Mechanism of Cesium Adsorption by Carbonized Rice Hull and Beech Sawdust
Abstract
:1. Introduction
2. Materials and Methods
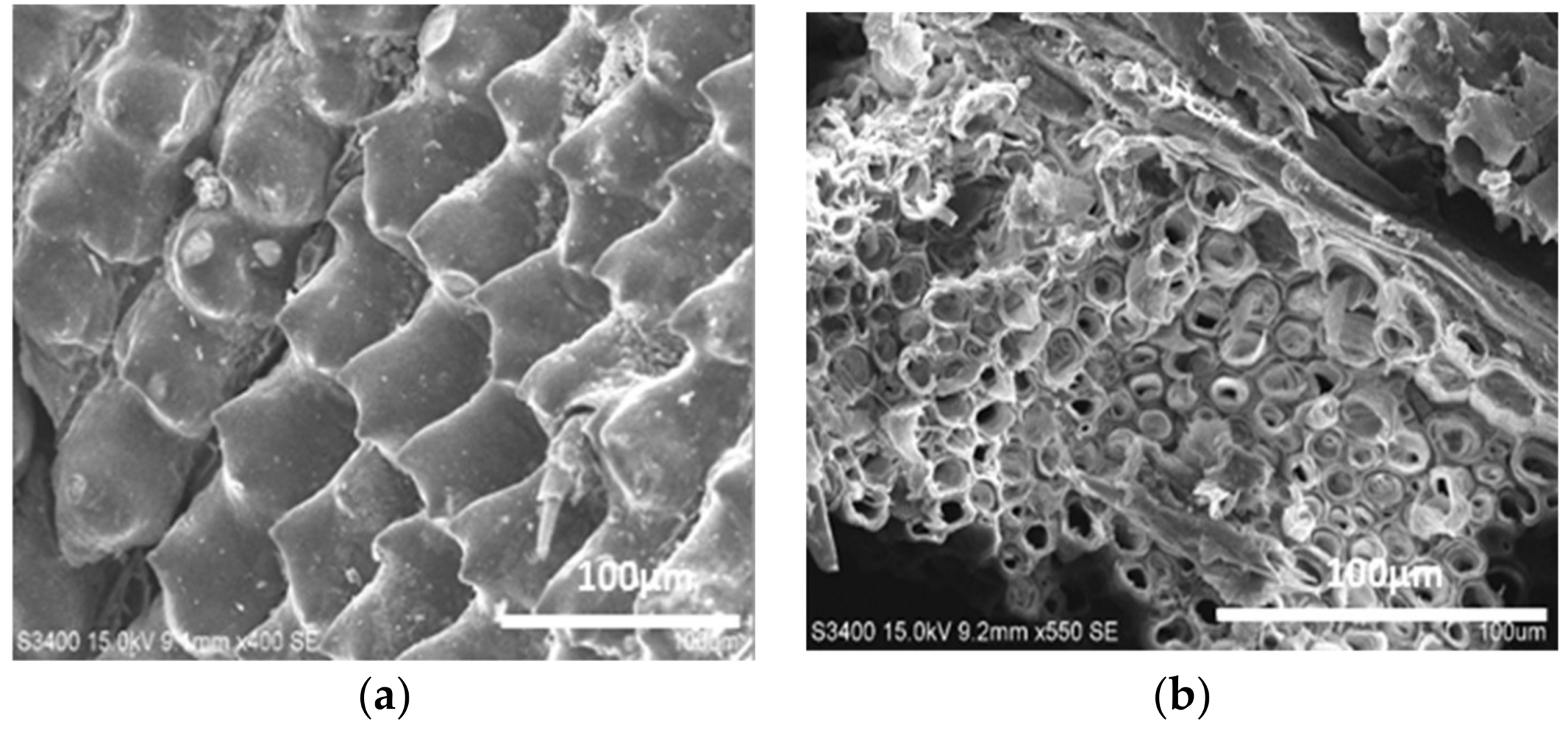
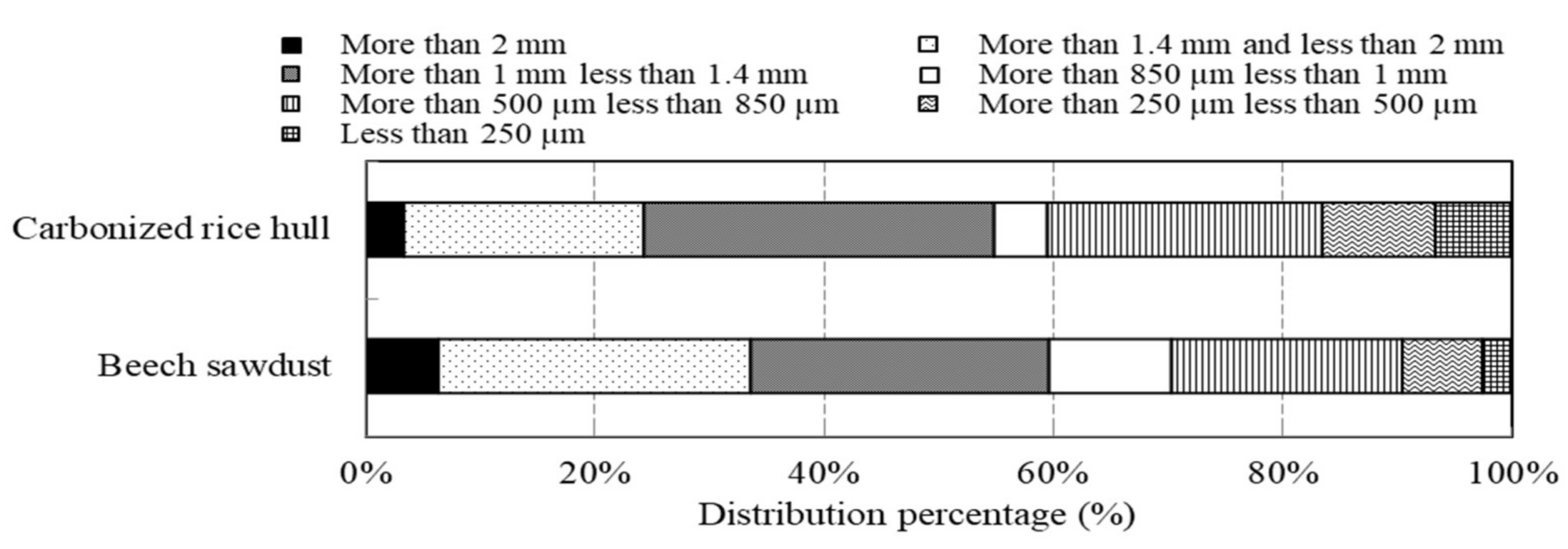
2.1. Materials
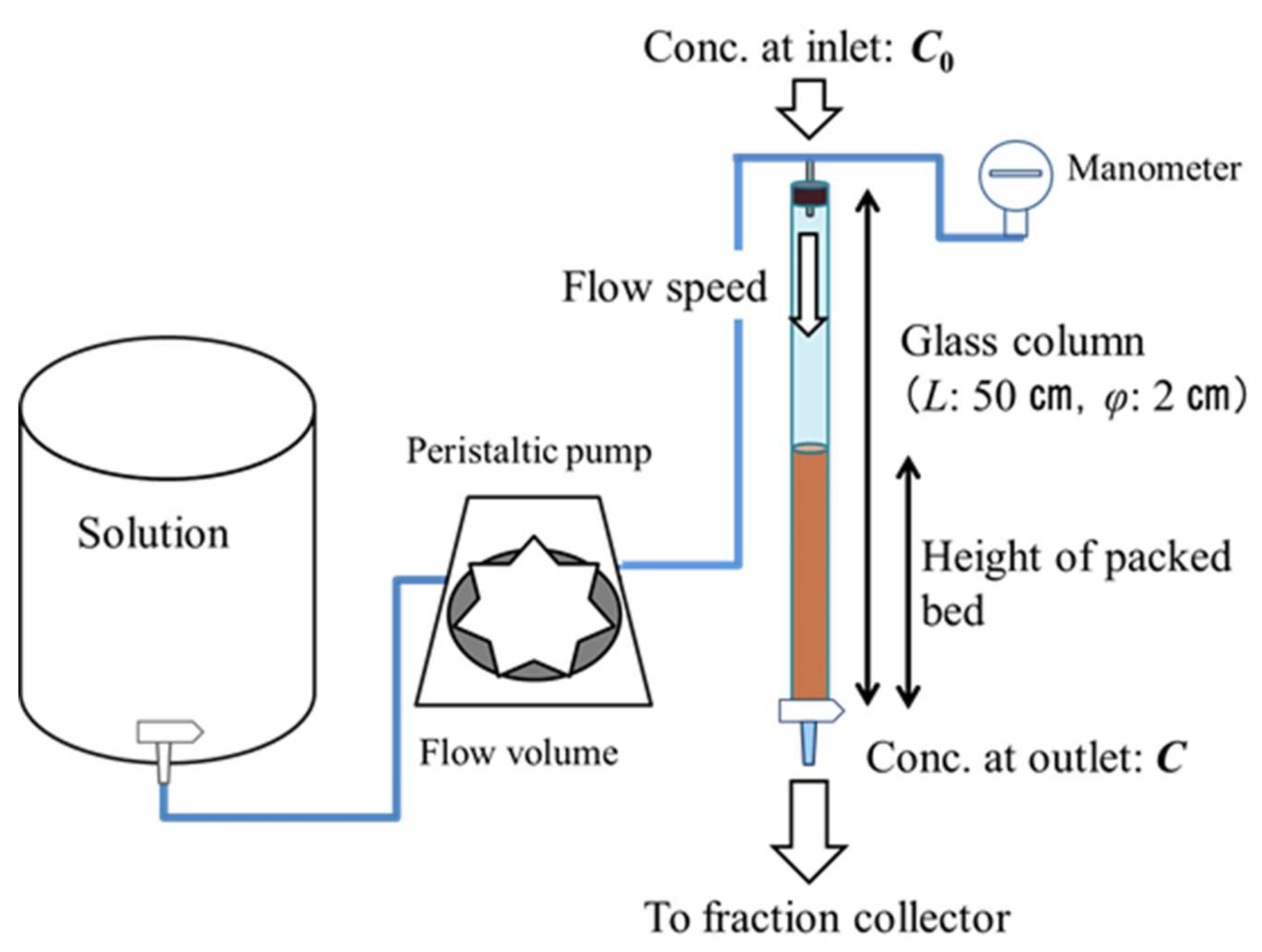
2.2. Fixed-Bed Adsorption Experiments
3. Results and Discussion
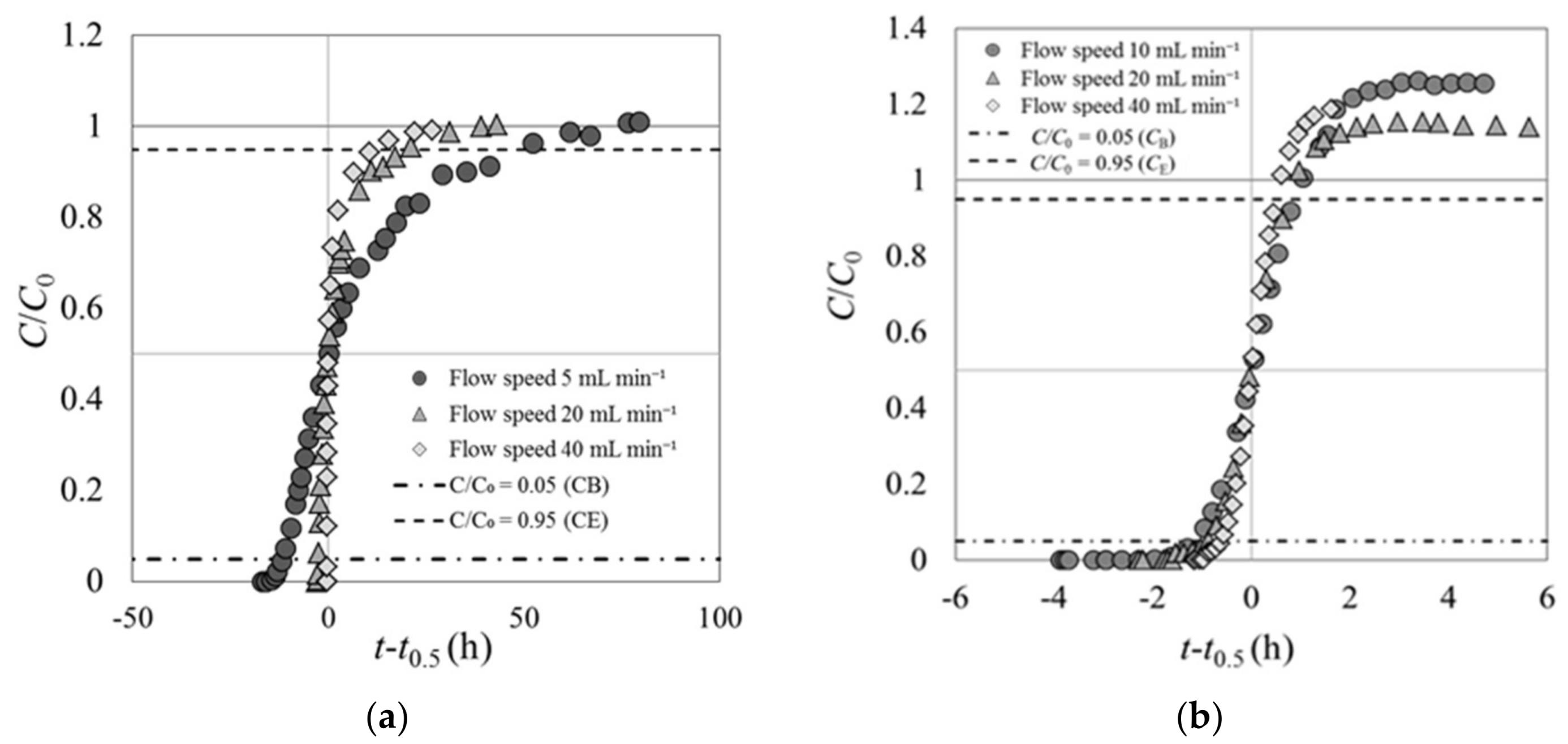
3.1. Breakthrough Curves for Cs Solutions at Various Flow Speeds (Experiment 1)
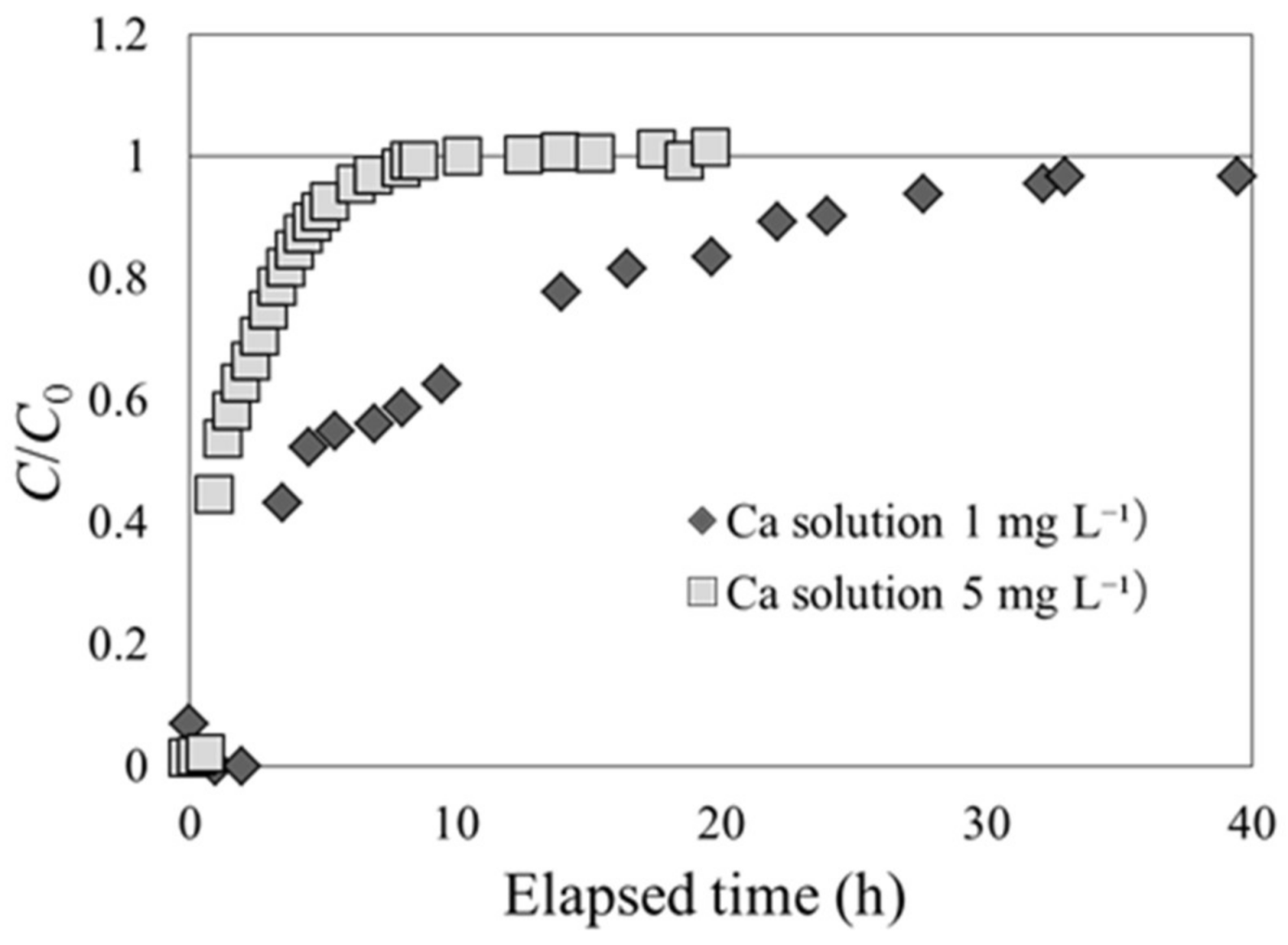
3.2. Cs Adsorption by Beech Sawdust (Experiments 2-1 and 2-2)
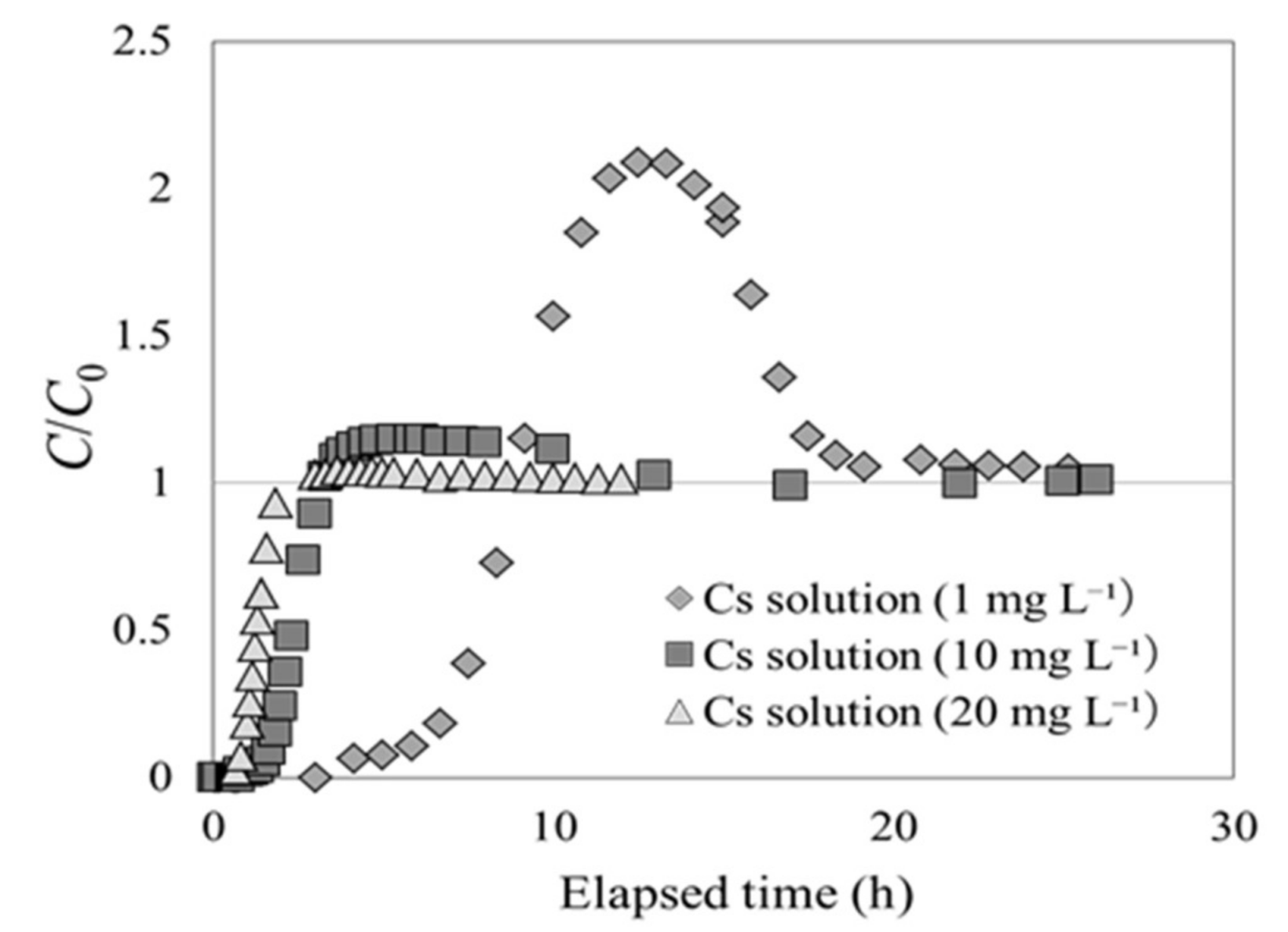
3.3. Overshoot in the Breakthrough Curve by Beech Sawdust
4. Conclusions
- (1)
- The shape of the breakthrough curve of carbonized rice hull indicates that although it could not adsorb Cs immediately after contact with the Cs solution, it could slowly adsorb Cs as the Cs solution passed through the fixed-bed layer. On the contrary, beech sawdust could rapidly adsorb Cs immediately upon contact with the Cs solution.
- (2)
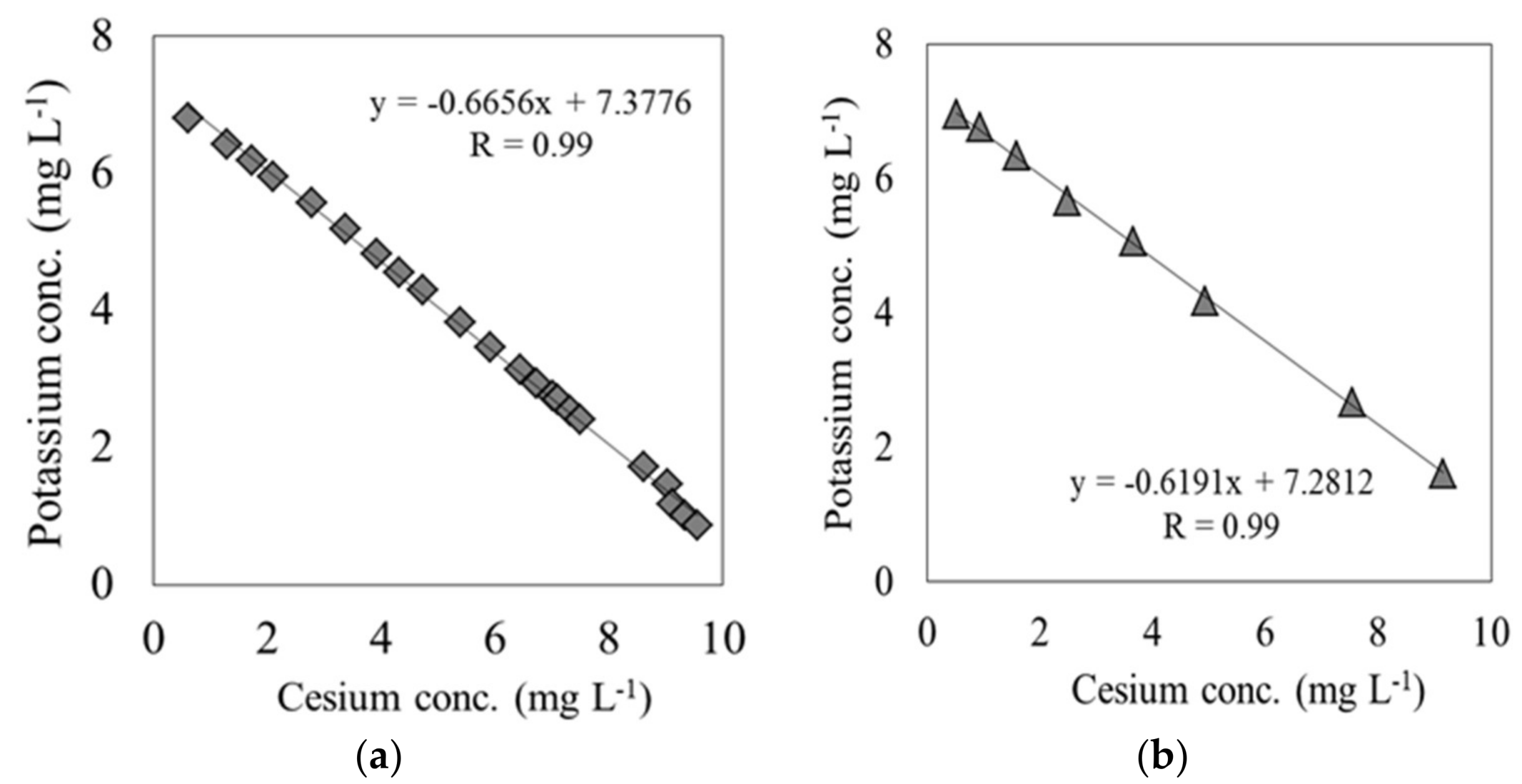
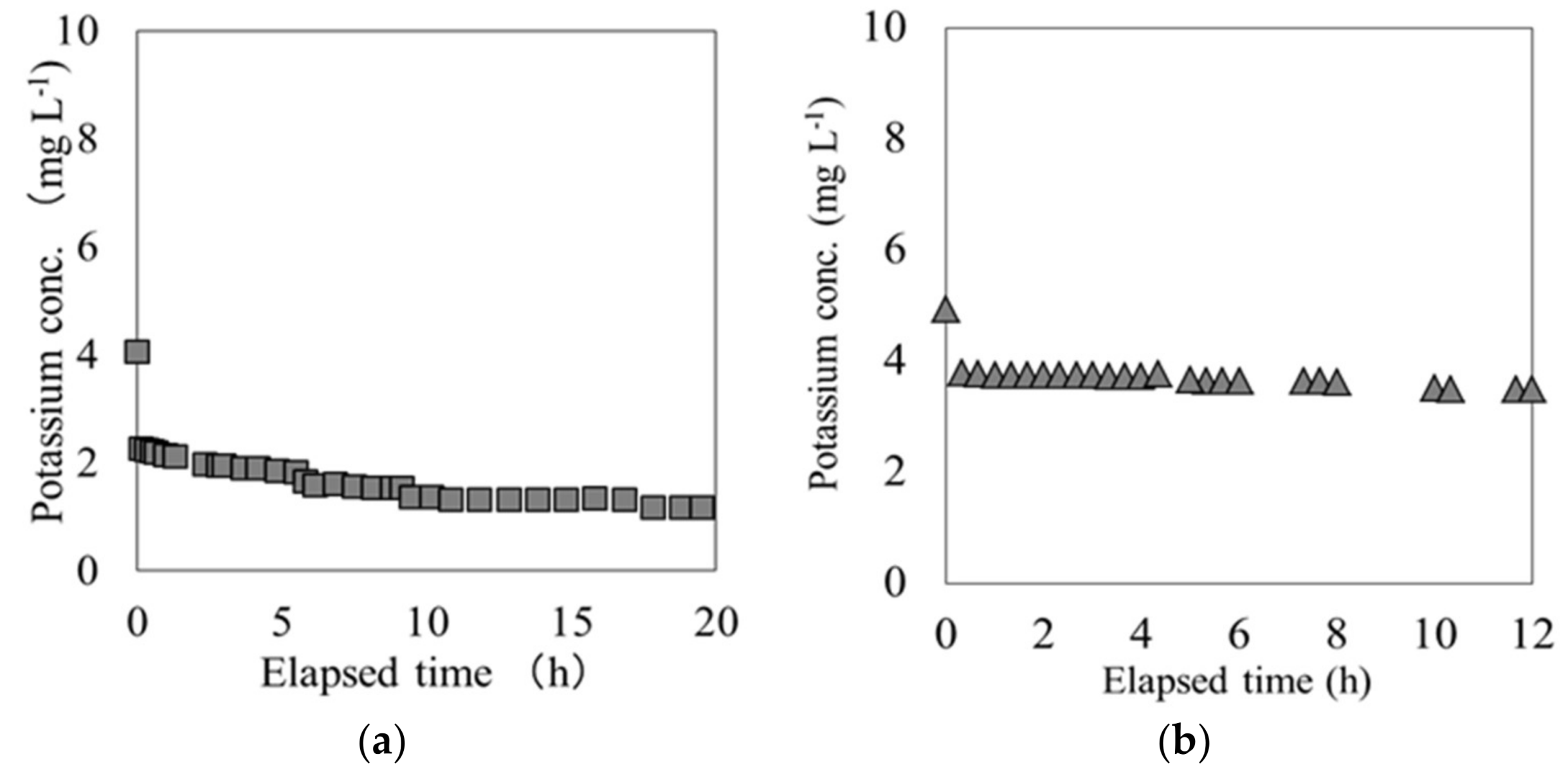
- Cs switched places with K through an ion-exchange reaction on the adsorption surface of each material.
- (3)
- The shape of the breakthrough curve of beech sawdust changed depending on the initial Cs concentration. As the Cs concentration at the column inlet was decreased, the value of C/C0 markedly exceeded 1 (overshoot). However, there was no change in pressure in the column throughout the experiment, indicating that overshoot did not occur due to the contraction or swelling of the material in the column.
- (4)
- Regardless of the experimental conditions for the breakthrough experiments, Cs adsorption by beech sawdust caused the overshoot phenomenon before reaching the equilibrium state.
Acknowledgments
Conflicts of Interest
References
- Fukushima Revitalization Station, Fukushima Prefecture Govt., Japan. Available online: http://www.pref.fukushima.lg.jp/site/portal/progress.html (accessed on 12 June 2017).
- Hashimoto, S.; Ugawa, S.; Nakano, K.; Shichi, K. The total amounts of radioactively contaminated materials in forests in Fukushima, Japan. Sci. Rep. 2012, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Yamaguchi, M.; Kurikami, H.; Yui, M.; Onishi, Y. Predicting sediment and cesium-137 discharge from catchments in eastern Fukushima. Anthropocene 2014, 5, 22–31. [Google Scholar] [CrossRef]
- Iijima, K. Status of the researches on the behavior in the environment of radioactive cesium transported from forests to river systems. Chikyukagaku 2015, 49, 203–215. [Google Scholar] [CrossRef]
- Kurikami, H.; Kitamura, A.; Yokuda, S.T.; Onishi, Y. Sediment and 137Cs behaviors in the Ogaki Dam Reservoir during a heavy rainfall event. J. Environ. Radioact. 2014, 137, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Fujiwara, K.; Harayama, T.; Okamura, Y.; Uchiyama, S.; Sugiyama, M.; Someya, T.; Amakai, W.; Umino, S.; Ono, T.; et al. Removal of cesium using cobalt-ferrocyanide-impregnated polymer-chain-grafted fibers. J. Nucl. Sci. Technol. 2011, 48, 1281–1284. [Google Scholar] [CrossRef]
- Saito, K. Removal of cesium from water using adsorptive fibers. Bull. Soc. Sea Water Sci. 2011, 65, 280–284. [Google Scholar]
- Shibata, J.; Furuyanaka, S.; Murayama, N.; Yoyogi, S. Development of Processing Technology for Aqueous Liquor Containing High Level Radioactive Elements Discharged from Fukushima Nuclear Power Plant; Disaster Prevention Research Institute, Kyoto University: Kyoto, Japan, 2012; pp. 1–20. [Google Scholar]
- Miura, A.; Kubota, T.; Hamada, K.; Hitomi, T. Adsorption efficiency of natural materials for low-concentration cesium in solution. Water Sci. Technol. 2016, 73, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Miura, A. Determination of cesium adsorption breakthrough curves using carbonized rice hull and beech sawdust as adsorbents. Environ. Ecol. Res. 2017, 5, 461–466. [Google Scholar] [CrossRef]
- Michaelas, A.S. Simplified method of interpreting kinetic data in fixed bed ion exchange. Ind. Eng. Chem. 1952, 44, 1922–1930. [Google Scholar] [CrossRef]
- Hashimoto, K. Design of fixed layer adsorption apparatus. Water Purif. Liq. Wastes Treat. 1972, 13, 37–47. [Google Scholar]
- Vincent, C.; Hertz, A.; Vincent, T.; Barré, Y.; Guibal, E. Immobilization of inorganic ion-exchanger into biopolymer foams-Application to cesium sorption. Chem. Eng. J. 2014, 236, 202–211. [Google Scholar] [CrossRef]
- Kim, T.Y.; An, S.S.; Shim, W.G.; Lee, J.W.; Cho, S.Y.; Kim, J.M. Adsorption and energetic heterogeneity properties of cesium ions on ion exchange resin. J. Ind. Eng. Chem. 2015, 27, 260–267. [Google Scholar] [CrossRef]
- Kleinubing, S.J.; d’Silva, E.A.; d’Silva, M.G.C.; Guibal, E. Equilibrium of Cu (II) and Ni (II) biosorption by marine alga Sargassum filipendula in adynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 102, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Figueira, M.M.; Volesky, B.; Climinelli, V.S.T.; Roddick, F.A. Biosorption of metals in brown seaweed biomass. Water Res. 2000, 34, 196–204. [Google Scholar] [CrossRef]
- Naja, G.; Volesky, B. Multi-metal biosorption in a fixed-bed flow-through column. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 281, 194–201. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Yousif, S.A.; Al-Faize, M.M. Single-multicomponent biosorption of lead mercury chromium and arsenic onto activated sludge in batch and fixed-bed adsorber. Desalin. Water Technol. 2015, 53, 3499–3512. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Chase, H.A. Simulation of the breakthrough curves for the adsorption of α-lactalbumin and β-lactoglobulin to SP Sepharose FF cation-exchanger. Biochem. Eng. J. 2010, 49, 221–228. [Google Scholar] [CrossRef]
- Hori, H.; Tanaka, I.; Akiyama, T. A simple estimation method of breakthrough time for multicomponent organic solvent vapors on activated carbon fixed bed adsorbers. Chem. Soc. Jpn. 1985, 11, 2087–2093. [Google Scholar] [CrossRef]
| Information for Materials | Carbonized Rice Hull | Beech Sawdust |
|---|---|---|
| Specific surface area; A (m2 g−1) | 3.4 | 0.35 |
| Bulk density (g L−1) | 122.6 | 211.7 |
| pH in solution | 7.25 | 3.42 |
| (a) Carbonized Rice Hull | Filled Adsorbent (g) | Height of Fixed Adsorbent Bed (cm) | Solution Flow Speed (mL min−1) | Cs Concentration (mg-Cs L−1) |
| C-1 | 10 | 17 | 5 | 10 |
| C-2 | 20 | |||
| C-3 | 40 | |||
| (b) Beech Sawdust | Filled Adsorbent (g) | Height of Fixed Adsorbent Bed (cm) | Solution Flow Speed (mL min−1) | Cs Concentration (mg-Cs L−1) |
| B-1 | 20 | 26 | 10 | 10 |
| B-2 | 20 | |||
| B-3 | 40 |
| Experiment 1 | Carbonized Rice Hull | Beech Sawdust | ||||
|---|---|---|---|---|---|---|
| u: Flow speed of Cs solution (mL min−1) | 5 | 20 | 40 | 10 | 20 | 40 |
| tB: Elapsed time at breakthrough point (h) | 4.6 | 0.2 | 2 × 10−2 | 2.5 | 1.4 | 0.5 |
| tE: Elapsed time at end point (h) | 65.5 | 23.0 | 12.1 | 4.6 | 3.0 | 1.7 |
| tB–E: Elapsed time between CB and CE (h) | 60.9 | 22.8 | 12.1 | 2.0 | 1.6 | 1.1 |
| Experiment 1 | Carbonized Rice Hull | Beech Sawdust | ||||
|---|---|---|---|---|---|---|
| Cs solution flow speed (mL min−1) | 5 | 20 | 40 | 10 | 20 | 40 |
| Total inflow of Cs (mg) | 204.7 | 273.4 | 290.2 | 12.1 | 19.8 | 26.8 |
| Total outflow of Cs (mg) | 129.7 | 213.2 | 245.3 | 5.4 | 9.7 | 12.4 |
| Total adsorbed Cs in column (mg) | 75.0 | 60.2 | 44.9 | 6.7 | 10.1 | 14.4 |
| Adsorbed Cs per unit weight of material (mg) | 7.5 | 6.0 | 4.5 | 0.3 | 0.5 | 0.7 |
| Cs adsorption rate in column (%) | 36.6 | 22.0 | 15.5 | 55.6 | 51.0 | 53.6 |
| Experimental Conditions (Beech Sawdust) | * Cs Solution Flow Speed (mL min−1) | C/C0 | * Initial Cs Concentration (mg-Cs L−1) | C/C0 | ** Height of Fixed Adsorbent Bed (cm) | C/C0 |
| 10 | 1.3 | 1 | 2.1 | 15 | 1.1 | |
| 20 | 1.2 | 10 | 1.1 | 20 | 1.2 | |
| 40 | 1.2 | 20 | 1.0 | 26 | 1.2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, A. Mechanism of Cesium Adsorption by Carbonized Rice Hull and Beech Sawdust. Separations 2018, 5, 22. https://doi.org/10.3390/separations5020022
Miura A. Mechanism of Cesium Adsorption by Carbonized Rice Hull and Beech Sawdust. Separations. 2018; 5(2):22. https://doi.org/10.3390/separations5020022
Chicago/Turabian StyleMiura, Asa. 2018. "Mechanism of Cesium Adsorption by Carbonized Rice Hull and Beech Sawdust" Separations 5, no. 2: 22. https://doi.org/10.3390/separations5020022




