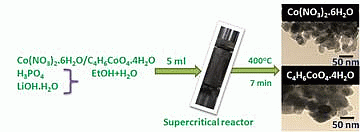
Supercritical Fluid Synthesis of LiCoPO4 Nanoparticles and Their Application to Lithium Ion Battery
Abstract
:1. Introduction
2. Results and Discussion
2.1. One Pot Synthesis

2.2. X-ray Powder Diffraction Analysis
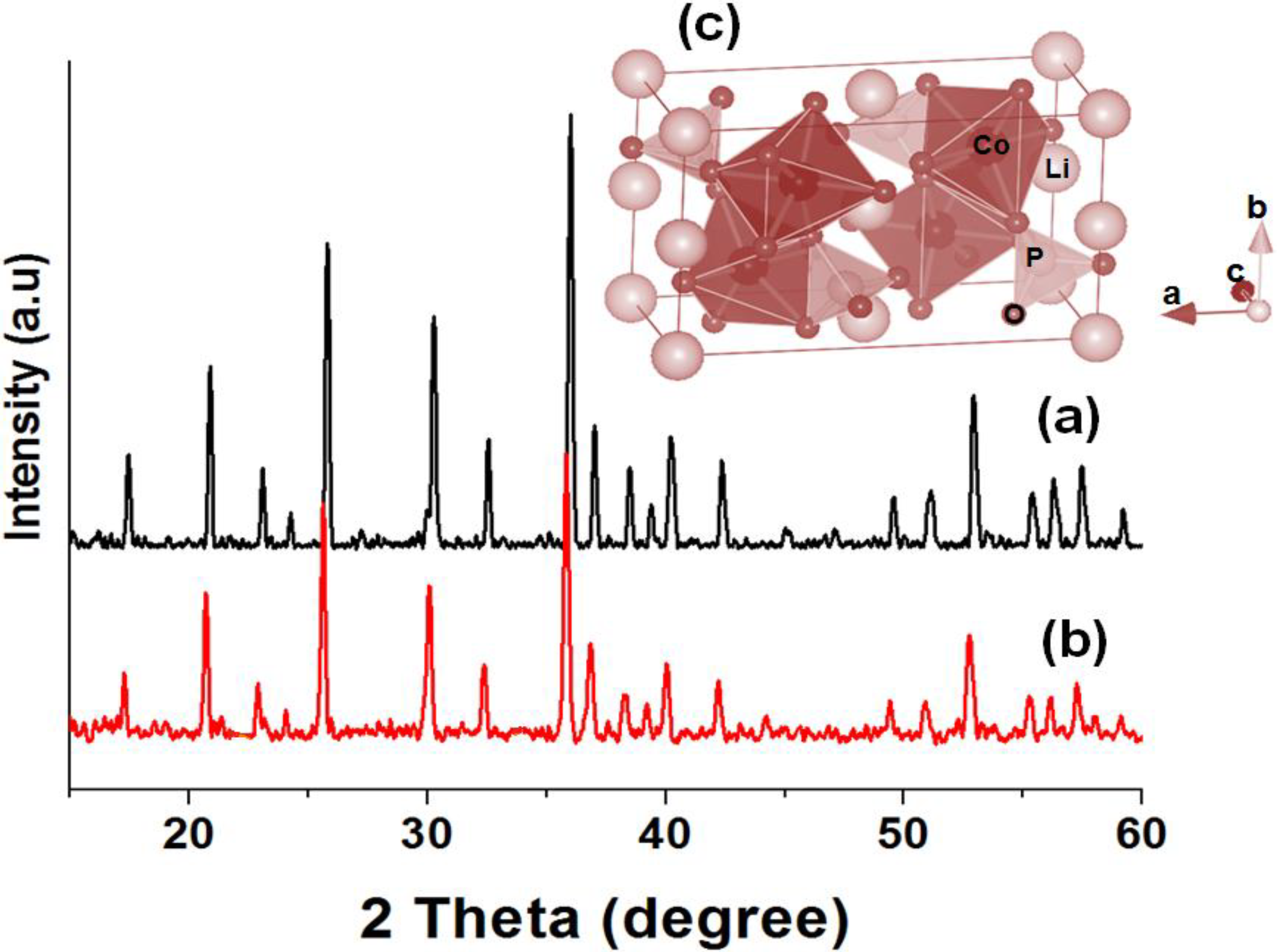
| Formula Crystal system Space group | LiCoPO4 (Using Co(NO3)2 6H2O) Orthorhombic Pnma | LiCoPO4 (Using C4H6CoO4 4H2O) Orthorhombic Pnma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atom | Site | X | Y | Z | g | B/Å2 | X | Y | Z | g | B/Å2 |
| Li | 4a | 0.5 | 0.5 | 0.5 | 1 | 0.8 | 0.5 | 0.5 | 0.5 | 1 | 0.8 |
| Co | 4c | 0.2778 | 0.25 | 0.9819 | 1 | 1.0 | 0.2783 | 0.25 | 0.9801 | 1 | 1.0 |
| P | 4c | 0.096 | 0.25 | 0.420 | 1 | 1.5 | 0.096 | 0.25 | 0.416 | 1 | 1.5 |
| O(1) | 4c | 0.091 | 0.25 | 0.736 | 1 | 1.7 | 0.085 | 0.25 | 0.728 | 1 | 1.7 |
| O(2) | 4c | 0.453 | 0.25 | 0.240 | 1 | 0.6 | 0.445 | 0.25 | 0.230 | 1 | 0.6 |
| O(3) | 8d | 0.160 | 0.029 | 0.278 | 1 | 0.9 | 0.157 | 0.0276 | 0.275 | 1 | 0.9 |
2.3. TEM and HRTEM Analysis

| Synthesis Temperature (°C) | Reaction Time (min) | Morphology | Particle size (nm) | XRD |
|---|---|---|---|---|
| 300 | 7,10 &15 | Sphere | 10-30 | nearly amorphous |
| 350 | 7,10 &15 | Sphere & rods | 10-30 | LiCoPO4, Li3PO4 |
| 400 | 7,10 &15 | Sphere, plates & rods | 10-30 | LiCoPO4 |
2.4. Elemental Mapping
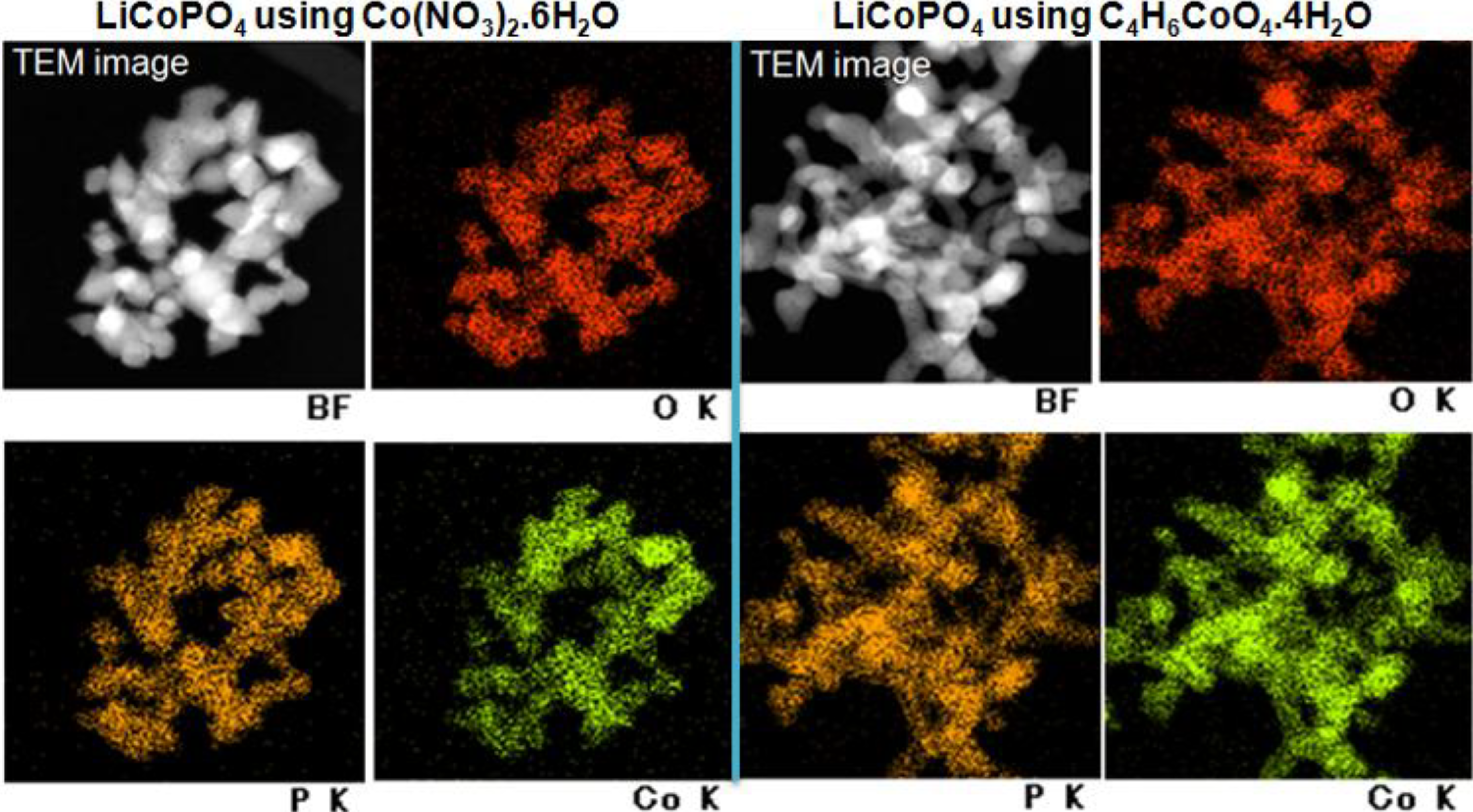
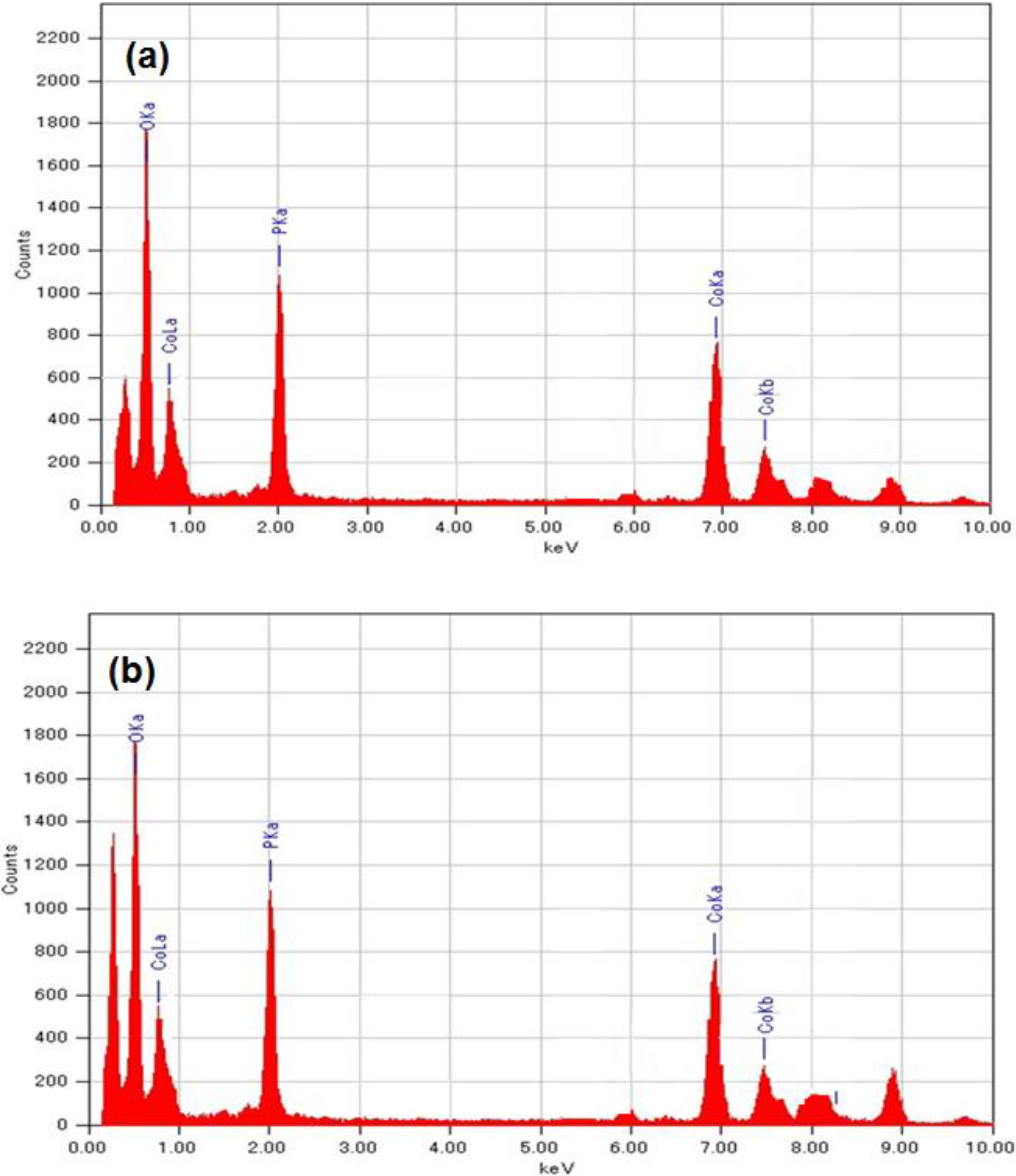
2.5. BET and TG-Analysis

2.6. Electrochemical Performance

3. Experimental Section
3.1. Synthesis of LiCoPO4 Nanoparticles
3.2. Material Characterization
3.3. Electrochemical Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ceder, G.; Chiang, Y.M.; Sadoway, D.R.; Aydinol, M.K.; Jang, Y.I.; Huang, B. Identification of cathode materials for lithium batteries guided by first-principles calculations. Nature 1998, 392, 694–696. [Google Scholar] [CrossRef]
- Andersson, A.M.; Abraham, D.P.; Haasch, R.; MacLaren, S.; Liu, J.; Amine, K. Surface characterization of electrodes from high power lithium-ion batteries. J. Electrochem. Soc. 2002, 149, A1358–A1369. [Google Scholar] [CrossRef]
- Chen, J.; Vacchio, M.J.; Wang, S.; Chernova, N.; Zavalij, P.Y.; Whittingham, M.S. The hydrothermal synthesis and characterization of olivines and related compounds for electrochemical applications. Solid State Ionics 2008, 178, 1676–1693. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.L.; Cao, M.H.; Guo, Y.G. Solvothermal synthesis of LiFePO4 hierarchically dumbbell-like microstructures by nanoplate self-assembly and their application as a cathode material in lithium-ion batteries. J. Phys. Chem. C 2009, 113, 3345–3351. [Google Scholar] [CrossRef]
- Delacourt, C.; Poizot, P.; Morcrette, M.; Tarascon, J.-M.; Masquelier, C. One-step low-temperature route for the preparation of electrochemically active LiMnPO4 powders. Chem. Mater. 2004, 16, 93–99. [Google Scholar]
- Fisher, C.A.J.; Prieto, V.M.H.; Islam, M.S. Lithium Battery Materials LiMPO4 (M = Mn, Fe, Co, Ni): Insights into Defect Association, Transport Mechanisms and Doping Behavior. Chem. Mater. 2008, 20, 5907–5915. [Google Scholar] [CrossRef]
- Ren, M.M.; Zhou, Z.; Gao, X.P.; Peng, W.X.; Wei, J.P. Core−shell Li3V2(PO4)3@C composites as cathode materials for lithium-ion batteries. J. Phys. Chem. C 2008, 112, 5689–5693. [Google Scholar] [CrossRef]
- Amine, A.; Yasuda, H.; Yamachi, M. Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries. Electrochem. Solid-State Lett. 2000, 3, 178. [Google Scholar]
- Rabanal, M.E.; Gutierrez, M.C.; Garcia-Alvarado, F.; Gonzalo, E.C.; Arroyo-de Dompablo, M.E. Improved electrode characteristics of olivine–LiCoPO4 processed by high energy milling. J. Power Sources 2006, 160, 523–528. [Google Scholar]
- Yang, J.; Xu, J.J. Synthesis and characterization of carbon-coated lithium transition metal phosphates LiMPO4 (M = Fe, Mn, Co, Ni) prepared via a nonaqueous sol-gel route. J. Electrochem. Soc. 2006, 153, A716–A723. [Google Scholar] [CrossRef]
- Piana, M.; Arrabito, M.; Bodoardo, S.; D’Epifanio, A.; Satolli, D.; Croce, F.; Scrosati, B. Characterization of phospho-olivines as materials for Li-ion cell cathodes. Ionics 2002, 8, 17–26. [Google Scholar]
- Wang, D.; Wang, Z.; Huang, X.; Chen, L. Cracking causing cyclic instability of LiFePO4 cathode material. J. Power Sources 2005, 146, 580–583. [Google Scholar] [CrossRef]
- Ruffo, R.; Mari, C.M.; Morazzoni, F.; Rosciano, F.; Scotti, R. Electrical and electrochemical behaviour of several LiFexCo1 − xPO4 solid solutions as cathode materials for lithium ion batteries. Ionics 2007, 13, 287–291. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Read, J.; Allen, J.L. Effect of carbon on the electronic conductivity and discharge capacity LiCoPO4. J. Power Sources 2007, 163, 1070–1073. [Google Scholar] [CrossRef]
- Jang, I.C.; Son, C.G.; Yang, A.J.W.; Lee, S.M.G.; Cho, A.R.; Aravindan, V.; Park, G.J.; Kang, K.S.; Kim, W.S.; Cho, W.I.; et al. LiFePO4 modified Li1.02(Co0.9Fe0.1)0.98PO4 cathodes with improved lithium storage properties. J. Mater. Chem. 2011, 21, 6510–6514. [Google Scholar] [CrossRef]
- Ju, H.; Wu, J.; Xu, Y. Lithium ion intercalation mechanism for LiCoPO4 electrode. Int. J. Energy Env. Eng. 2013, 4, 22. [Google Scholar] [CrossRef]
- Yujuan, Z.; Suijun, W.; Chunsong, Z.; Dingguo, X. Synthesis and electrochemical performance of LiCoPO4 micron-rods by dispersant-aided hydrothermal method for lithium ion batteries. Rare Met. 2009, 28, 117–121. [Google Scholar] [CrossRef]
- Huang, X.; Ma, J.; Wu, P.; Hu, Y.; Dai, J.; Zhu, Z.; Chen, H.; Wang, H. Hydrothermal synthesis of LiCoPO4 cathode materials for rechargeable lithium ion batteries. Mater. Lett. 2005, 59, 578–582. [Google Scholar] [CrossRef]
- Kotobuki, M. Hydrothermal synthesis of carbon-coated LiCoPO4 cathode material from various Co sources. Int. J. Energy Env. Eng. 2013, 4, 25. [Google Scholar] [CrossRef]
- Gangulibabu, D.; Bhuvaneswari, N.; Kalaiselvi, N.; Jayaprakash, P.; Periasamy, J. CAM sol–gel synthesized LiMPO4 (M=Co, Ni) cathodes for rechargeable lithium batteries. Sol–Gel Sci. Technol. 2009, 49, 137–144. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Nithya, V.D.; Karthikeyan, K.; Sanjeeviraja, K.C.; Lee, Y.S.; Kalai Selvan, R. Physicochemical properties of V5+ doped LiCoPO4 as cathode materials for Li-ion batteries. J. Sol-Gel Sci. Technol. 2013, 65, 399–410. [Google Scholar] [CrossRef]
- Sahnmukaraj, D.; Murugan, R. Synthesis and characterization of LiNiyCo1−yPO4 (y = 0–1) cathode materials for lithium secondary batteries. Ionics 2004, 10, 88–92. [Google Scholar] [CrossRef]
- Saint-Martin, R.; Franger, S. Growth of LiCoPO4 single crystals using an optical floating-zone technique. J. Cryst. Growth 2008, 310, 861–864. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion diffusion kinetics in LiCoPO4 thin films deposited on NASICON-type glass ceramic electrolytes by magnetron sputtering. J. Power Sources 2009, 192, 689–692. [Google Scholar] [CrossRef]
- Shui, J.L.; Yu, Y.; Yang, X.F.; Chen, C.H. LiCoPO4-based ternary composite thin-film electrode for lithium secondary battery. Electrochem. Commun. 2006, 8, 1087–1091. [Google Scholar] [CrossRef]
- Li, H.H.; Jin, J.; Wei, J.P.; Zhou, Z.; Yan, J.J. Fast synthesis of core-shell LiCoPO4/C nanocomposite via microwave heating and its electrochemical Li intercalation performances. Electrochem. Commun. 2009, 11, 95–98. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Taniguchi, I. Cathode performance of LiMnPO4/C nanocomposites prepared by a combination of spray pyrolysis and wet ball-milling followed by heat treatment. J. Power Sources 2011, 196, 1399–1408. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Honma, I. Hydrothermal and solvothermal process towards development of LiMPO4 (M = Fe, Mn) nanomaterials for lithium-ion batteries. Adv. Energy Mater. 2012, 2, 284–297. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Sathish, M.; Honma, I. Handbook of Sustainable Engineering; Kauffman, J., Lee, K.-M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1149–1173. [Google Scholar]
- Devaraju, M.K.; Rangappa, D.; Honma, I. Controlled synthesis of plate-like LiCoPO4 nanoparticles via supercritical method and their electrode property. Electrochem. Acta 2012, 85, 548. [Google Scholar] [CrossRef]
- Hong, S.; Kim, S.J.; Chung, K.Y.; Chung, M.S.; Lee, B.G.; Kim, J. Continuous synthesis of lithium iron phosphate (LiFePO4) nanoparticles in supercritical water: Effect of mixing tee. J. Supercrit. Fluids 2013, 73, 70–79. [Google Scholar] [CrossRef]
- Adschiri, T.; Lee, Y.W.; Goto, M.; Takamib, S. Green materials synthesis with supercritical water. Green Chem. 2011, 13, 1380–1390. [Google Scholar] [CrossRef]
- Hong, S.; Kim, S.J.; Chung, K.Y.; Lee, Y.W.; Kim, J.; Sang, B. Continuous synthesis of lithium iron phosphate nanoparticles in supercritical water: Effect of process parameters. Chem. Eng. J. 2013, 229, 313–323. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, S.J.; Kyung, W.C.; Chung, Y.; Kim, J. Facile synthesis of hierarchical mesoporous Li4Ti5O12 microspheres in supercritical methanol. J. Power Sources 2013, 244, 164–169. [Google Scholar] [CrossRef]
- Nugroho, A.; Kim, S.J.; Chung, K.Y.; Kim, J. Synthesis of Li4Ti5O12 in Supercritical water for Li-Ion batteries: Reaction mechanism and high-rate performance. Electrochim. Acta 2012, 78, 623–632. [Google Scholar] [CrossRef]
- Nugroho, A.; Chang, W.; Kim, S.J.; Chung, K.Y.; Kim, J. Superior high rate performance of core–shell Li4Ti5O12/Carbon Nanocomposite synthesized by a supercritical alcohol approach. RSC Adv. 2012, 2, 10805–10808. [Google Scholar] [CrossRef]
- Jensen, K.; Christensen, M.; Tyrsted, C.; Brummerstedt Iversen, B. Real-time synchrotron powder X-ray diffraction study of the antisite defect formation during sub- and supercritical synthesis of LiFePO4 and LiFe1−xMnx PO4 nanoparticles. J. Appl. Crystallogr. 2011, 44, 287–294. [Google Scholar] [CrossRef]
- Rangappa, D.; Sone, K.; Kudo, T.; Honma, I. Directed growth of nanoarchitectured LiFePO4 electrode by solvothermal synthesis and their cathode properties. J. Power Sources 2010, 195, 6167–6171. [Google Scholar] [CrossRef]
- Rangappa, D.; Sone, K.; Ichihara, M.; Kudo, T.; Honma, I. Rapid one-pot synthesis of LiMPO4 (M = Fe, Mn) colloidal nanocrystals by supercritical ethanol process. Chem. Commun. 2010, 46, 7548. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Dinesh, R.; Honma, I. Controlled synthesis of nanocrystalline Li2MnSiO4 particles for high capacity cathode application in lithium-ion batteries. Chem. Commun. 2012, 48, 2698–2700. [Google Scholar] [CrossRef]
- Dinesh, R.; Devaraju, M.K.; Tomai, T.; Unemoto, A.; Honma, I. Ultrathin nanosheets of Li2MSiO4 (M = Fe, Mn) as high-capacity Li-ion battery electrode. Nano Lett. 2012, 12, 1146. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Tomai, T.; Unemoto, A.; Honma, I. Novel processing of lithium manganese silicate nanomaterials for Li-ion battery applications. RSC Adv. 2013, 3, 608–615. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Tomai, T.; Honma, I. Supercritical hydrothermal synthesis of rod like Li2FeSiO4 particles for cathode application in lithium ion batteries. Electrochem. Acta 2013, 109, 75–78. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Truong, Q.D.; Honma, I. Synthesis of Li2CoSiO4 nanoparticles and structure observation by annular bright and dark field electron microscopy. RSC Adv. 2013, 43, 20633–20638. [Google Scholar] [CrossRef]
- Devaraju, M.K.; Honma, I. One-pot synthesis of Li2FePO4F nanoparticles via a supercritical fluid process and characterization for application in lithium-ion batteries. RSC Adv. 2013, 43, 19849–19852. [Google Scholar] [CrossRef]
- Liu, J.; Conry, T.E.; Song, X.Y.; Yang, L.; Doeff, M.M.; Richardson, T.J. Spherical nanoporous LiCoPO4/C composites as high performance cathode materials for rechargeable lithium-ion batteries. J. Mater. Chem. 2011, 21, 9984–9987. [Google Scholar] [CrossRef]
- Doan, T.N.L.; Taniguchi, I. Preparation of LiCoPO4/C nanocomposite cathode of lithium batteries with high rate performance. J. Power Sources 2011, 196, 5679–5684. [Google Scholar] [CrossRef]
- Truong, Q.D.; Devaraju, M.K.; Tomai, T.; Honma, I. Direct observation of antisite defects in LiCoPO4 cathode materials by annular dark and bright field electron microscopy. ACS Appl. Mater. Interfaces 2013, 5, 9926–9932. [Google Scholar]
- Han, D.W.; Kang, Y.M.; Yin, R.Z.; Song, M.S.; Kwon, H.S. Effects of Fe doping onthe electrochemical performance of LiCoPO4/C composites for high power-density cathode materials. Eletrochem. Commun. 2009, 11, 137–140. [Google Scholar]
- Ehrenberg, H.; Bramnik, N.N.; Senyshyn, A.; Fuess, H. Crystal and magnetic structures of electrochemically delithiated Li1−xCoPO4 phases. Solid State Sci. 2009, 11, 18–23. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Nikolowski, K.; Baehtz, C.; Bramnik, K.G.; Ehrenberg, H. Phase transitions occurring upon lithium insertion−extraction of LiCoPO4. Chem. Mater. 2007, 19, 908–915. [Google Scholar] [CrossRef]
- Izumi, F.; Ikeda, T. A Rietvled-analysis program REITAN-98 and its applications to zeolites. Mater. Sci. Forum 2000, 321–324, 198–203. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Devaraju, M.K.; Truong, Q.D.; Hyodo, H.; Tomai, T.; Honma, I. Supercritical Fluid Synthesis of LiCoPO4 Nanoparticles and Their Application to Lithium Ion Battery. Inorganics 2014, 2, 233-247. https://doi.org/10.3390/inorganics2020233
Devaraju MK, Truong QD, Hyodo H, Tomai T, Honma I. Supercritical Fluid Synthesis of LiCoPO4 Nanoparticles and Their Application to Lithium Ion Battery. Inorganics. 2014; 2(2):233-247. https://doi.org/10.3390/inorganics2020233
Chicago/Turabian StyleDevaraju, Murukanahally Kempaiah, Quang Duc Truong, Hiroshi Hyodo, Takaaki Tomai, and Itaru Honma. 2014. "Supercritical Fluid Synthesis of LiCoPO4 Nanoparticles and Their Application to Lithium Ion Battery" Inorganics 2, no. 2: 233-247. https://doi.org/10.3390/inorganics2020233