Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases
Abstract
:1. Introduction
2. Basic Technology of LSFG-NAVI
≈ <In,m,t>2/< (In,m,t − <In,m,t>) 2>
= <In,m,t>2/(<I2n,m,t> − <In,m,t>2)
= 2 {<In,m,t>2/(<I2n,m,t> − <In,m,t>2)} = 2 SBRn,m,t
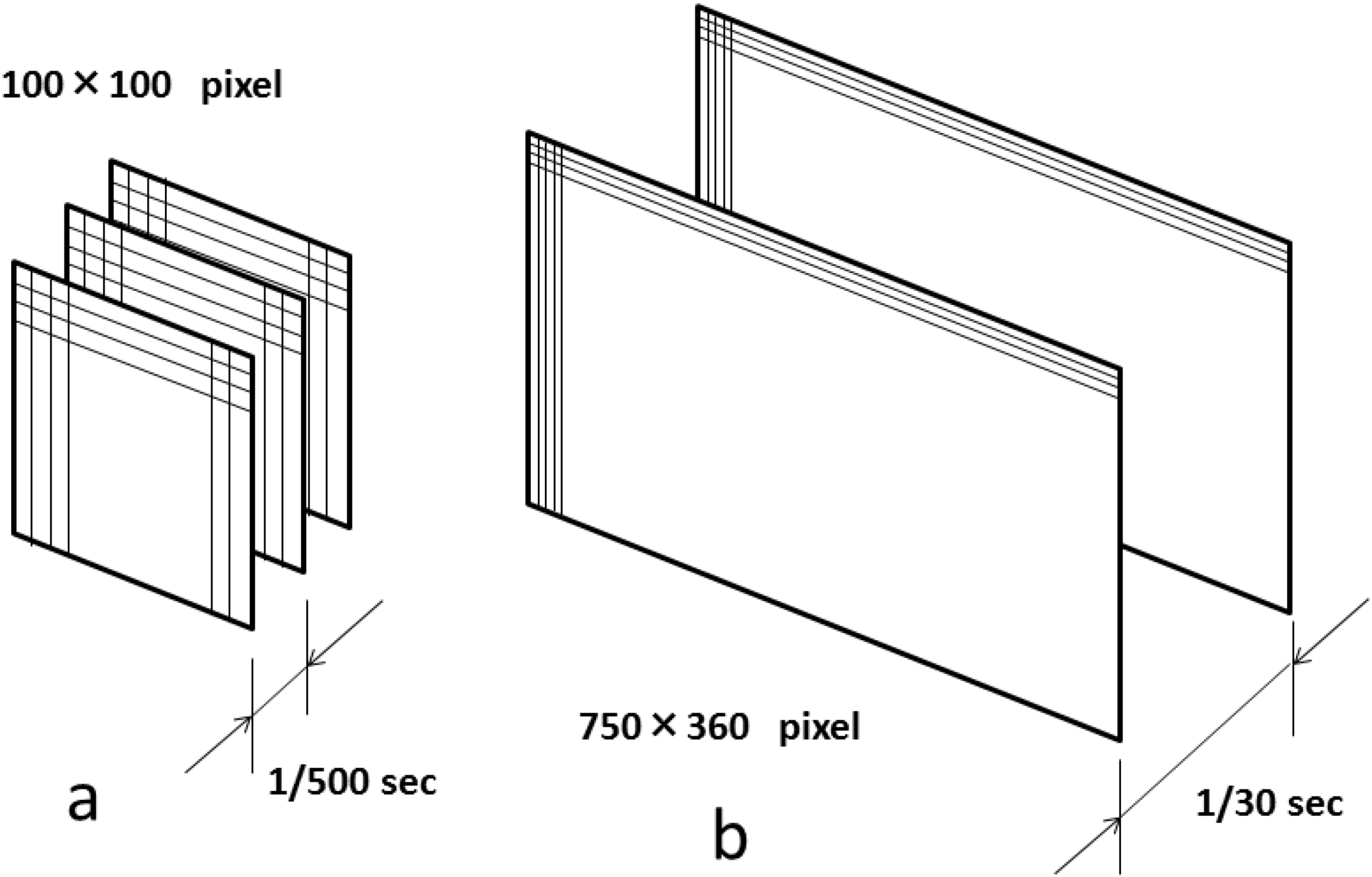
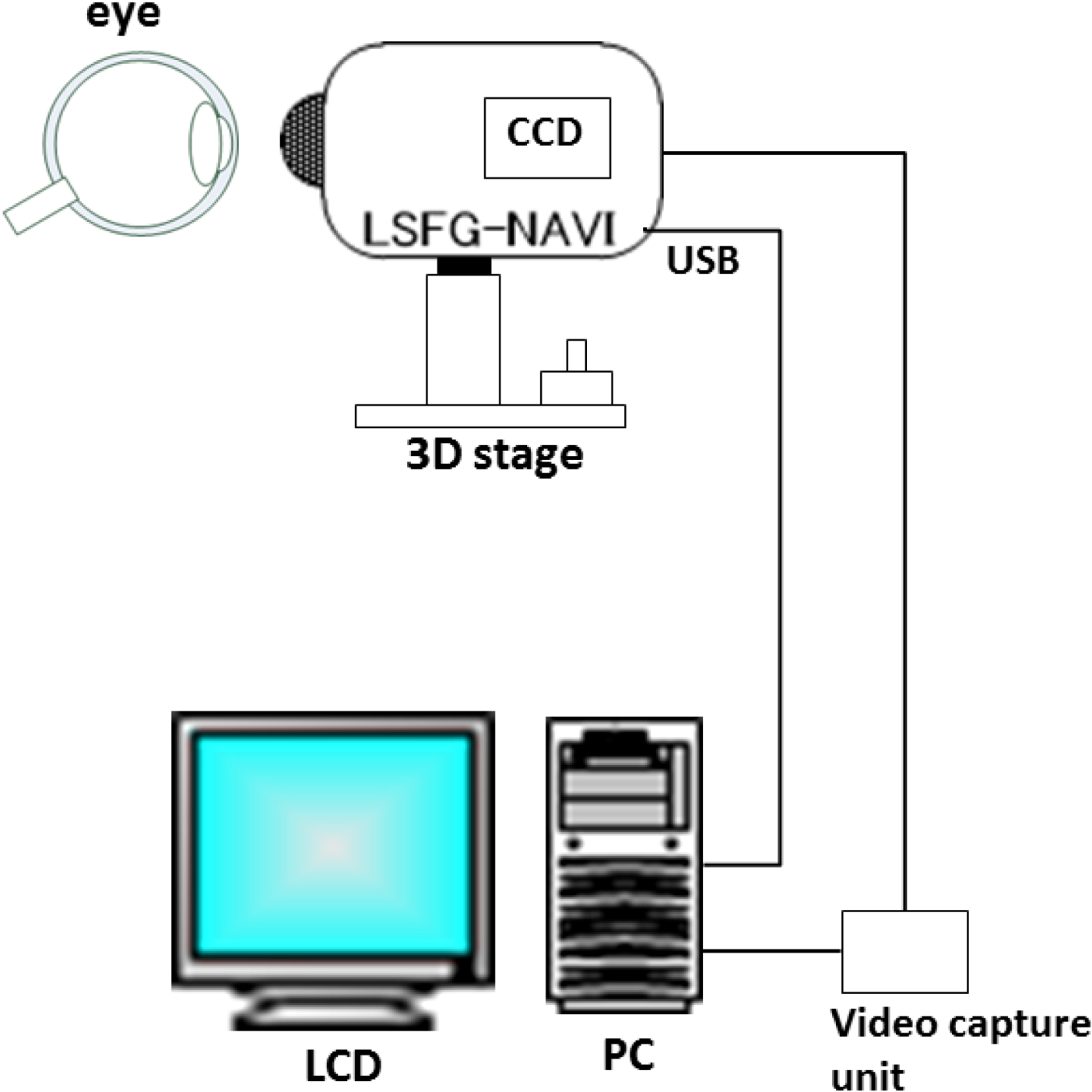
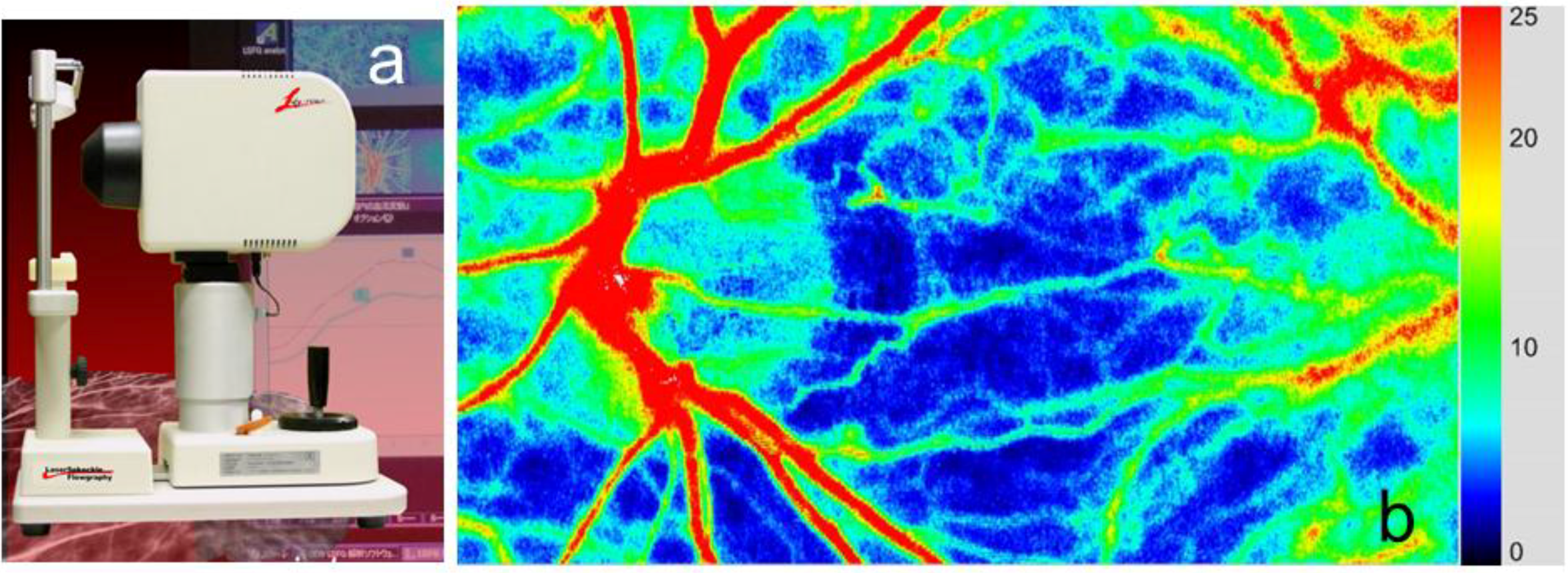
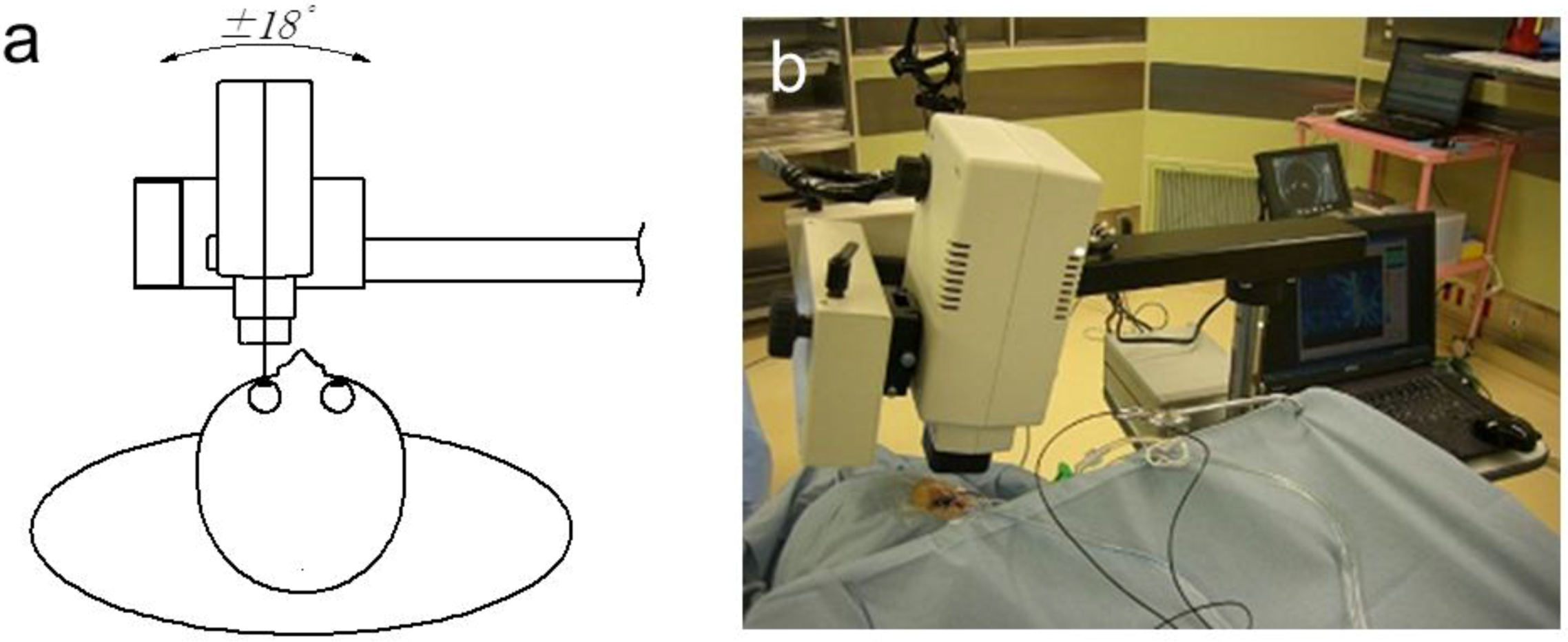
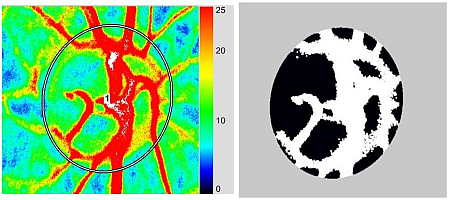
3. Application of LSFG-NAVI to Basic and Clinical Research
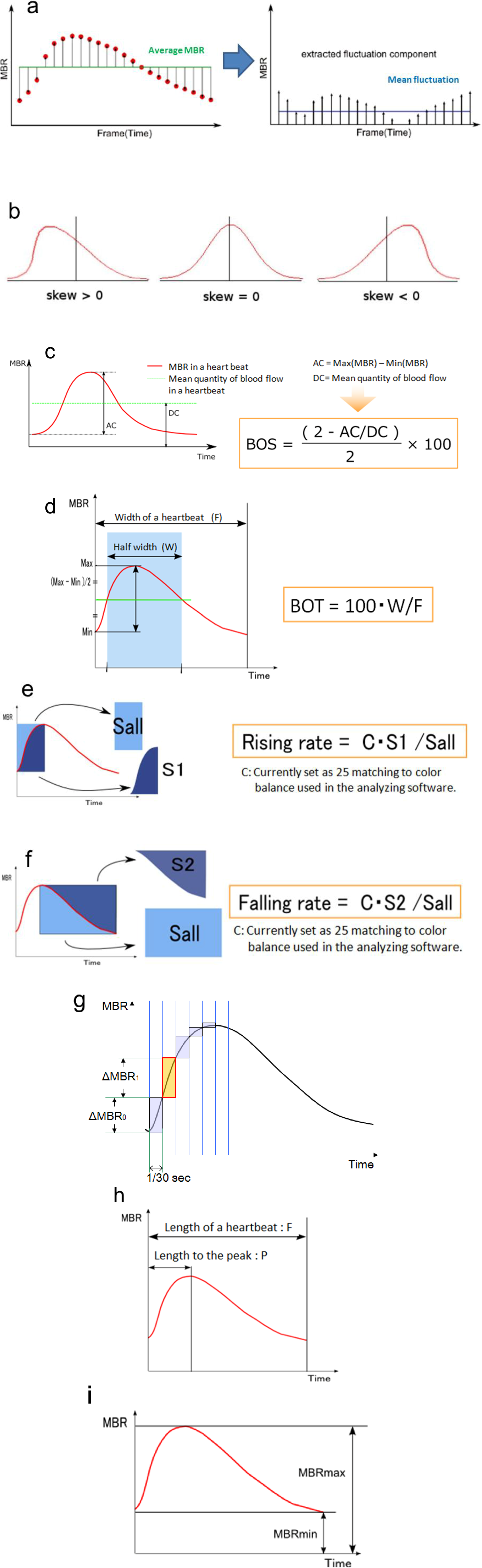
3.1. Specific Parameters in LSFG-NAVI
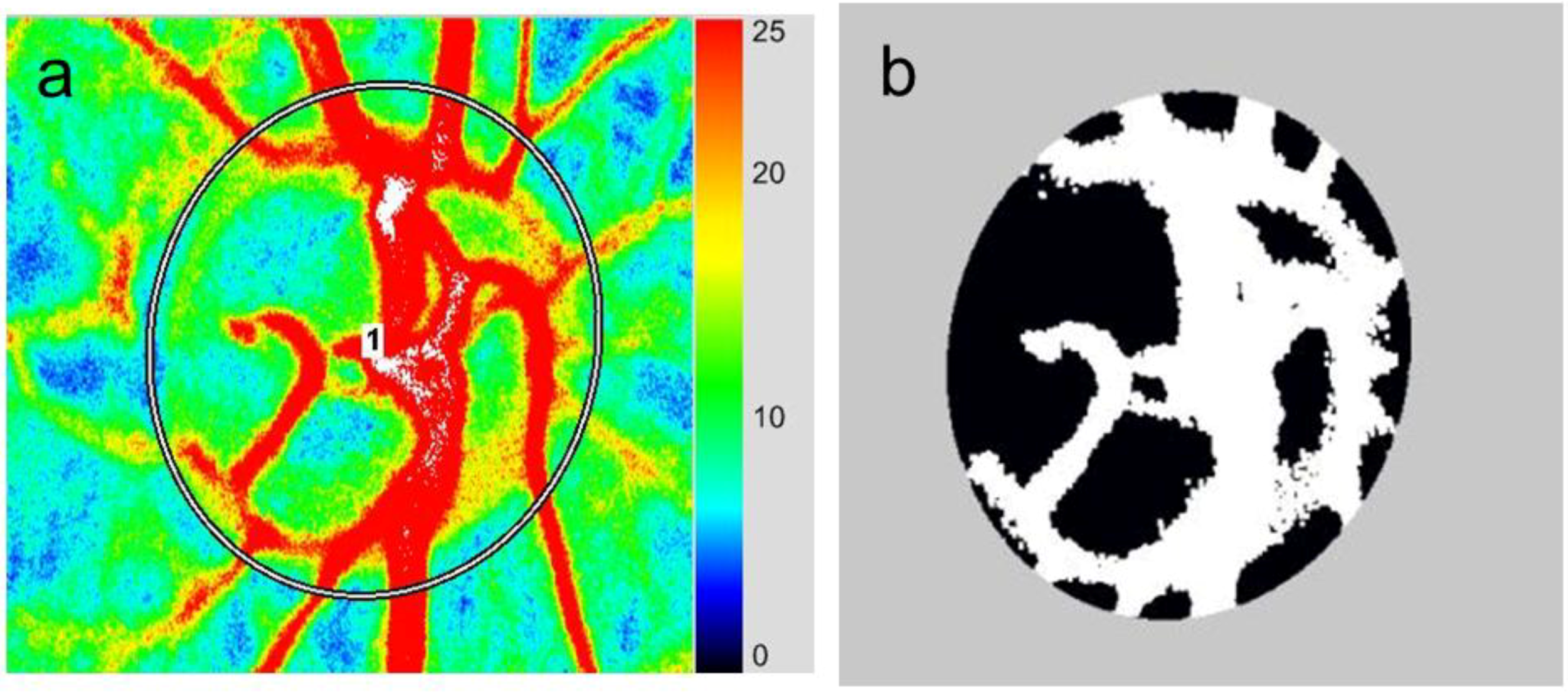
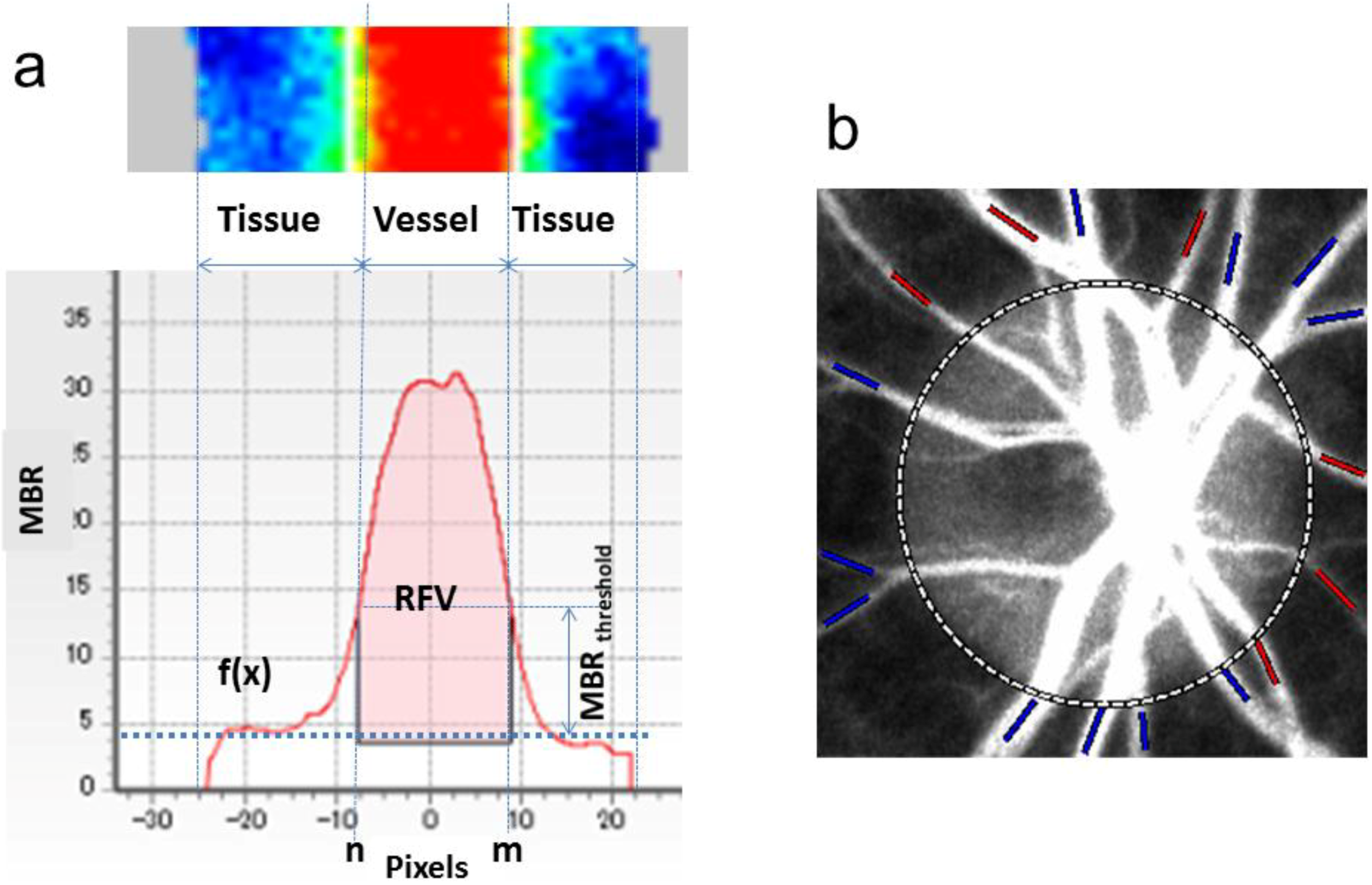
3.2. Application of LSFG-NAVI to Basic Research
3.3. Application of LSFG-NAVI to Glaucoma and Other Optic Nerve Diseases
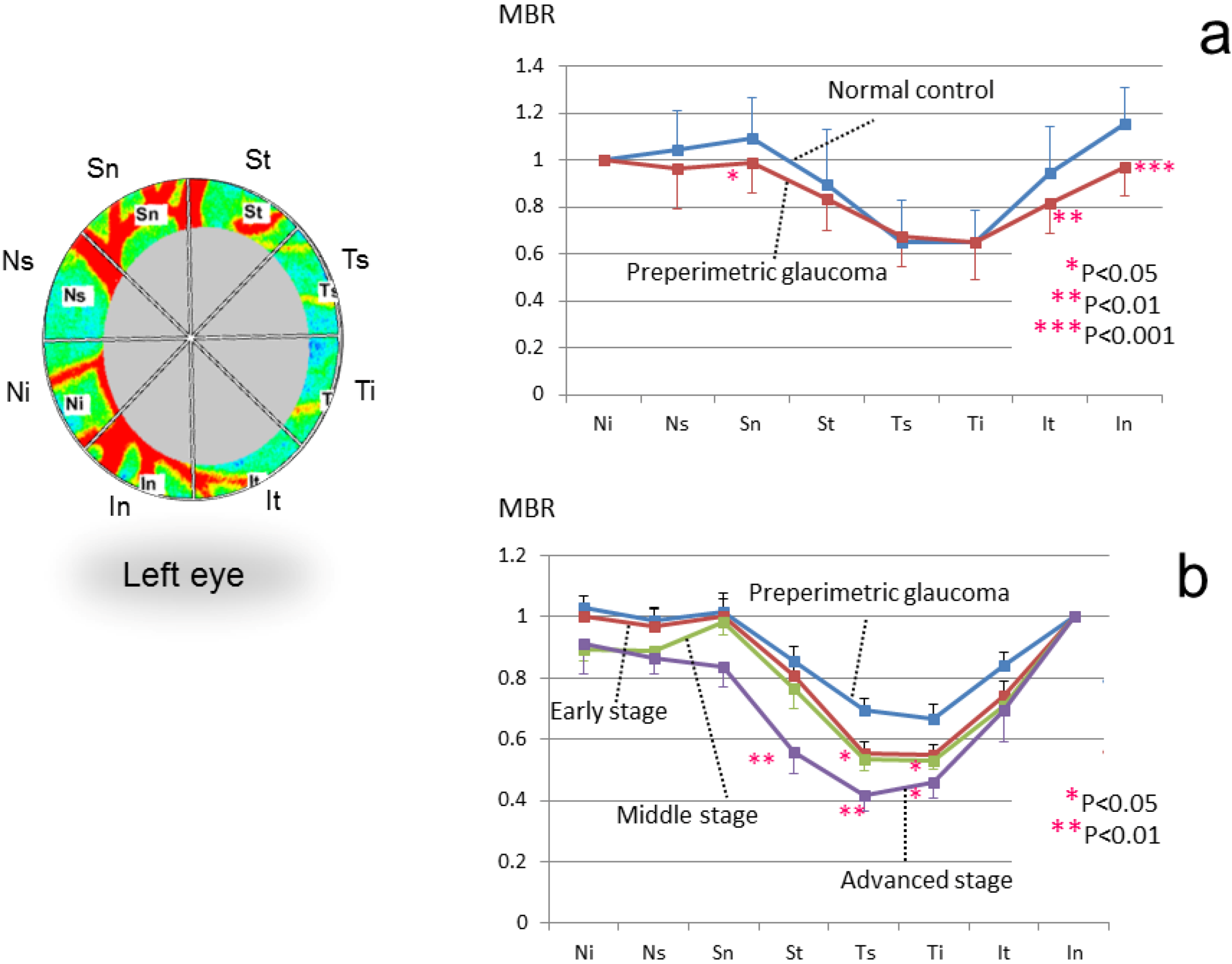
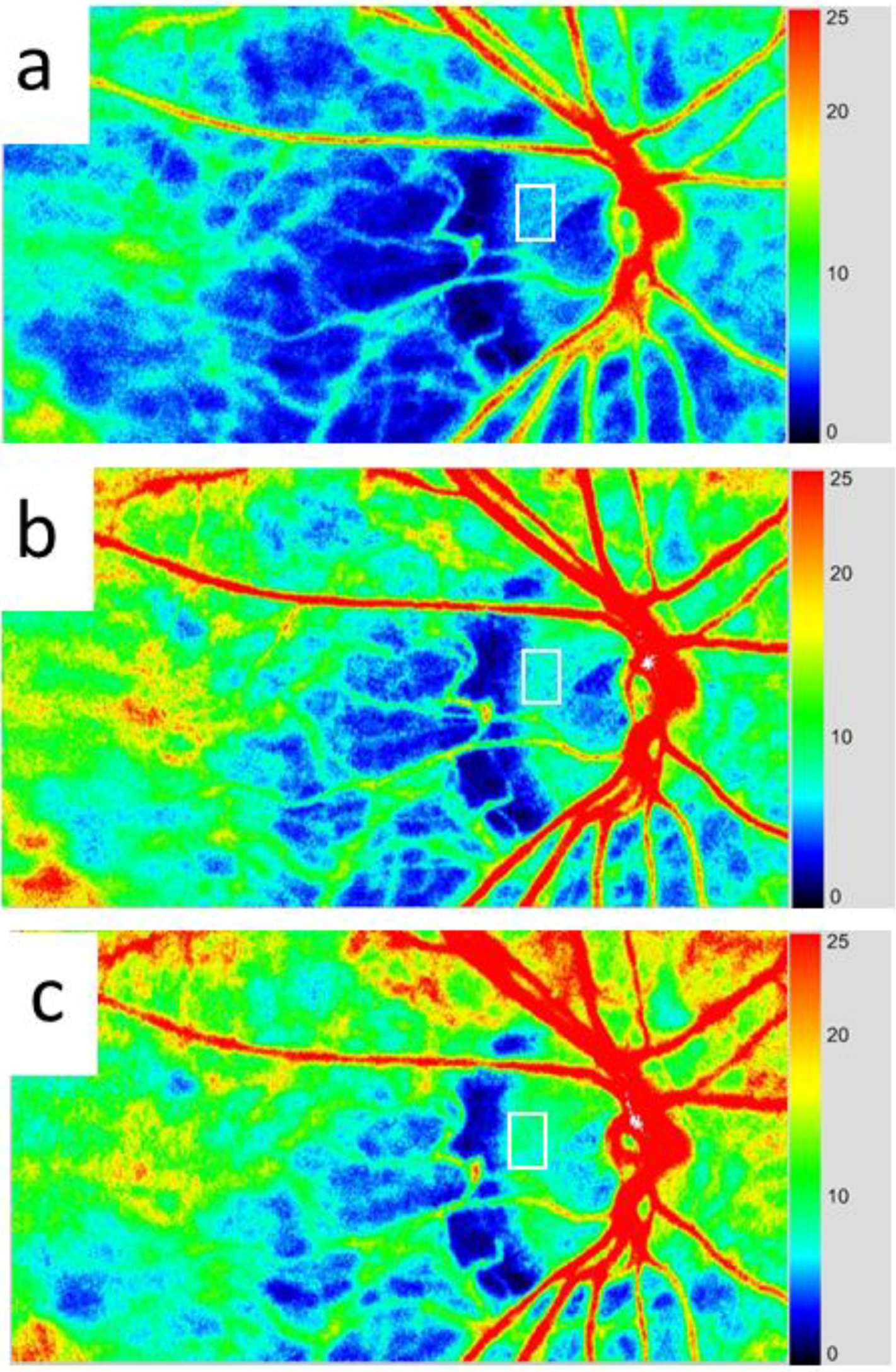
3.4. Application of LSFG-NAVI to Retinal and Choroidal Diseases
4. Conclusions
Conflicts of Interest
References
- Fercher, A.F.; Briers, J.D. Flow visualization by means of single-exposure speckle photography. Opt. Commun. 1981, 37, 326–330. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Kawamoto, E.; Fujii, H. Non-contact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Investig. Ophthalmol. Vis. Res. 1994, 35, 3825–3834. [Google Scholar]
- Tamaki, Y.; Araie, M.; Kawamoto, E.; Eguchi, S.; Fujii, H. Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp. Eye Res. 1995, 60, 373–384. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Tomita, K.; Nagahara, M.; Tomidokoro, A.; Fujii, H. Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn. J. Ophthalmol. 1997, 41, 49–54. [Google Scholar] [CrossRef]
- Fujii, H. Laser speckle blood flowgraphy. In Medical Diagnostic Techniques and Procedures; Narosa Publishing House: New Delhi, India; London, UK, 2000; pp. 216–224. [Google Scholar]
- Sugiyama, T.; Araie, M.; Riva, C.E.; Schmetterer, L.; Orgul, S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 2010, 88, 723–729. [Google Scholar] [CrossRef]
- Konishi, N.; Tokimoto, Y.; Kohra, K.; Fujii, H. New laser speckle flowgraphy system using CCD camera. Opt. Rev. 2002, 9, 163–169. [Google Scholar] [CrossRef]
- Ubuka, M.; Sugiyama, T.; Onoda, Y.; Shiba, T.; Hori, Y.; Maeno, T. Changes in the blood flow of the optic nerve head induced by different concentrations of epinephrine in intravitreal infusion during vitreous surgery. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1625–1629. [Google Scholar] [CrossRef]
- Aizawa, N.; Yokoyama, Y.; Chiba, N.; Omodaka, K.; Yasuda, M.; Otomo, T.; Nakamura, M.; Fuse, N.; Nakazawa, T. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVIin patients with glaucoma. Clin. Ophthalmol. 2011, 5, 1171–1176. [Google Scholar]
- Tsuda, S.; Kunikata, H.; Shimura, M.; Aizawa, N.; Omodaka, K.; Shiga, Y.; Yasuda, M.; Yokoyama, Y.; Nakazawa, T. Pulse-wave analysis of normal population using laser speckle flowgraphy. Curr. Eye Res. 2014, in press. [Google Scholar]
- Shiga, Y.; Asano, T; Kunikata, H.; Nitta, F.; Sato, H.; Nakazawa, T.; Shimura, M. Relative flow volume, a novel blood flow index in the human retina derived from laser speckle flowgraphy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3899–3904. [Google Scholar] [CrossRef]
- Takahashi, H.; Sugiyama, T.; Tokushige, H.; Maeno, T.; Nakazawa, T.; Ikeda, T.; Araie, M. Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp. Eye Res. 2013, 108, 10–15. [Google Scholar] [CrossRef]
- Liang, Y.; Fortune, B.; Cull, G.; Cioffi, G.A.; Wang, L. Quantification of dynamic blood flow autoregulation in optic nerve head of rhesus monkeys. Exp. Eye Res. 2010, 90, 203–209. [Google Scholar]
- Shiga, Y.; Omodaka, K.; Kunikata, H.; Ryu, M.; Yokoyama, Y.; Tsuda, S.; Asano, T.; Maekawa, S.; Maruyama, K.; Nakazawa, T. Waveform analysis of ocular blood flow and the early detection of normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7699–7706. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Hori, Y.; Maeno, T.; Shirai, K. Optic nerve head circulation determined by pulse wave analysis is significantly correlated with cardio ankle vascular index, left ventricular diastolic function, and age. J. Atheroscler. Thromb. 2012, 19, 999–1005. [Google Scholar]
- Shiba, T.; Takahashi, M.; Hori, Y.; Maeno, T. Pulse-wave analysis of optic nerve head circulation is significantly correlated with brachial-ankle pulse-wave velocity, carotid intima-media thickness, and age. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1275–1281. [Google Scholar] [CrossRef]
- Shiba, T.; Takahashi, M.; Maeno, T. Pulse-wave analysis of optic nerve head circulation is significantly correlated with kidney function in patients with and without chronic kidney disease. J. Ophthalmol. 2014, 2014, 291687. [Google Scholar]
- Tamura, A.; Kogure, A.; Watanabe, G.; Kishi, S.; Hori, S. Association between age and chorioretinal hemodynamics in normal volunteers examined with laser speckle flowgraphy. J. Jpn. Ophthalmol. Soc. 2013, 117, 110–116. [Google Scholar]
- Wang, L.; Cull, G.A.; Piper, C.; Burgoyne, C.F.; Fortune, B. Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and Microsphere Method. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8303–8309. [Google Scholar] [CrossRef]
- Cull, G.; Burgoyne, C.F.; Fortune, B.; Wang, L. Longitudinal hemodynamic changes within the optic nerve head in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4271–4277. [Google Scholar] [CrossRef]
- Shibata, M.; Sugiyama, T.; Kojima, S.; Ueki, M.; Ikeda, T. Sectorial analysis of optic nerve head rim blood flow using laser speckle flowgraphy. J. Eye 2010, 27, 1279–1285. [Google Scholar]
- Yokoyama, Y.; Aizawa, N.; Chiba, N.; Omodaka, K.; Nakamura, M.; Otomo, T.; Yokokura, S.; Fuse, N.; Nakazawa, T. Significant correlations between optic nerve head microcirculation and visual field defects and nerve fiber layer loss in glaucoma patients with myopic glaucomatous disk. Clin. Ophthalmol. 2011, 5, 1721–1727. [Google Scholar]
- Chiba, N.; Omodaka, K.; Yokoyama, Y.; Aizawa, N.; Tsuda, S.; Yasuda, M.; Otomo, T.; Yokokura, S.; Fuse, N.; Nakazawa, T. Association between optic nerve blood flow and objective examinations in glaucoma patients with generalized enlargement disc type. Clin. Ophthalmol. 2011, 5, 1549–1556. [Google Scholar]
- Aizawa, N.; Kunikata, H.; Yokoyama, Y.; Nakazawa, T. Correlation between optic disc microcirculation in glaucoma measured with laser speckle flowgraphy and fluorescein angiography, and the correlation with mean deviation. Clin. Exp. Ophthalmol. 2013, 42, 293–294. [Google Scholar]
- Sugiyama, T.; Shibata, M.; Kojima, S.; Ueki, M.; Ikeda, T. Analysis of waveforms obtained from periodic change in optic nerve head blood flow of glaucoma patients using laser speckle flowgraphy-NAVI™. J. Eye 2012, 29, 984–987. [Google Scholar]
- Shibata, M.; Sugiyama, T.; Kojima, S.; Ueki, M.; Ikeda, T. Optic nerve head blood flow changes induced by long-acting beta-blocker addition to latanoprost in primary open-angle glaucoma. J. Eye 2011, 28, 1017–1021. [Google Scholar]
- Sugiyama, T.; Shibata, M.; Kojima, S.; Ueki, M.; Ikeda, T. Changes in microcirculation in the optic nerve head following topical tafluprost in eyes with primary open-angle glaucoma. Jpn. J. Clin. Ophthalmol. 2011, 65, 475–479. [Google Scholar]
- Tsuda, S.; Yokoyama, Y.; Chiba, N.; Aizawa, N.; Shiga, Y.; Yasuda, M.; Yokokura, S.; Otomo, T.; Fuse, N.; Nakazawa, T. Effect of topical tafluprost on optic nerve head blood flow in patients with myopic disc type. J. Glaucoma 2013, 22, 398–403. [Google Scholar] [CrossRef]
- Kojima, S.; Sugiyama, T.; Shibata, M.; Ueki, M.; Komoto, R.; Ikeda, T. Changes in visual field, morphology and microcirculation of the optic nerve head after instillation of tafluprost for one year. Jpn. J. Clin. Ophthalmol. 2014, 68, 895–902. [Google Scholar]
- Aizawa, N.; Kunikata, H.; Omodaka, K.; Nakazawa, T. Optic disc microcirculation in superior segmental optic hypoplasia assessed with laser speckle flowgraphy. Clin. Exp. Ophthalmol. 2014. [Google Scholar] [CrossRef]
- Maekubo, T.; Chuman, H.; Nao-I, N. Laser speckle flowgraphy for differentiating between nonarteritic ischemic optic neuropathy and anterior optic neuritis. Jpn. J. Ophthalmol. 2013, 57, 385–390. [Google Scholar] [CrossRef]
- Isono, H.; Kishi, S.; Kimura, Y.; Hagiwara, N.; Konishi, N.; Fujii, H. Observation of choroidal circulation using index of erythrocytic velocity. Arch. Ophthalmol. 2003, 121, 225–231. [Google Scholar] [CrossRef]
- Watanabe, G.; Fujii, H.; Kishi, S. Imaging of choroidal hemodynamics in eyes with polypoidal choroidal vasculopathy using laser speckle phenomenon. Jpn. J. Ophthalmol. 2008, 52, 175–181. [Google Scholar] [CrossRef]
- Hirose, S.; Saito, W.; Yoshida, K.; Saito, M.; Dong, Z.; Namba, K.; Satoh, H.; Ohno, S. Elevated choroidal blood flow velocity during systemic corticosteroid therapy in Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2008, 86, 902–907. [Google Scholar]
- Maeda, K.; Ishikawa, F.; Ohguro, H. Ocular blood flow levels and visual prognosis in a patient with nonischemic type central retinal vein occlusion. Clin. Ophthalmol. 2009, 3, 489–491. [Google Scholar] [CrossRef]
- Enaida, H.; Okamoto, K.; Fujii, H.; Ishibashi, T. LSFG findings of proliferative diabetic retinopathy after intravitreal injection of bevacizumab. Ophthalmic Surg. Lasers Imaging 2010, 41, e1–e3. [Google Scholar]
- Hashimoto, Y.; Saito, W.; Mori, S.; Saito, M.; Ishida, S. Increased macular choroidal blood flow velocity during systemic corticosteroid therapy in a patient with acute macular neuroretinopathy. Clin. Ophthalmol. 2012, 6, 1645–1649. [Google Scholar]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Fujiya, A.; Noda, K.; Ishida, S. Macular choroidal blood flow velocity decreases with regression of acute central serous chorioretinopathy. Br. J. Ophthalmol. 2013, 97, 775–780. [Google Scholar] [CrossRef]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Shinmei, Y.; Noda, K.; Ishida, S. Correlation between decreased choroidal blood flow velocity and the pathogenesis of acute zonal occult outer retinopathy. Clin. Exp. Ophthalmol. 2014, 42, 139–150. [Google Scholar]
- Hirooka, K.; Saito, W.; Hashimoto, Y.; Saito, M.; Ishida, S. Increased macular choroidal blood flow velocity and decreased choroidal thickness with regression of punctate inner choroidopathy. BMC Ophthalmol. 2014, 14, 73. [Google Scholar] [CrossRef]
- Nitta, F.; Kunikata, H.; Aizawa, N.; Omodaka, K.; Shiga, Y.; Yasuda, M.; Nakazawa, T. The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin. Ophthalmol. 2014, 8, 1119–1127. [Google Scholar]
- Matsumoto, M.; Suzuma, K.; Fukazawa, Y.; Yamada, Y.; Tsuiki, E.; Fujikawa, A.; Kitaoka, T. Retinal blood flow levels measured by Laser Speckle Flowgraphy in patients who received intravitreal bevacizumab injection for macular edema secondary to central retinal vein occlusion. Retinal Cases Brief Rep. 2014, 8, 1–87. [Google Scholar] [CrossRef]
- Saito, M.; Yoshida, K.; Saito, W.; Fujiya, A.; Ohgami, K.; Kitaichi, N.; Tsukahara, H.; Ishida, S.; Ohno, S. Astaxanthin increases choroidal blood flow velocity. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 239–245. [Google Scholar] [CrossRef]
- Akiyama, M.; Ikeda, Y.; Yoshida, N.; Notomi, S.; Murakami, Y.; Hisatomi, T.; Enaida, H.; Ishibashi, T. Therapeutic efficacy of topical unoprostone isopropyl in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, e229–e234. [Google Scholar] [CrossRef]
- Okamoto, M.; Matsuura, T.; Ogata, N. Ocular blood flow before, during, and after vitrectomy determined by laser speckle flowgraphy. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 118–124. [Google Scholar]
© 2014 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sugiyama, T. Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases. Photonics 2014, 1, 220-234. https://doi.org/10.3390/photonics1030220
Sugiyama T. Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases. Photonics. 2014; 1(3):220-234. https://doi.org/10.3390/photonics1030220
Chicago/Turabian StyleSugiyama, Tetsuya. 2014. "Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases" Photonics 1, no. 3: 220-234. https://doi.org/10.3390/photonics1030220