Supercritical CO2 Extracts and Volatile Oil of Basil (Ocimum basilicum L.) Comparison with Conventional Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Supercritical Fluid Extraction and Conventional Extractions
2.3. Gas Chromatography and Gas Chromatography-Mass Spectrometry Analysis
2.4. Determination of Total Phenolic and Flavonoid Content
2.5. Antioxidant Activity Determinations
2.6. Acetylcholinesterase and Tyrosinase Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
3.2. Quantitative Analysis of the Essential Oil and the Volatile Oil
3.3. Caracterization of Plant Extract
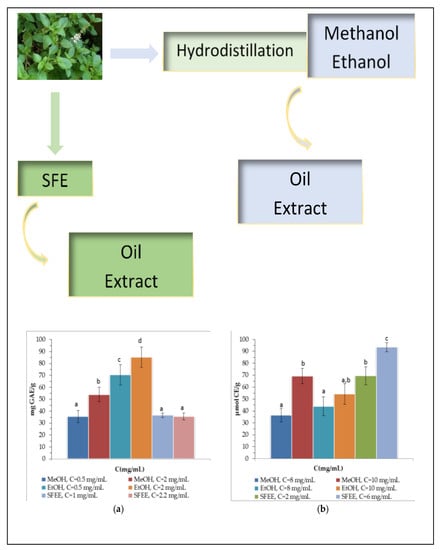
3.3.1. Total Phenolic and Flavonoid Content and Antioxidant Activity
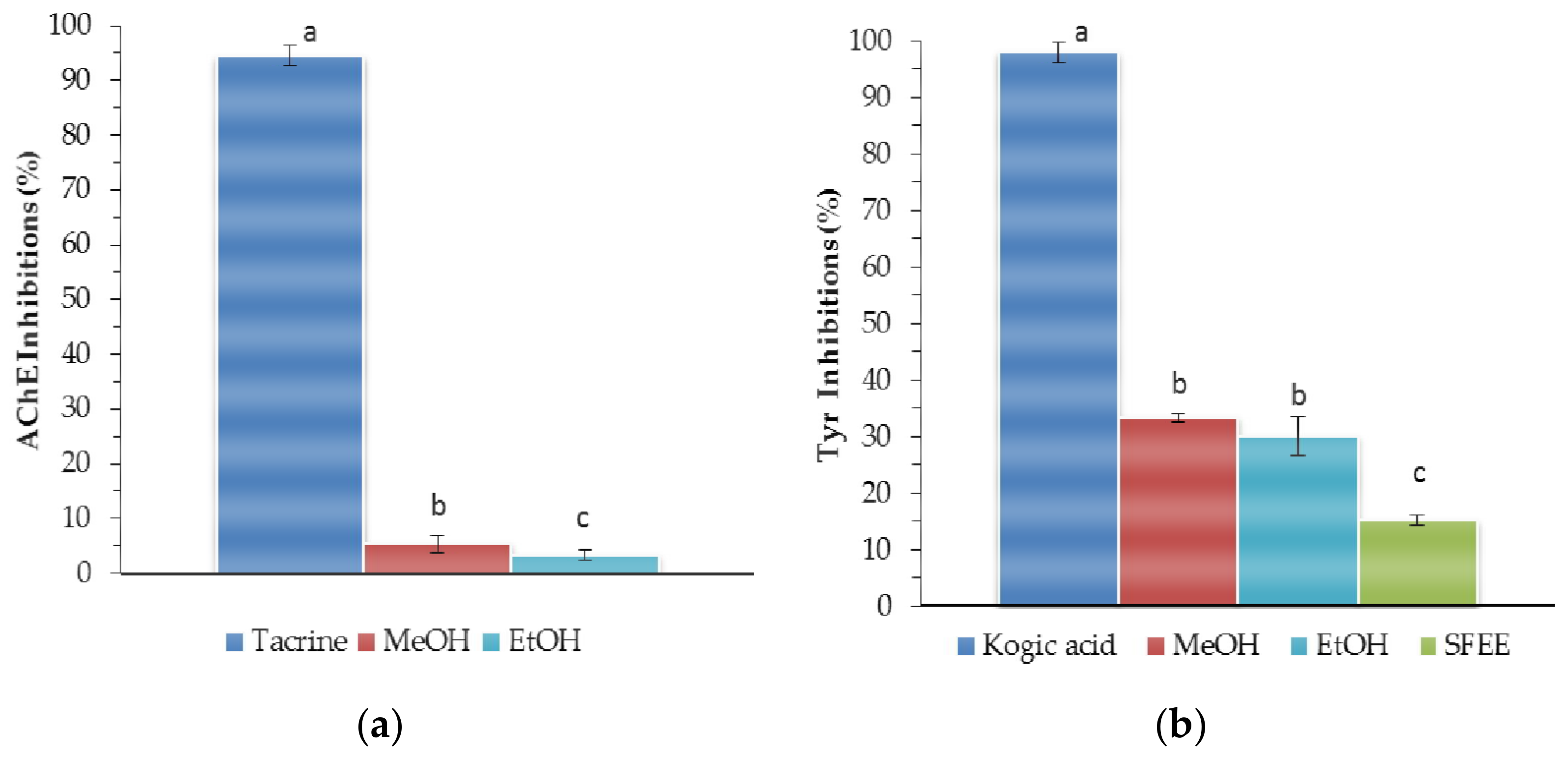
3.3.2. Acetylcholinesterase and Tyrosinase Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Avanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) Found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Ismail, M. Central properties and chemical composition of Ocimum basilicum essential oil. Pharm. Biol. 2006, 44, 619–626. [Google Scholar] [CrossRef]
- Ijaz, A.; Anwar, F.; Tufail, S.; Sherazi, H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and oil composition of 38 basil (Ocimum basilicum L.) accessions grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Dev, N.; Das, A.K.; Hossain, M.A.; Rahman, S.M.M. Chemical compositions of different extracts of Ocimum basilicum leaves. J. Sci. Res. 2011, 3, 197–206. [Google Scholar] [CrossRef]
- Marwat, S.K.; Fazal-Ur-Rehman; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K. Phytochemical Constituents and pharmacological activities of sweet basil—Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2011, 23, 3773–3782. [Google Scholar]
- Khair-ul-Bariyah, S.; Ahmed, D.; Ikram, M. Ocimum basilicum: A review on phytochemical and pharmacological studies. Pak. J. Chem. 2012, 2, 78–85. [Google Scholar] [CrossRef]
- El-Azim, M.H.; Abdelgawad, A.A.; MohamedEl-Gerby; Ali, S.; El-Mesallamy, A. Chemical composition and antimicrobial activity of essential oil of egyptiam Ocimum. Indo Am. J. Pharm. Sci. 2015, 2, 837–842. [Google Scholar]
- El-soud, N.H.A.; Deabes, M.; El-kassem, L.A.; Khalil, M. Chemical composition and antifungal activity of Ocimum basilicum L. essential oil. J. Med. Sci. 2015, 3, 374–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menaker, A.; Kravets, M.; Koel, M.; Orav, A. Identification and characterization of supercritical fluid extracts from herbs. Comptes Rendus Chim. 2004, 7, 629–633. [Google Scholar] [CrossRef]
- Mazutti, M.; Beledelli, B.; Mossi, A.J.; Cansian, R.L.; Dariva, C.; Oliveira, V. De Caracterização química de extratos de Ocimum basilicum L. obtidos através de extração com CO2 a altas pressões. Quim. Nova 2006, 29, 1198–1202. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Evans, W.B.; Ebelhar, M.W.; Coker, C. Yield and Composition of Ocimum basilicum L. and Ocimum sanctum L. grown at four locations. Hortic. Sci. 2008, 43, 737–741. [Google Scholar]
- Barbalho, S.M.; Maria, F.; Farinazzi, V.; Rodrigues, S.; Pereira, H.; Goulart, R.D.A. Sweet basil (Ocimum basilicum): Much more than a condiment. TANG. Humanit. Med. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Barros, N.A.; Assis, A.R.; Mendes, M.F. Extração do óleo de manjericão usando fluido supercrítico: Analise experimental e matemática. Ciência Rural 2014, 44, 1499–1505. [Google Scholar] [CrossRef]
- Sales, K.C.; Rosa, F.; Sampaio, P.N.; Fonseca, L.P.; Lopes, M.B.; Calado, C.R.C. In situ near-infrared (NIR) versus high-throughput mid-infrared (MIR) spectroscopy to monitor biopharmaceutical production. Appl. Spectrosc. 2015, 69, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.F.; Maia, N.B.; Carmello, Q.A.C.; Catharino, R.R.; Eberlin, M.N.; Meireles, M.A.A. Sweet basil (Ocimum basilicum) extracts obtained by supercritical fluid extraction (SFE): Global yields, chemical composition, antioxidant activity, and estimation of the cost of manufacturing. Food Bioprocess Technol. 2008, 1, 326–338. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Meas. J. Int. Meas. Confed. 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Vitor, R.F.; Reis, C.P.; Roberto, A.; Rijo, P. Antioxidant activity and rosmarinic acid content of ultrasound-assisted ethanolic extracts of medicinal plants. Measurement 2016, 89, 328–332. [Google Scholar] [CrossRef]
- Karmali, A. Process for Simultaneous Extraction and Purification of Fine Chemicals from Either Spent Mushroom Compost, Mushroom Stems or Partially Degraded Mushroom Fruiting Bodies. European Patent No. EP 2078755, 18 December 2009. [Google Scholar]
- Arteiro, J.M.S.; Martins, M.R.; Salvador, C.; Candeias, M.F.; Karmali, A.; Caldeira, A.T. Protein-polysaccharides of Trametes versicolor: Production and biological activities. Med. Chem. Res. 2011, 21, 937–943. [Google Scholar] [CrossRef]
- Silva, S.; Martins, S.; Karmali, A.; Rosa, E. Production, purification and characterisation of polysaccharides from Pleurotus ostreatus with antitumour activity. J. Sci. Food Agric. 2012, 92, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid. Med. Cell. Longev. 2016, 2016, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Palavra, A.M.F.; Coelho, J.P.; Barroso, J.G.; Rauter, A.P.; Fareleira, J.M.N.A.; Mainar, A.; Urieta, J.S.; Nobre, B.P.; Gouveia, L.; Mendes, R.L.; et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids 2011, 60, 21–27. [Google Scholar] [CrossRef]
- Coelho, J.P.; Palavra, A.F. Supercritical fluide of compounds from spices and herbs. High Press. Fluid Technol. Green Food Process. 2015, 357–396. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Campardelli, R.; Della Porta, G.; De Marco, I.; Scognamiglio, M. Supercritical fluids based techniques to process pharmaceutical products difficult to micronize: Palmitoylethanolamide. J. Supercrit. Fluids 2015, 102, 24–31. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vazquez, E.; Garcia-Risco, M.; Reglero, G. Isolation of essential oil from different plants and herbes by supercritical fluid extraction. J. Chromatogr. 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Coelho, J.A.; Grosso, C.; Pereira, A.P.; Burillo, J.; Urieta, J.S.; Figueiredo, A.C.; Barroso, J.G.; Mendes, R.L.; Palavra, A.M.F. Supercritical carbon dioxide extraction of volatiles from Satureja fruticosa Béguinot. Flavour Fragr. J. 2007, 22. [Google Scholar] [CrossRef]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.N.A.; et al. Extraction of volatile oil from aromatic plants with supercritical carbon dioxide: Experiments and modeling. Molecules 2012, 17, 10550–10573. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.P.P.; Bernotaityte, K.; Miraldes, M.A.A.; Mendonca, A.F.; Stateva, R.P.P.; Mendonça, A.F.F.; Stateva, R.P.P. Solubility of ethanamide and 2-propenamide in supercritical carbon dioxide. Measurements and correlation. J. Chem. Eng. Data 2009, 54. [Google Scholar] [CrossRef]
- Marques, A.J.V.; Coelho, J.A.P. Determination of fat contents with supercritical CO2 extraction in two commercial powder chocolate products: Comparison with NP-1719. J. Food Process Eng. 2011, 34. [Google Scholar] [CrossRef]
- Coelho, J.P.; Mendonça, A.F.; Palavra, A.F.; Stateva, R.P. On the solubility of three disperse anthraquinone dyes in supercritical carbon dioxide: New experimental data and correlation. Ind. Eng. Chem. Res. 2011, 50. [Google Scholar] [CrossRef]
- Mota, L.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Ascensão, L. Glandular trichomes, histochemical localization of secretion, and essential oil composition in Plectranthus grandidentatus growing in Portugal. Flavour Fragr. J. 2013, 28, 393–401. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-throughput micro-plate HCl-vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, M.; Kalaimagal, C. In vitro antioxidant activity of ethanolic extract of a medicinal mushroom, Ganoderma lucidum. J. Pharm. Sci. Res. 2011, 3, 1427–1433. [Google Scholar]
- Reis, F.S.; Pereira, E.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 2011, 16, 4328–4338. [Google Scholar] [CrossRef] [PubMed]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.E. Unravelling New Ethnopharmacological Roles of Plectranthus Species: Biological Activity Screening. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Chang, C.; Chang, W.; Hsu, J.; Shih, Y.; Chou, S. Chemical composition and tyrosinase inhibitory activity of Cinnamomum cassia essential oil. Bot. Stud. 2013, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Yim, E.-Y.; Song, G.; Lee, N.H.; Hyun, C.-G. Screening of elastase and tyrosinase inhibitory activity from Jeju Island plants. EurAsian J. Biosci. 2010, 53, 41–53. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mitsunaga, T.; Batubara, I. Isolation, Identification and tyrosinase inhibitory activities of the extractives from Allamanda cathartica. Nat. Resour. 2011, 2, 167–172. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Sovilj, M.N.; Nikolovski, B.G.; Spasojević, M.Đ. Critical review of supercritical fluid extraction of selected spice plant materials. Maced. J. Chem. Chem. Eng. 2011, 30, 197–220. [Google Scholar]
- Filip, S.; Vidović, S.; Adamović, D.; Zeković, Z. Fractionation of non-polar compounds of basil (Ocimum basilicum L.) by supercritical fluid extraction (SFE). J. Supercrit. Fluids 2014, 86, 85–90. [Google Scholar] [CrossRef]
- Filip, S.; Vidović, S.; Vladić, J.; Pavlić, B.; Adamović, D.; Zeković, Z. Chemical composition and antioxidant properties of Ocimum basilicum L. extracts obtained by supercritical carbon dioxide extraction: Drug exhausting method. J. Supercrit. Fluids 2016, 109, 20–25. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC—Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Reverchon, E.; Donsi, G.; Osseo, L.S. Modeling of supercritical fluid extraction from herbaceous matrices. Ind. Eng. Chem. Res. 1993, 32, 2721–2726. [Google Scholar] [CrossRef]
- Poletto, M.; Reverchon, E. Comparison of models for supercritical fluid extraction of seed and essential oils in relation to the mass-transfer rate. Ind. Eng. Chem. Res. 1996, 5885, 3680–3686. [Google Scholar] [CrossRef]
- Özcan, M.; Chalchat, J.-C. Essential oil composition of Ocimum basilicum L. and Ocimum minimum L. in Turkey. Czech J. Food Sci. 2002, 20, 223–228. [Google Scholar] [CrossRef]
- Occhipinti, A.; Capuzzo, A.; Bossi, S.; Milanesi, C.; Maffei, M.E. Comparative analysis of supercritical CO2 extracts and essential oils from an Ocimum basilicum chemotype particularly rich in T-cadinol. J. Essent. Oil Res. 2013, 25, 272–277. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluids 1997, 10, 1–37. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Correa, H.A.; Magalhães, P.M.; Queiroga, C.L.; Peixoto, C.A.; Oliveira, A.L.; Cabral, F.A. Extracts from pitanga (Eugenia uniflora L.) leaves: Influence of extraction process on antioxidant properties and yield of phenolic compounds. J. Supercrit. Fluids 2011, 55, 998–1006. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, V.; Shri, R. In Vitro Evaluation of acetylcolinesterase inhibition by standardized extracts of selected plants. Int. J. Biol. Pharm. Res. 2015, 6, 247–250. [Google Scholar]
- Curtis, B.J.; Radek, K.A. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: New perspectives in epidermal immunity and disease. J. Investig. Dermatol. 2012, 132, 28–42. [Google Scholar] [CrossRef] [PubMed]

| Extraction Method | Sample Identification | Yield (%) |
|---|---|---|
| Hydrodistillation (Fresh plant) | HDF | 0.35 ± 0.02 |
| Hydrodistillation (Dry plant) | HDD | 0.32 ± 0.02 |
| SFE (9.0 MPa, 40 °C) | SFEO | 0.39 ± 0.02 |
| Soxhlet (Methanol) | MeOH | 17.8 ± 0.9 |
| Soxhlet (Ethanol) | EtOH | 9.6 ± 0.4 |
| SFE (40.0 MPa, 40 °C) | SFEE | 2.2 ± 0.1 |
| Ocimum basilicum L. | |||||
|---|---|---|---|---|---|
| Components | RI | HDF | HDD | SFEO | Waxes |
| 2nd S | 1st S | ||||
| α-Pinene | 930 | 0.3 | 0.6 | t | t |
| Camphene | 938 | 0.1 | 0.2 | t | t |
| Sabinene | 958 | 0.2 | 0.4 | 0.1 | t |
| 1-Octen-3-ol | 961 | 0.2 | 0.2 | 0.1 | t |
| β-Pinene | 963 | 0.6 | 1.3 | 0.2 | t |
| β-Myrcene | 975 | 0.8 | 0.7 | 0.2 | t |
| 1,8-Cineole | 1005 | 7.6 | 11.0 | 5.8 | t |
| trans-β-Ocimene | 1027 | 1.5 | 0.8 | 0.3 | t |
| γ-Terpinene | 1035 | 0.2 | 0.2 | 0.1 | t |
| trans-Sabinene hydrate | 1037 | 0.1 | 0.1 | 0.1 | t |
| Terpinolene | 1064 | 0.4 | 0.4 | 0.1 | t |
| Linalool | 1074 | 18.1 | 18.8 | 12.6 | t |
| Camphor | 1102 | 0.7 | 0.9 | 0.5 | t |
| Borneol | 1134 | 0.6 | 0.6 | 0.4 | t |
| Terpinen-4-ol | 1148 | 0.3 | 0.4 | 0.1 | t |
| α-Terpineol | 1159 | 0.9 | 1.0 | 0.7 | t |
| Methyl chavicol (Estragole) | 1163 | 13.5 | 19.9 | 12.6 | t |
| Bornyl acetate | 1265 | 0.4 | 0.5 | 0.3 | t |
| Eugenol | 1327 | 6.9 | 3.9 | 6.5 | t |
| trans-Methyl cinnamate | 1346 | 0.1 | 0.2 | 0.2 | t |
| α-Copaene | 1375 | 0.1 | t | 0.1 | t |
| Methyl eugenol | 1377 | 34.0 | 30.1 | 29.4 | 1.3 |
| trans-α-Bergamotene | 1434 | 2.8 | 2.4 | 5.6 | 0.1 |
| α-Humulene | 1447 | 0.5 | 0.3 | 0.6 | 0.4 |
| trans-β-Farnesene | 1455 | 1.3 | 1.1 | 3.4 | 1.3 |
| Germacrene D | 1474 | 1.4 | 0.3 | 1.7 | 1.6 |
| Bicyclogermacrene | 1487 | 0.5 | 0.1 | 0.5 | 0.2 |
| γ-Cadinene | 1500 | 0.4 | 0.3 | 0.6 | 0.3 |
| β-Sesquiphellandrene | 1508 | 0.6 | 0.4 | 0.9 | 0.7 |
| Spathulenol | 1551 | 0.2 | 0.1 | 0.2 | t |
| T-Cadinol | 1616 | 1.7 | 0.9 | 1.1 | 0.3 |
| Phytol acetate 2 | 2101 | 0.4 | 0.3 | 5.4 | 3.9 |
| n-Heptacosane | 2700 | t | 0.1 | 0.2 | 17.8 |
| n-Nonacosane | 2900 | t | t | 0.2 | 15.4 |
| n-Triacontane | 2000 | t | t | 0.1 | 8.3 |
| n-Hentriacontane | 3100 | t | t | 0.1 | 11.6 |
| n-Dotriacontane | 3200 | t | t | 0.2 | 23.8 |
| Identified Compounds | 97.4 | 98.5 | 91.2 | 87.2 | |
| Grouped components | |||||
| Monoterpene hydrocarbons | 4.2 | 4.7 | 1.1 | t | |
| Oxygen-containing monoterpenes | 28.4 | 32.9 | 20.2 | t | |
| Sesquiterpene hydrocarbons | 7.7 | 5.0 | 13.5 | 4.6 | |
| Oxygen-containing sesquiterpenes | 2.3 | 1.3 | 6.7 | 4.4 | |
| Phenylpropanoids | 54.4 | 53.9 | 48.5 | 1.3 | |
| Others | 0.4 | 0.7 | 1.2 | 76.9 | |
| Sample | DPPH-IC50 (mg/mL) | ABTS-IC50 (mg/mL) | Reduction Power (μmol TE/g) |
|---|---|---|---|
| MeOH | 3.05 ± 0.36 a | 4.26 ± 0.22 a | 306.8 ± 21.8 a |
| EtOH | 3.99 ± 0.55 a | 5.38 ± 0.23 b | 285.1 ± 18.1 a |
| SFEE | 5.63 ± 0.20 b | 1.74 ± 0.05 c | 111.7 ± 7.3 b |
| Trolox | 0.471 ± 0.088 c | 0.425 ± 0.084 d | ----- |
| Ascorbic acid | 0.266 ± 0.022 d | 0.331 ± 0.050 e | ----- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.; Veiga, J.; Karmali, A.; Nicolai, M.; Pinto Reis, C.; Nobre, B.; Palavra, A. Supercritical CO2 Extracts and Volatile Oil of Basil (Ocimum basilicum L.) Comparison with Conventional Methods. Separations 2018, 5, 21. https://doi.org/10.3390/separations5020021
Coelho J, Veiga J, Karmali A, Nicolai M, Pinto Reis C, Nobre B, Palavra A. Supercritical CO2 Extracts and Volatile Oil of Basil (Ocimum basilicum L.) Comparison with Conventional Methods. Separations. 2018; 5(2):21. https://doi.org/10.3390/separations5020021
Chicago/Turabian StyleCoelho, José, Jerson Veiga, Amin Karmali, Marisa Nicolai, Catarina Pinto Reis, Beatriz Nobre, and António Palavra. 2018. "Supercritical CO2 Extracts and Volatile Oil of Basil (Ocimum basilicum L.) Comparison with Conventional Methods" Separations 5, no. 2: 21. https://doi.org/10.3390/separations5020021