Photothermal Microscopy for High Sensitivity and High Resolution Absorption Contrast Imaging of Biological Tissues
Abstract
:1. Introduction
2. Experimental
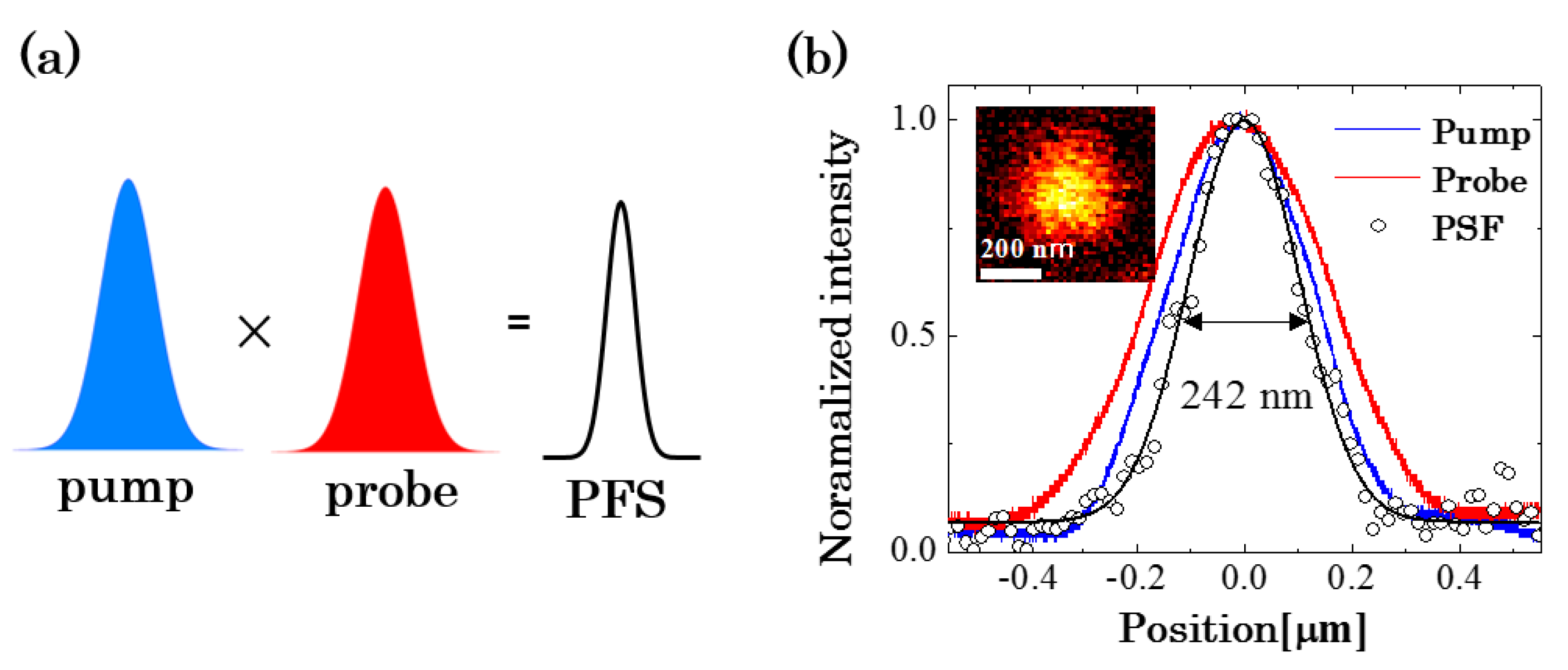
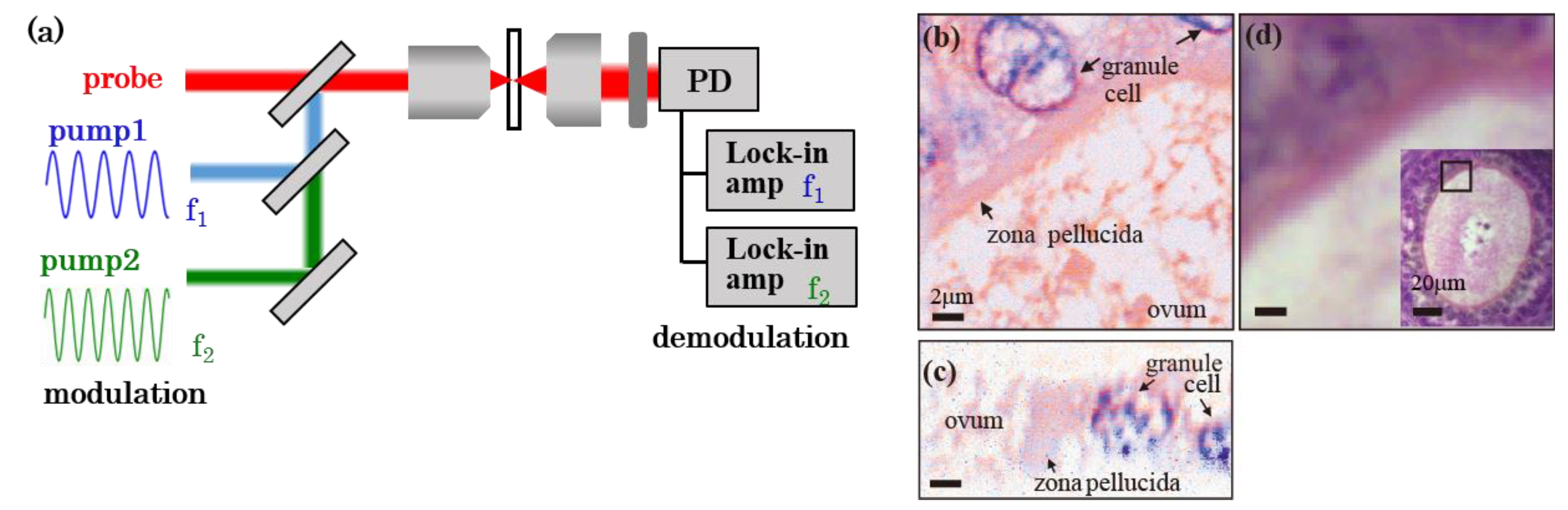
2.1. Principle of PT Microscopy
2.2. Experimental Setup
3. Results and Discussion
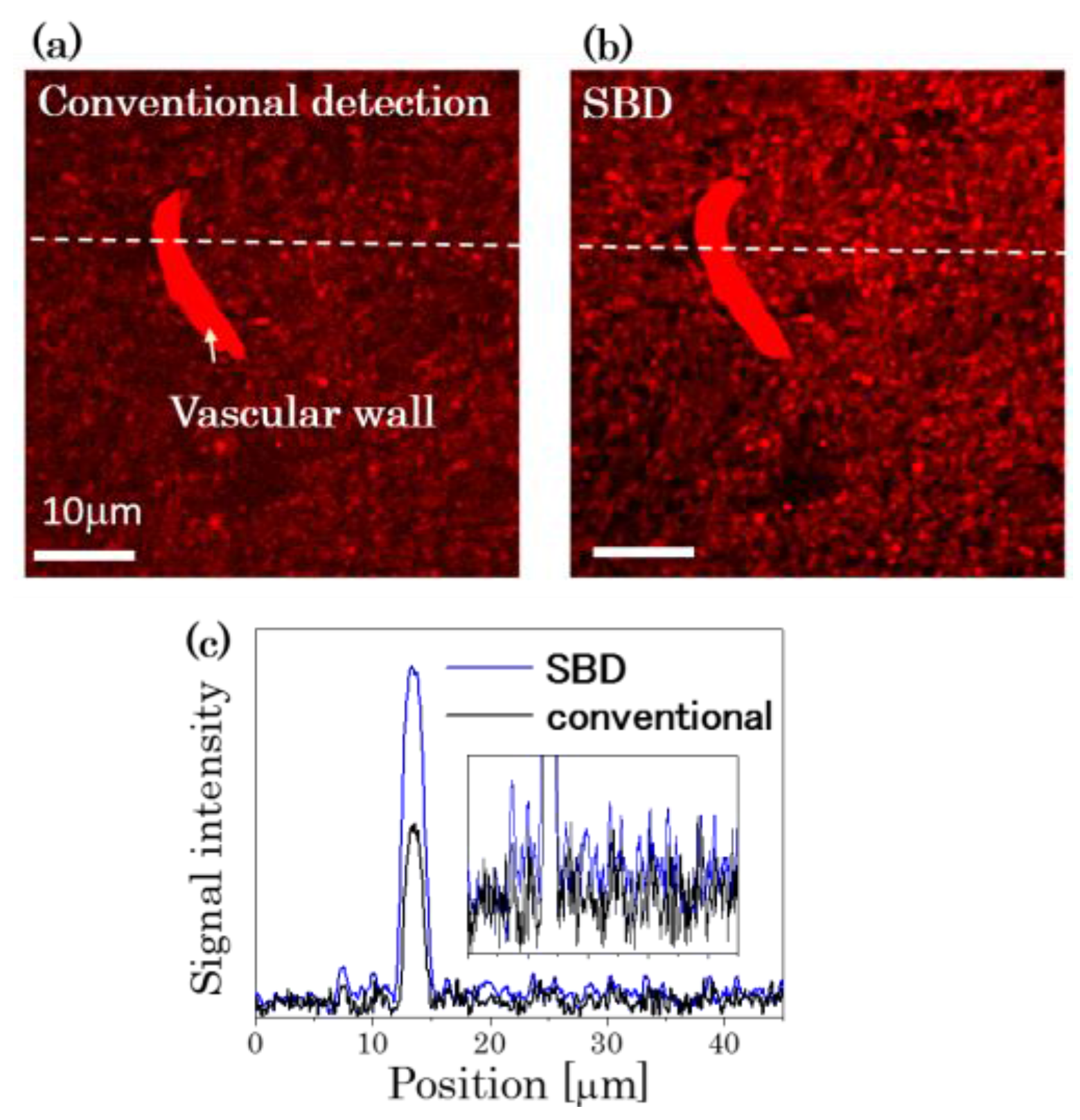
3.1. Improvement in SNR by the Spatially Segmented Balanced Deteciton
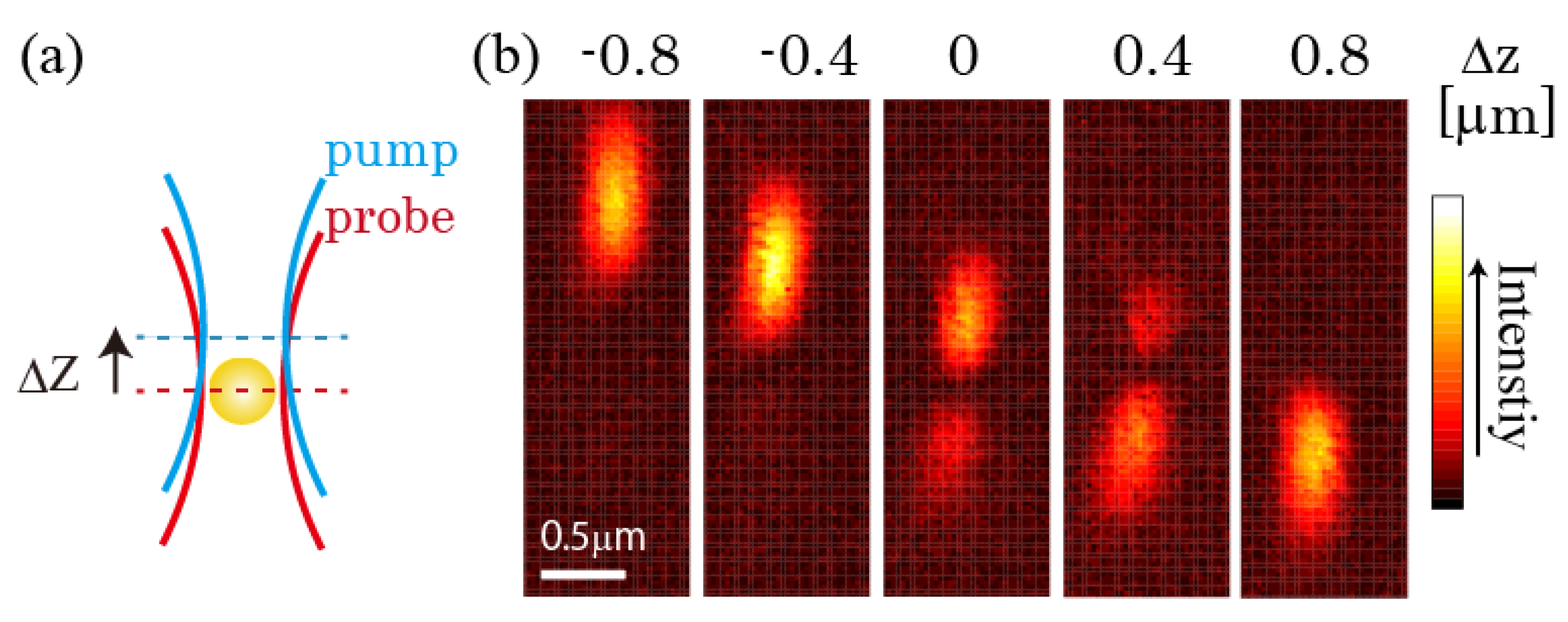
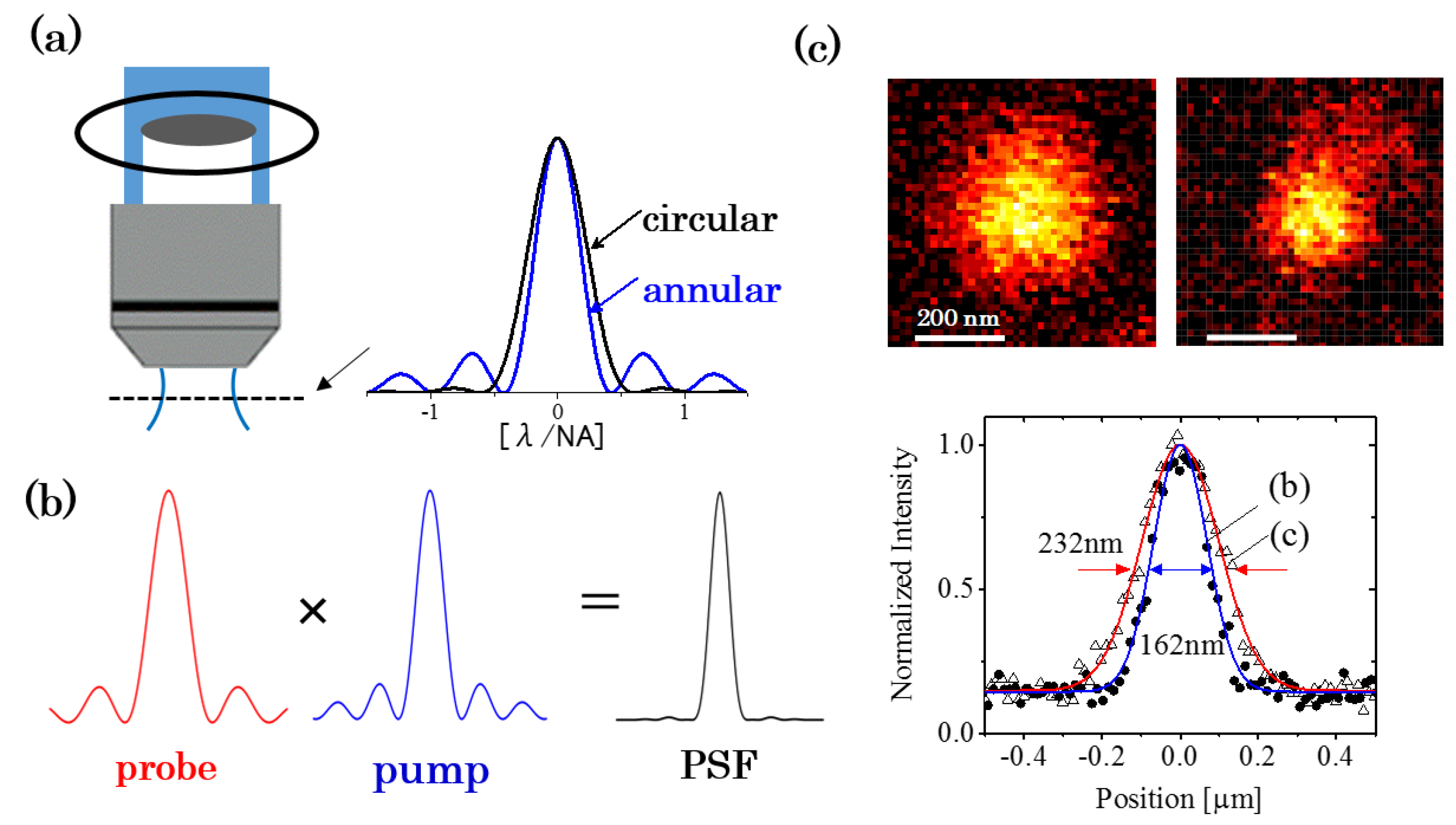
3.2. Improvement in the Spatial Resolution
3.3. Multiwavelength Imaging
3.4. Multimodal Imaging of Biological Tissue
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tamaki, E.; Sato, K.; Tokeshi, M.; Sato, K.; Aihara, M.; Kitamori, T. Single-cell analysis by a scanning thermal lens microscope with a microchip: Direct monitoring of cytochrome c distribution during apoptosis process. Anal. Chem. 2002, 74, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Lasne, D.; Blab, G.A.; De Giorgi, F.; Ichas, F.; Lounis, B.; Cognet, L. Label-free optical imaging of mitochondria in live cells. Opt. Express 2007, 15, 14184–14193. [Google Scholar] [CrossRef] [PubMed]
- Brusnichkin, A.V.; Nedosekin, D.A.; Galanzha, E.I.; Vladimirov, Y.A.; Shevtsova, E.F.; Proskurnin, M.A.; Zharov, V.P. Ultrasensitive label-free photothermal imaging, spectral identification, and quantification of cytochrome c in mitochondria, live cells, and solutions. J. Biophotonics 2010, 3, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Nedosekin, D.A.; Galanzha, E.I.; Ayyadevara, S.; Shmookler Reis, R.J.; Zharov, V.P. Photothermal confocal spectromicroscopy of multiple cellular chromophores and fluorophores. Biophys. J. 2012, 102, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Min, W.; Chong, S.; Holtom, G.R.; Xie, X.S. Label-free imaging of heme proteins with two-photon excited photothermal lens microscopy. Appl. Phys. Lett. 2010, 96, 113701. [Google Scholar] [CrossRef]
- Tong, L.; Liu, Y.; Dolash, B.D.; Jung, Y.; Slipchenko, M.N.; Bergstrom, D.E.; Cheng, J.X. Label-free imaging of semiconducting and metallic carbon nanotubes in cells and mice using transient absorption microscopy. Nat. Nanotechnol. 2012, 7, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nedosekin, D.A.; Shashkov, E.V.; Galanzha, E.I.; Hennings, L.; Zharov, V.P. Photothermal multispectral image cytometry for quantitative histology of nanoparticles and micrometastasis in intact, stained and selectively burned tissues. Cytom. Part A 2010, 77, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Cognet, L.; Tardin, C.; Boyer, D.; Choquet, D.; Tamarat, P.; Lounis, B. Single metallic nanoparticle imaging for protein detection in cells. Proc. Natl. Acad. Sci. USA 2003, 100, 11350–11355. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Jung, J.M.; Carney, R.P.; Stellacci, F.; Lounis, B. Direct investigation of intracellular presence of gold nanoparticles via photothermal heterodyne imaging. ACS Nano 2011, 5, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Si, S.; Gautier, J.; Soto-Ribeiro, M.; Wehrle-Haller, B.; Gautreau, A.; Giannone, G.; Cognet, L.; Lounis, B. A highly specific gold nanoprobe for live-cell single-molecule imaging. Nano Lett. 2013, 13, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Lasne, D.; Blab, G.A.; Berciaud, S.; Heine, M.; Groc, L.; Choquet, D.; Cognet, L.; Lounis, B. Single nanoparticle photothermal tracking (SNaPT) of 5-nm gold beads in live cells. Biophys. J. 2006, 91, 4598–4604. [Google Scholar] [CrossRef] [PubMed]
- Octeau, V.; Cognet, L.; Duchesne, L.; Lasne, D.; Schaeffer, N.; Fernig, D.G.; Lounis, B. Photothermal absorption correlation spectroscopy. ACS Nano 2009, 3, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, P.; Cognet, L.; Lounis, B. Photothermal microscopy: Optical detection of small absorbers in scattering environments. J. Microsc. 2014, 254, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Min, W. Pump-probe optical microscopy for imaging nonfluorescent chromophores. Anal. Bioanal. Chem. 2012, 403, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Selmke, M.; Braun, M.; Cichos, F. Nano-lens diffraction around a single heated nano particle. Opt. Express 2012, 20, 8055. [Google Scholar] [CrossRef] [PubMed]
- Selmke, M.; Braun, M.; Cichos, F. Photothermal single-particle microscopy: Detection of a nanolens. ACS Nano 2012, 6, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Paulo, P.M.R.; Gaiduk, A.; Kulzer, F.; Krens, S.F.G.; Spaink, H.P.; Schmidt, T.; Orrit, M. Photothermal correlation spectroscopy of gold nanoparticles in solution. J. Phys. Chem. C 2009, 113, 11451–11457. [Google Scholar] [CrossRef]
- Chang, W.-S.; Link, S. Enhancing the sensitivity of single-particle photothermal imaging with thermotropic liquid crystals. J. Phys. Chem. Lett. 2012, 3, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Parra-Vasquez, A.N.G.; Oudjedi, L.; Cognet, L.; Lounis, B. Nanoscale thermotropic phase transitions enhancing photothermal microscopy signals. J. Phys. Chem. Lett. 2012, 3, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Proskurnin, M.A.; Volkov, D.S.; Gor'kova, T.A.; Bendrysheva, S.N.; Smirnova, A.P.; Nedosekin, D.A. Advances in thermal lens spectrometry. J. Anal. Chem. 2015, 70, 249–276. [Google Scholar] [CrossRef]
- Uchiyama, K.; Hibara, A.; Kimura, H.; Sawada, T.; Kitamori, T. Thermal lens microscope. Jpn. J. Appl. Phys. 2000, 39, 5316–5322. [Google Scholar] [CrossRef]
- Miyazaki, J.; Tsurui, H.; Kawasumi, K.; Kobayashi, T. Sensitivity enhancement of photothermal microscopy with radially segmented balanced detection. Opt. Lett. 2015, 40, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Lu, S.; Chong, S.; Roy, R.; Holtom, G.R.; Xie, X.S. Imaging chromophores with undetectable fluorescence by stimulated emission microscopy. Nature 2009, 461, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, Y.; Kitagawa, Y.; Sumimura, K.; Nishizawa, N.; Umemura, W.; Kajiyama, S.; Fukui, K.; Itoh, K. Stimulated Raman scattering microscope with shot noise limited sensitivity using subharmonically synchronized laser pulses. Opt. Express 2010, 18, 13708–13719. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Cognet, L.; Blab, G.A.; Lounis, B. Photothermal heterodyne imaging of individual nonfluorescent nanoclusters and nanocrystals. Phys. Rev. Lett. 2004, 93, 257402. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Lasne, D.; Blab, G.A.; Cognet, L.; Lounis, B. Photothermal heterodyne imaging of individual metallic nanoparticles: Theory versus experiment. Phys. Rev. B 2006, 73, 045424. [Google Scholar] [CrossRef]
- Selmke, M.; Heber, A.; Braun, M.; Cichos, F. Photothermal single particle microscopy using a single laser beam. Appl. Phys. Lett. 2014, 105, 013511. [Google Scholar] [CrossRef]
- Seto, K.; Okuda, Y.; Tokunaga, E.; Kobayashi, T. Multiplex stimulated Raman imaging with white probe-light from a photonic-crystal fibre and with multi-wavelength balanced detection. J. Phys. D Appl. Phys. 2014, 47, 345401. [Google Scholar] [CrossRef]
- Celebrano, M.; Kukura, P.; Renn, A.; Sandoghdar, V. Single-molecule imaging by optical absorption. Nat. Photonics 2011, 5, 95–98. [Google Scholar] [CrossRef]
- Hu, C.R.; Slipchenko, M.N.; Wang, P.; Wang, P.; Lin, J.D.; Simpson, G.; Hu, B.; Cheng, J.X. Stimulated Raman scattering imaging by continuous-wave laser excitation. Opt. Lett. 2013, 38, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Freudiger, C.W.; Yang, W.; Holtom, G.R.; Peyghambarian, N.; Xie, X.S.; Kieu, K.Q. Stimulated Raman scattering microscopy with a robust fibre laser source. Nat. Photonics 2014, 8, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Iida, T.; Tanaka, S.; Hayashi-Takagi, A.; Kasai, H.; Okabe, S.; Kobayashi, T. Fast 3D visualization of endogenous brain signals with high-sensitivity laser scanning photothermal microscopy. Biomed. Opt. Express 2016, 7, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J. Improvement of signal-to-noise ratio in photothermal microscopy by optimizing detection aperture. Opt. Commun. 2017, 390, 99–104. [Google Scholar] [CrossRef]
- Miyazaki, J.; Tsurui, H.; Kobayashi, T. Reduction of distortion in photothermal microscopy and its application to the high-resolution three-dimensional imaging of nonfluorescent tissues. Biomed. Opt. Express 2015, 6, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Loriette, V. Confocal dual-beam thermal-lens microscope: Model and experimental results. Jpn. J. Appl. Phys. 2006, 45, 7141–7151. [Google Scholar] [CrossRef]
- Moreau, J.; Loriette, V. Confocal thermal-lens microscope. Opt. Lett. 2004, 29, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Serrels, K.A.; Ramsay, E.; Reid, D.T. 70 nm resolution in subsurface optical imaging of silicon integrated-circuits using pupil-function engineering. Appl. Phys. Lett. 2009, 94, 073113. [Google Scholar] [CrossRef]
- Hell, S.W.; Hanninen, P.E.; Kuusisto, A.; Schrader, M.; Soini, E. Annular aperture two-photon excitation microscopy. Opt. Commun. 1995, 117, 20–24. [Google Scholar] [CrossRef]
- Mondal, P.P.; Diaspro, A. Lateral resolution improvement in two-photon excitation microscopy by aperture engineering. Opt. Commun. 2008, 281, 1855–1859. [Google Scholar] [CrossRef]
- Sick, B.; Hecht, B.; Novotny, L. Orientational imaging of single molecules by annular illumination. Phys. Rev. Lett. 2000, 85, 4482–4485. [Google Scholar] [CrossRef] [PubMed]
- Botcherby, E.J.; Juškaitis, R.; Wilson, T. Scanning two photon fluorescence microscopy with extended depth of field. Opt. Commun. 2006, 268, 253–260. [Google Scholar] [CrossRef]
- Purnapatra, S.B.; Bera, S.; Mondal, P.P. Spatial filter based bessel-like beam for improved penetration depth imaging in fluorescence microscopy. Sci. Rep. 2012, 2, 692. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Tsurui, H.; Kawasumi, K.; Kobayashi, T. Simultaneous dual-wavelength imaging of nonfluorescent tissues with 3D subdiffraction photothermal microscopy. Opt. Express 2015, 23, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Gaiduk, A.; Ruijgrok, P.V.; Yorulmaz, M.; Orrit, M. Making gold nanoparticles fluorescent for simultaneous absorption and fluorescence detection on the single particle level. Phys. Chem. Chem. Phys. 2011, 13, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.C.; Duff, K.; Gouras, G.K.; Webb, W.W. Optical visualization of Alzheimer’s pathology via multiphoton-excited intrinsic fluorescence and second harmonic generation. Opt. Express 2009, 17, 3679–3689. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.R., Jr.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Tang, Y.; Zhou, S.; Toh, B.H.; McLean, C.; Li, H. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol. Cell. Neurosci. 2010, 43, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Benseny-Cases, N.; Klementieva, O.; Cotte, M.; Ferrer, I.; Cladera, J. Microspectroscopy (mu FTIR) reveals co-localization of lipid oxidation and amyloid plaques in human alzheimer disease brains. Anal. Chem. 2014, 86, 12047–12054. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, J.; Kobayahsi, T. Photothermal Microscopy for High Sensitivity and High Resolution Absorption Contrast Imaging of Biological Tissues. Photonics 2017, 4, 32. https://doi.org/10.3390/photonics4020032
Miyazaki J, Kobayahsi T. Photothermal Microscopy for High Sensitivity and High Resolution Absorption Contrast Imaging of Biological Tissues. Photonics. 2017; 4(2):32. https://doi.org/10.3390/photonics4020032
Chicago/Turabian StyleMiyazaki, Jun, and Takayoshi Kobayahsi. 2017. "Photothermal Microscopy for High Sensitivity and High Resolution Absorption Contrast Imaging of Biological Tissues" Photonics 4, no. 2: 32. https://doi.org/10.3390/photonics4020032





