL (0.008 μg/mL) 0.025 μg/mL (0.008 μg/mL) | [63] | |
| Benzodiazepines | sol-gel PEG | Blood serum | 50 μL | 0.03 μg/mL (0.01 μg/mL) | [64] | |
| Selected estrogens | sol-gel PTHF | Urine | 10 mL | 0.066 ng/mL (0.020 ng/mL) | [65] | |
| Androgens and progestogens | sol-gel PTHF | Urine | 2 mL | 29.7 ng/L (8.9 ng/L) | [66] | |
| Food and Food Supplements | Non-volatile plastic additives | sol-gel PDMS | Aqueous food simulants | 10 mL | 3 ng/g (1 ng/g) | [67] |
| Amphenicols | sol-gel PEG | Milk | 0.5 g | 20 μg/kg | [68] | |
| Sulfonamides residues | sol–gel short-chain PEG | Milk | 1 g | 30 μg/kg (n.r.) | [69] | |
| Volatile compounds | sol-gel Carbowax® 20 M | Orange | 75 mL | n.r. | [70] | |
| Penicillin antibiotics | sol-gel PEG | Milk | 0.5 g | 10 μg/kg (3 μg/kg) | [71] | |
| Bisphenol A and residual dental restorative material | sol–gel graphene | Cow and human breast milk | 0.5 g | 50 μg/kg (16.7 μg/kg) | [72] | |
| Environmental | Pharmaceuticals and personal care products | sol-gel Carbowax® 20 M | Water | 50 mL | 20 ng/mL (2 ng/mL) | [73] |
| Selected estrogens | sol-gel PTHF | Water | n.r. | 0.066 ng/mL (0.020 ng/mL) | [65] | |
| Alkyl phenols | sol-gel PTHF | Water Soil | n.r. 1 g | n.r. (0.161 ng/mL) n.r. (1 ng/g) | [74] | |
| NSAIDs | sol-gel PTHF | Water | 30 mL | 3 ng/L (0.8 ng/L) | [75] | |
| Triazine herbicides | sol-gel PTHF | Water | 100 mL | 0.26 μg/L | [76] | |
| Benzotriazole UV stabilizers | sol–gel PDMDPS | Sewage | 10 mL | 24.5 ng/L (7.34 ng/L) | [77,78] | |
| Pharmaceuticals and personal care products | sol-gel Carbowax® 20 M | Water | 10 mL | 0.1 μg/L (0.01 μg/L) | [79] | |
| Cadmium | sol-gel PDMDPS | Water | 13.5 mL | 1.2 μg/L (0.4 μg/L) | [80] | |
| Androgens and progestogens | sol-gel PTHF | Waters | 2 L | 5.7 ng/L (1.7 ng/L) | [66] | |
| Co(II), Ni(II) and Pd(II) | sol-gel PTHF | Water | 10 mL | 1 ng/mL (n.r.) | [81] | |
| Pheromones | sol-gel PDMDPS | Air | - | 2.6 μg (0.8 μg) | [82] |
| Feature | MEPS | FPSE | DLLME | SPE | SPME |
|---|---|---|---|---|---|
| Phase amount | 0.5–4 mg | n.a. | n.a. | 50–10,000 mg | 150 mm thickness |
| Principle-separation | no emulsion | no emulsion | emulsion | no emulsion | no emulsion |
| Procedure time | 1–2 min | 5–30 min | 5–15 min | 10–15 min | 10–40 min |
| Re-use | 40–100 times | 30–50 times | Single use | Single use | 50–100 times |
| Recovery | + | + | + | + | − |
| Carryover | − | − | n.a. | + | + |
| Solvent consumption | − | +/− | + | + | solventless |
| Sensitivity | − | + | + | + | − |
| Easy-to-use | − | + | − | + | − |
| Sample quantity | − | +/− | +/− | + | + |
| Easily adaptable to | GC or HPLC | GC or HPLC | GC or HPLC | GC or HPLC | GC |
| Automatable | + | − | − | + | + |
| Target analytes | polar and charged analytes may be extracted | polar and charged analytes may be extracted | polar analytes difficult to extract | polar and charged analytes may be extracted | polar and charged analytes may be extracted |
| Cost | − | n.a. | + | + | + |
| Commercially available | + | − | + | + | + |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. https://doi.org/10.3390/separations4040036
Kabir A, Locatelli M, Ulusoy HI. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations. 2017; 4(4):36. https://doi.org/10.3390/separations4040036
Chicago/Turabian StyleKabir, Abuzar, Marcello Locatelli, and Halil Ibrahim Ulusoy. 2017. "Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation" Separations 4, no. 4: 36. https://doi.org/10.3390/separations4040036







