The Role of Lead (Pb) in the High Temperature Formation of MoS2 Nanotubes
Abstract
:1. Introduction
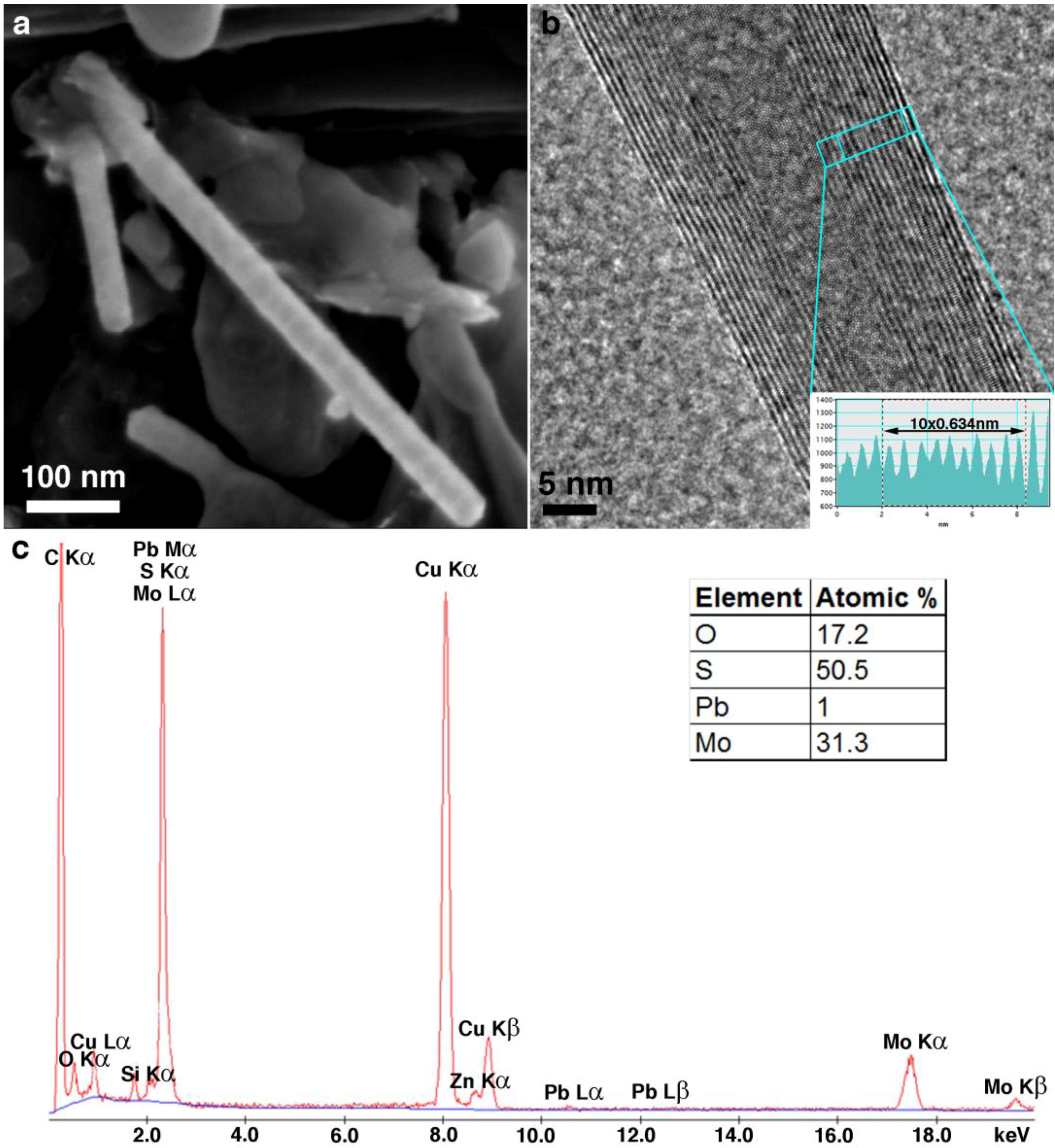
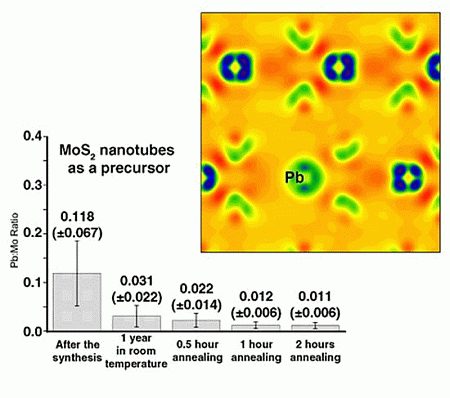
2. Results and Discussion
2.1. Annealing
| Precursor | After the Solar Ablation Synthesis | After a Year in Ambient Conditions | 30 min Annealing in H2S | 1 h Annealing in H2S | 2 h Annealing in H2S |
|---|---|---|---|---|---|
| MoO3−x | MoO3−x (1) | MoO3−x (2) | MoS2 (3a) | MoS2 (3b) | MoS2 (3c) |
| MoS2 | MoS2 (1) | MoS2 (2) | MoS2 (4a) | MoS2 (4b) | MoS2 (4c) |
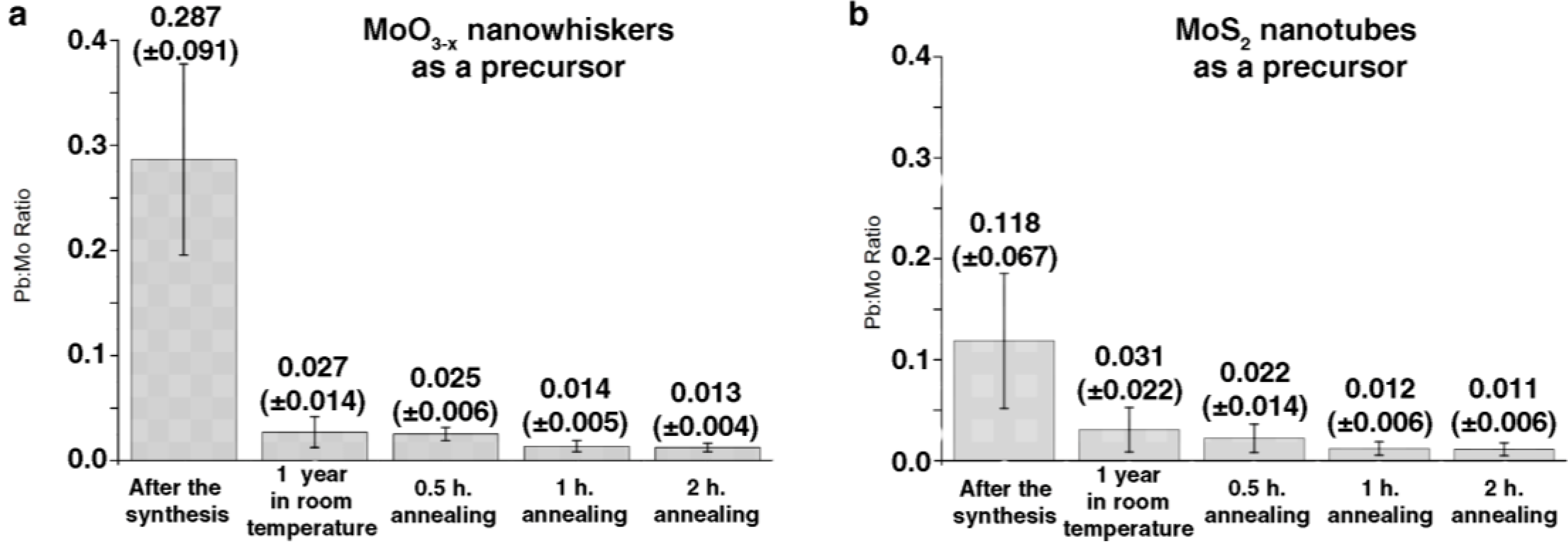
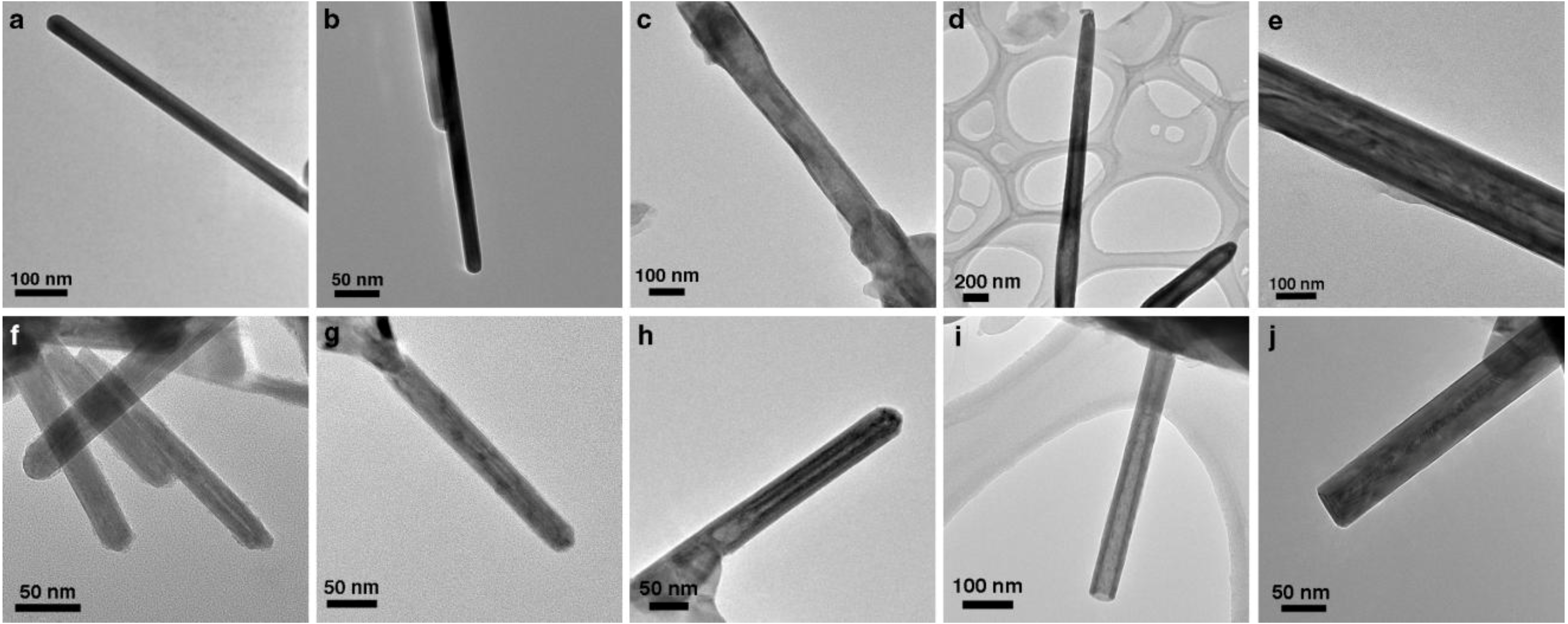
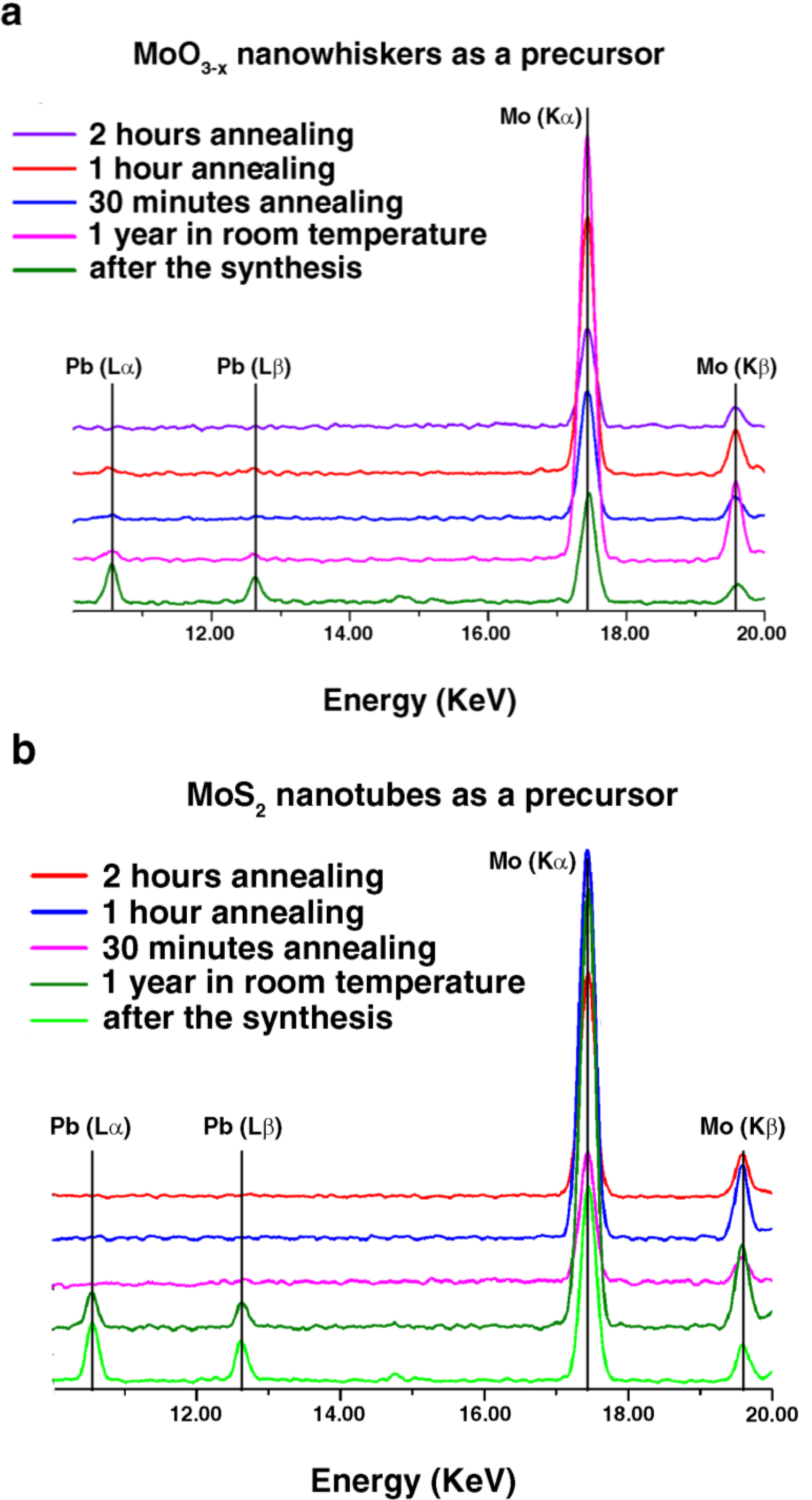
2.2. DFT Calculations of Mo1−xPbxS2 Solid Solutions and Pby/MoS2 Intercalates
2.2.1. Electronic Structure
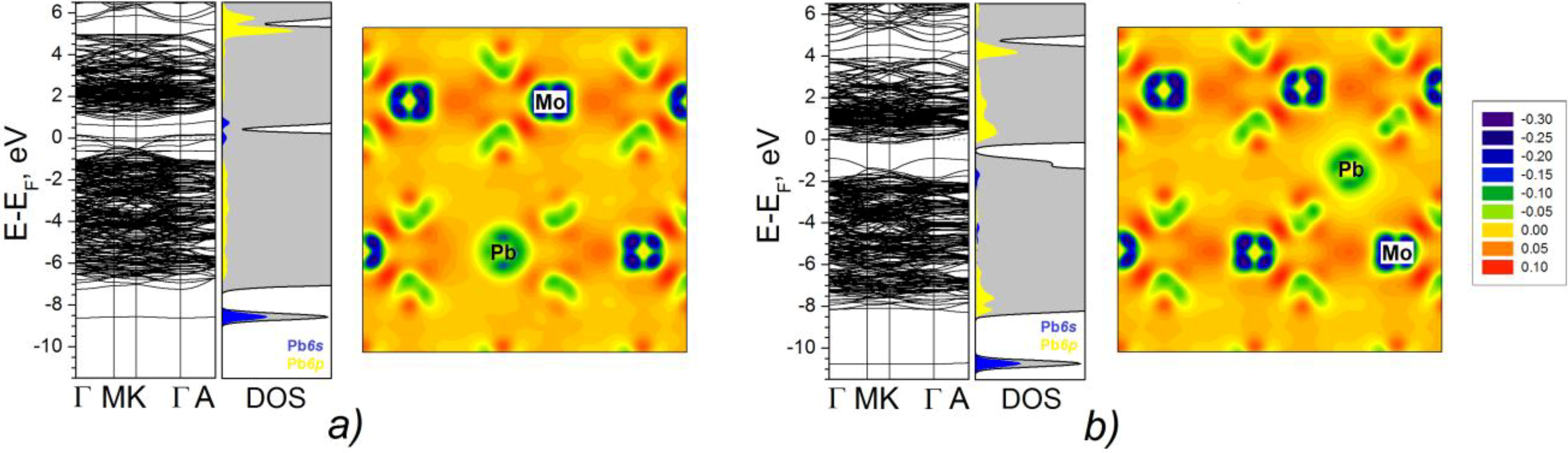
2.2.2. Estimation of the Stability Limit for Lead Atoms in the MoS2 Lattices
| Isomer | Pb atoms Arrangement | Supercell Composition | Ecoh, eV/atom | ΔE, eV/Pb |
| no Pb atoms | Mo32S64, x,y = 0.00 | −1.58 | - | |
| Mo1−xPbxS2 Doped by | ||||
| D1 | single Pb atom | Pb1Mo31S64, x = 0.031 | −1.50 | 4.65 |
| D2a | two neighbor Pb atoms within the same layer | Pb2Mo30S64, x = 0.063 | −1.45 | 3.75 |
| D2b | two distant Pb atoms within the same layer | Pb2Mo30S64, x = 0.063 | −1.43 | 4.63 |
| D2c | two neighbor Pb atoms in different layers | Pb2Mo30S64, x = 0.063 | −1.43 | 4.42 |
| D2d | two distant Pb atoms in different layers | Pb2Mo30S64, x = 0.063 | −1.43 | 4.46 |
| D3a | three neighbor Pb atoms within the same layer | Pb3Mo29S64, x = 0.094 | −1.39 | 3.34 |
| D4a | four neighbor Pb atoms within the same layer | Pb4Mo28S64 , x = 0.125 | −1.33 | 3.18 |
| Pby/MoS2 Intercalated by | ||||
| I1 | single Pb atom | Pb1/Mo32S64, y = 0.031 | −1.51 | 5.72 |
| I2a | two neighbor Pb atoms within the same vdW gap | Pb2/Mo32S64, y = 0.063 | −1.47 | 3.79 |
| I2b | two distant Pb atoms within the same vdW gap | Pb2/Mo32S64, y = 0.063 | −1.46 | 4.28 |
| I2c | two neighbor Pb atoms in different vdW gaps | Pb2/Mo32S64, y = 0.063 | −1.44 | 5.66 |
| I2d | two distant Pb atoms in different vdW gaps | Pb2/Mo32S64, y = 0.063 | −1.43 | 5.73 |

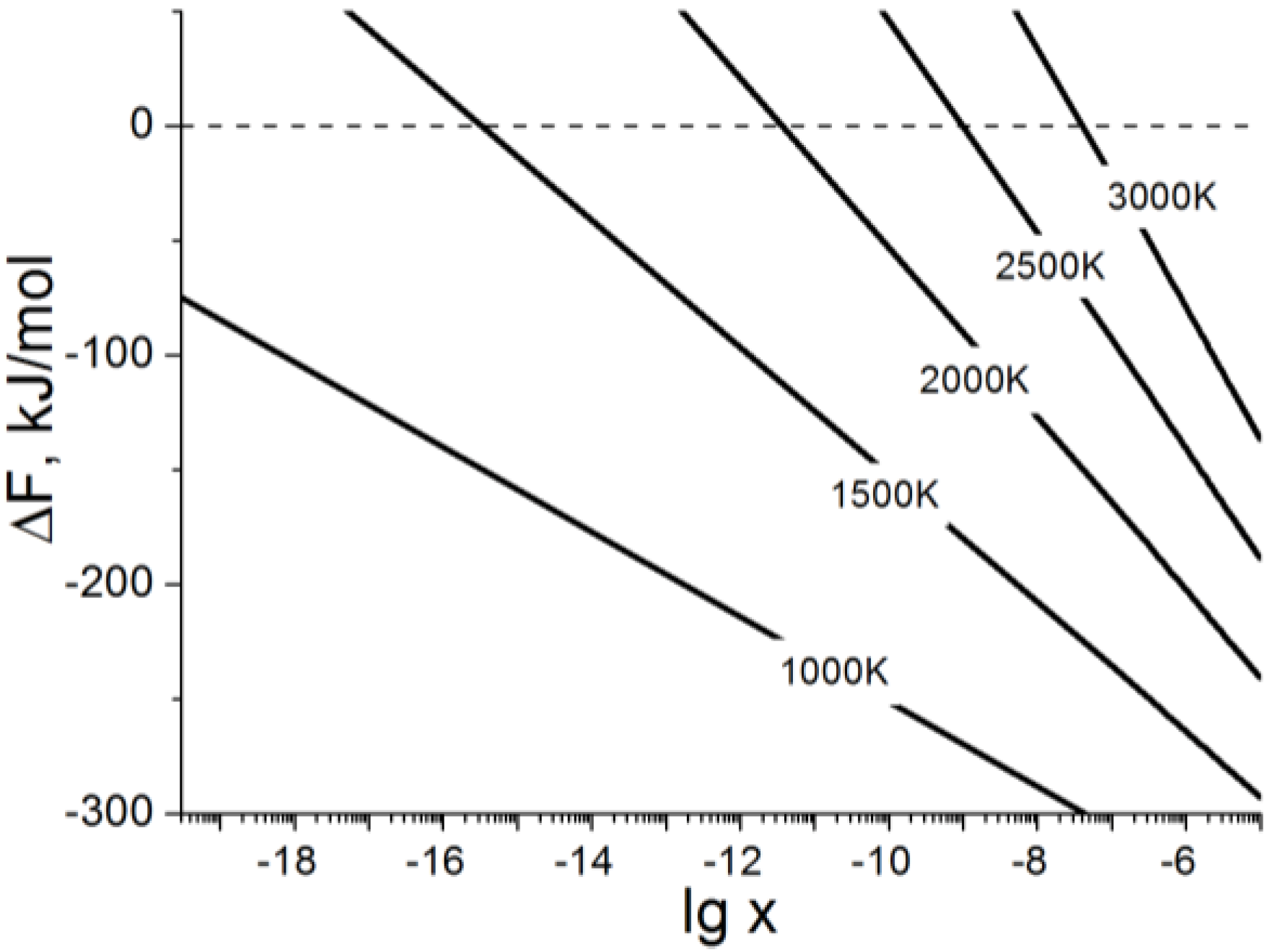
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaplan-Ashiri, I.; Cohen, S.; Gartsman, K.; Rosentsveig, R.; Seifert, G.; Tenne, R. Mechanical behavior of individual WS2 nanotubes. J. Mater. Res. 2004, 19, 454–459. [Google Scholar] [CrossRef]
- Kaplan-Ashiri, I.; Cohen, S.R.; Gartsman, K.; Ivanovskaya, V.; Heine, T.; Seifert, G.; Wiesel, I.; Wagner, H.; Tenne, R. On the mechanical behavior of WS2 nanotubes under axial tension and compression. Proc. Natl. Acad. Sci. USA 2006, 103, 523. [Google Scholar] [CrossRef]
- Nagapriya, K.S.; Goldbart, O.; Kaplan-Ashiri, I.; Seifert, G.; Tenne, R.; Joselevich, E. Torsional stick-slip behavior in WS2 nanotubes. Phys. Rev. Lett. 2008, 101, 195501. [Google Scholar] [CrossRef]
- Frey, G.L.; Tenne, R.; Matthews, M.J.; Dresselhaus, M.S.; Dresselhaus, G. Optical properties of MS2 (M = Mo, W) inorganic fullerenelike and nanotube material optical absorption and resonance raman measurements. J. Mater. Res. 1998, 13, 2412–2417. [Google Scholar] [CrossRef]
- Staiger, M.; Rafailov, P.; Gartsman, K.; Telg, H.; Krause, M.; Radovsky, G.; Zak, A.; Thomsen, C. Excitonic resonances in WS2 nanotubes. Phys. Rev. B 2012, 86. [Google Scholar] [CrossRef]
- Virsek, M.; Jesih, A.; Milosevic, I.; Damnjanovic, M.; Remskar, M. Raman scattering of the MoS2 and WS2 single nanotubes. Surf. Sci. 2007, 601, 2868–2872. [Google Scholar] [CrossRef]
- Enyashin, A.; Gemming, S.; Seifert, G. Nanosized allotropes of molybdenum disulfide. Eur. Phys. J.-Spec. Top. 2007, 149, 103–125. [Google Scholar] [CrossRef]
- Levi, R.; Bitton, O.; Leitus, G.; Tenne, R.; Joselevich, E. Field-effect transistors based on WS2 nanotubes with high current-carrying capacity. Nano Lett. 2013, 13, 3736–3741. [Google Scholar] [CrossRef]
- Remskar, M.; Skraba, Z.; Cleton, F.; Sanjines, R.; Levy, F. MoS2 as microtubes. Appl. Phys. Lett. 1996, 69, 351–353. [Google Scholar] [CrossRef]
- Remskar, M.; Mrzel, A.; Virsek, M.; Godec, M.; Krause, M.; Kolitsch, A.; Singh, A.; Seabaugh, A. The MoS2 nanotubes with defect-controlled electric properties. Nanoscale Res. Lett. 2010, 6, 26. [Google Scholar]
- Yella, A.; Mugnaioli, E.; Panthöfer, M.; Therese, H.A.; Kolb, U.; Tremel, W. Bismuth-catalyzed growth of SnS2 nanotubes and their stability. Angew. Chem. Int. Ed. 2009, 48, 6426–6430. [Google Scholar] [CrossRef]
- Radovsky, G.; Popovitz-Biro, R.; Stroppa, D.G.; Houben, L.; Tenne, R. Nanotubes from chalcogenide misfit compounds: Sn–S and Nb–Pb–S. Acc. Chem. Res. 2013, 47, 406–416. [Google Scholar]
- Radovsky, G.; Popovitz-Biro, R.; Staiger, M.; Gartsman, K.; Thomsen, C.; Lorenz, T.; Seifert, G.; Tenne, R. Synthesis of copious amounts of SnS2 and SnS2/SnS nanotubes with ordered superstructures. Angew. Chem. Int. Ed. 2011, 50, 12316–12320. [Google Scholar] [CrossRef]
- Zak, A.; Sallacan-Ecker, L.; Margolin, A.; Genut, M.; Tenne, R. Insight into the growth mechanism of WS2 nanotubes in the scaled-up fluidized bed reactor. Nano 2009, 4, 91–98. [Google Scholar] [CrossRef]
- Brontvein, O.; Stroppa, D.G.; Popovitz-Biro, R.; Albu-Yaron, A.; Levy, M.; Feuerman, D.; Houben, L.; Tenne, R.; Gordon, J.M. New high-temperature Pb-catalyzed synthesis of inorganic nanotubes. J. Am. Chem. Soc. 2012, 134, 16379–16386. [Google Scholar] [CrossRef]
- Brontvein, O.; Jayaram, V.; Reddy, K.P.J.; Gordon, J.M.; Tenne, R. Two-step synthesis of MoS2 nanotubes using shock waves with lead as growth promoter. Z. Anorg. Allg. Chem. 2014, 640, 1152–1158. [Google Scholar]
- Feldman, Y.; Wasserman, E.; Srolovitz, D.; Tenne, R. High-rate, gas-phase growth of MoS2 nested inorganic fullerenes and nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef]
- Yamazoe, N.; Kihlborg, L. Mo5O14—Twinning and three-dimensional structure, determined from a partly tantalum-substituted crystal. Acta Crystallogr. Sect. B 1975, 31, 1666–1672. [Google Scholar] [CrossRef]
- Yamazoe, N.; Ekstrom, T.; Kihlborg, L. Structural effects of vanadium substitution in molybdenum oxide (Mo17O47). Acta Chem. Scand. Ser. A 1975, 29, 404–408. [Google Scholar] [CrossRef]
- Kihlborg, L. Stabilization of the tunnel structure of molybdenum oxide (Mo5O14) by partial metal atom substitution. Acta Chem. Scand. 1969, 23, 1834–1835. [Google Scholar] [CrossRef]
- Boker, T.; Severin, R.; Muller, A.; Janowitz, C.; Manzke, R.; Voss, D.; Kruger, P.; Mazur, A.; Pollmann, J. Band structure of MoS2, MoSe2, and α-MoTe2: Angle-resolved photoelectron spectroscopy and ab initio calculations. Phys. Rev. B 2001, 64, 235305. [Google Scholar] [CrossRef]
- Walsh, A. Effects of reduced dimensionality on the electronic structure and defect chemistry of semiconducting hybrid organic-inorganic PbS solids. Proc. R. Soc. A 2011, 467, 1970–1985. [Google Scholar] [CrossRef]
- Ordejon, P.; Artacho, E.; Soler, J.M. Self-consistent order-N density-functional calculations for very large systems. Phys. Rev. B 1996, 53, 10441–10444. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garcia, A.; Junquera, J.; Ordejon, P.; Sanchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys.-Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef]
- Moreno, J.; Soler, J.M. Optimal meshes for integrals in real-space and reciprocal-space unit cells. Phys. Rev. B 1992, 45, 13891–13898. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brontvein, O.; Tenne, R.; Enyashin, A. The Role of Lead (Pb) in the High Temperature Formation of MoS2 Nanotubes. Inorganics 2014, 2, 363-376. https://doi.org/10.3390/inorganics2020363
Brontvein O, Tenne R, Enyashin A. The Role of Lead (Pb) in the High Temperature Formation of MoS2 Nanotubes. Inorganics. 2014; 2(2):363-376. https://doi.org/10.3390/inorganics2020363
Chicago/Turabian StyleBrontvein, Olga, Reshef Tenne, and Andrey Enyashin. 2014. "The Role of Lead (Pb) in the High Temperature Formation of MoS2 Nanotubes" Inorganics 2, no. 2: 363-376. https://doi.org/10.3390/inorganics2020363