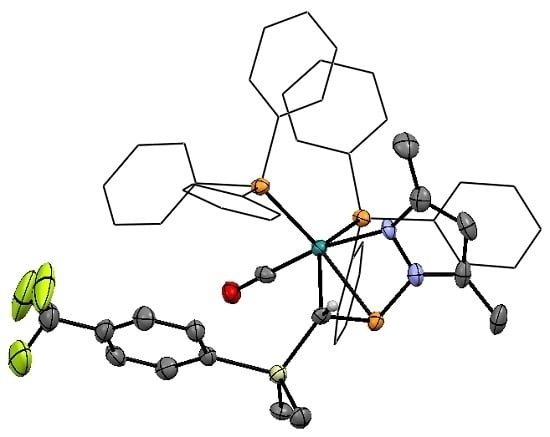
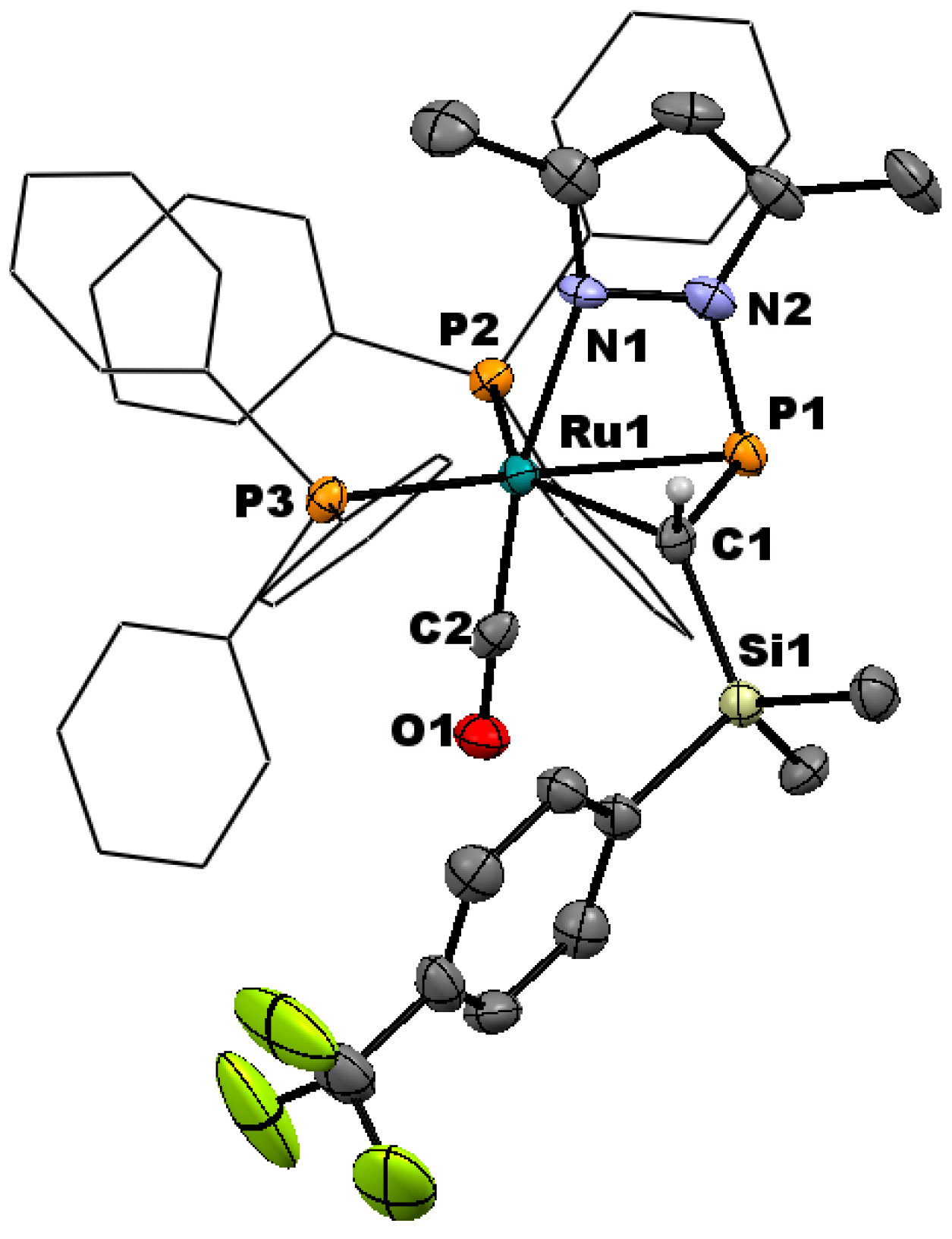
η1:η2-P-Pyrazolylphosphaalkene Complexes of Ruthenium(0)
Abstract
:1. Introduction
2. Results and Discussion
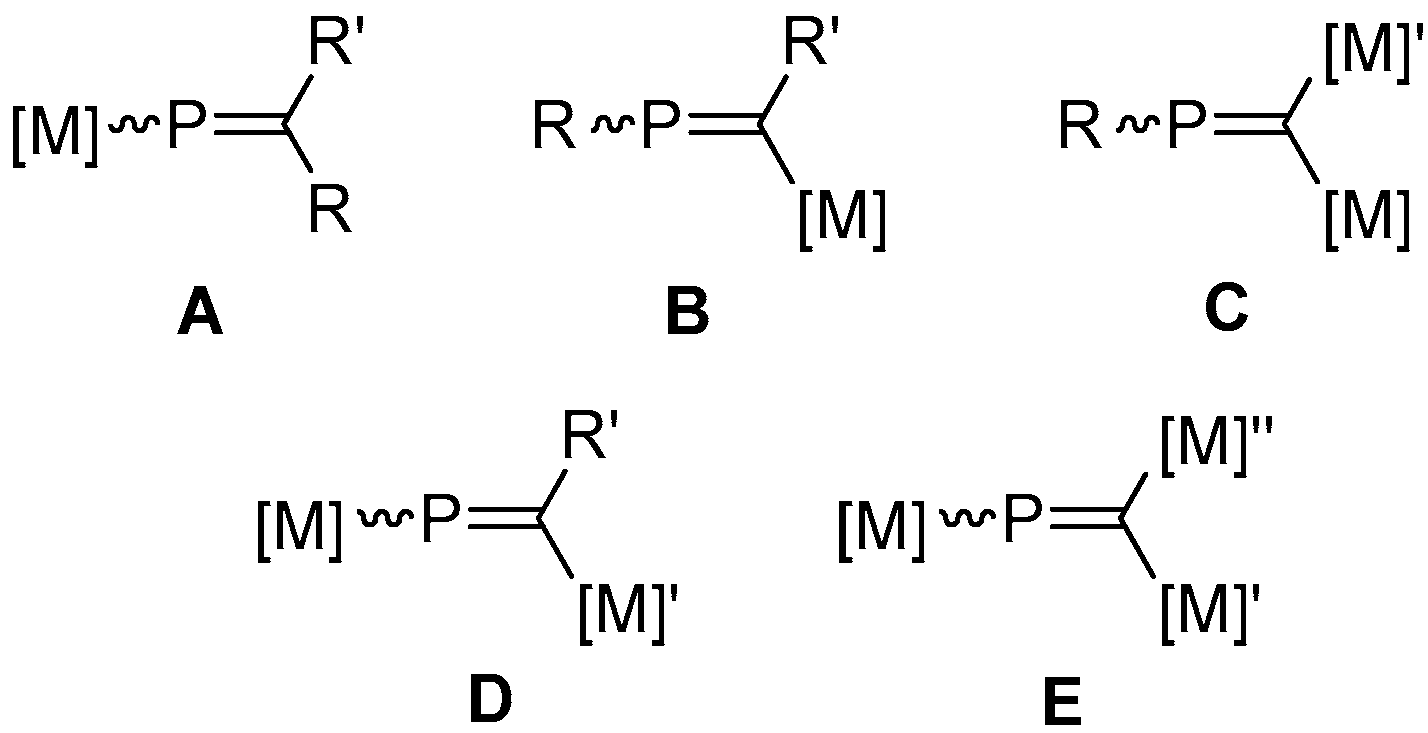
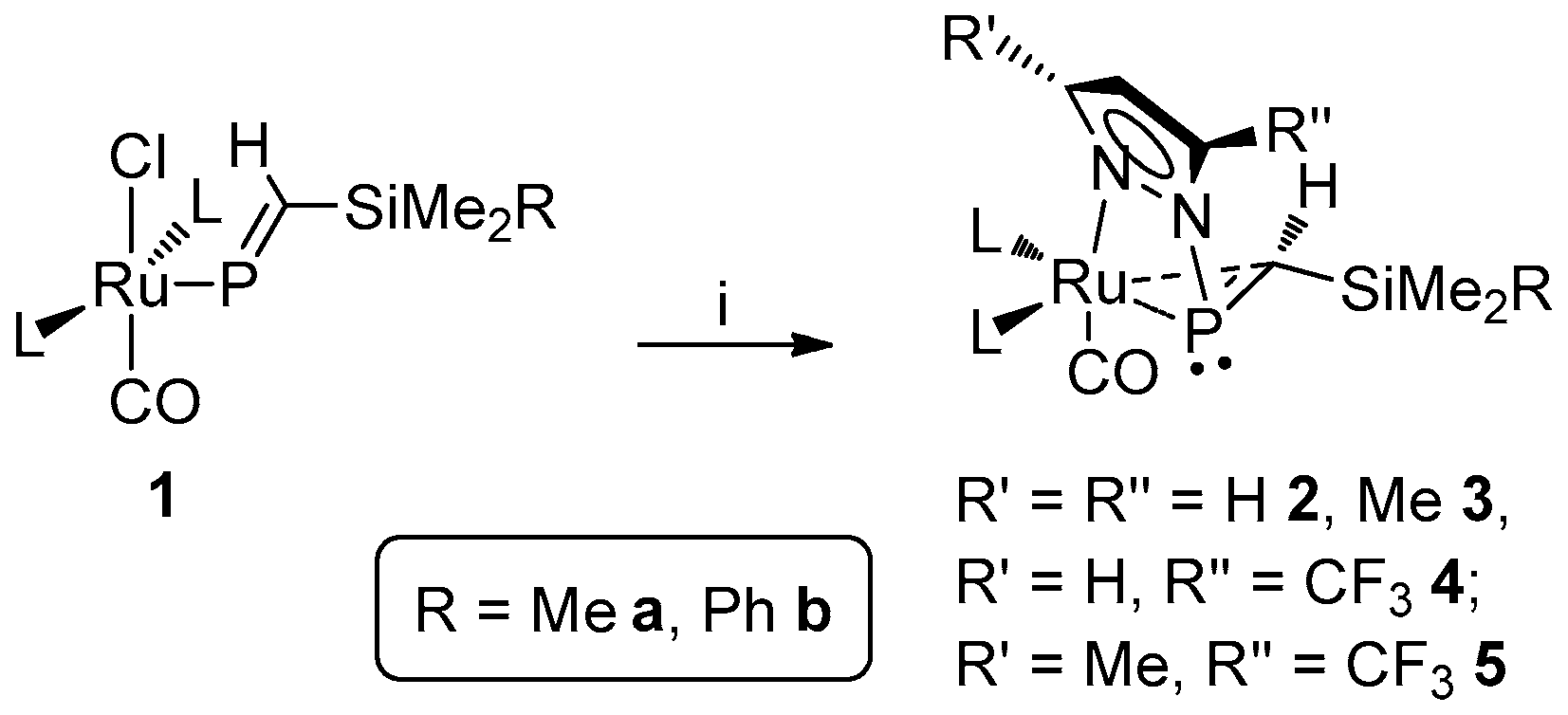
2.1. Synthesis and Characterization of η2-Pyrazolylphosphaalkene Complexes
2.2. Spectroscopic Features and Trends
3. Materials and Methods
3.1. General Methods
3.2. X-Ray Crystallography
3.3. Syntheses and Characterisation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Waterman, R. Phosphorus chemistry: Discoveries and advances. Dalton Trans. 2016, 45, 1801–1803. [Google Scholar] [CrossRef] [PubMed]
- Mathey, F. Phospha-Organic Chemistry: Panorama and Perspectives. Angew. Chem. Int. Ed. 2003, 42, 1578–1604. [Google Scholar] [CrossRef] [PubMed]
- Dillon, K.B.; Mathey, F.; Nixon, J.F. Phosphorus: The Carbon Copy; Wiley: Chichester, UK, 1998. [Google Scholar]
- Nixon, J.F. Recent developments in the organometallic chemistry of phospha-alkynes, RC-P. Coord. Chem. Rev. 1995, 145, 201–258. [Google Scholar]
- Appel, R. Multiple Bonds and Low Coordination in Phosphorus Chemistry; Regitz, M., Scherer, O.J., Eds.; Thieme: Stutgart, Germany, 1990. [Google Scholar]
- Markovski, L.N.; Romanenko, V.D. Phosphaalkynes and phosphaalkenes. Tetrahedron 1989, 45, 6019–6090. [Google Scholar] [CrossRef]
- Nixon, J.F. Coordination chemistry of compounds containing phosphorus–carbon multiple bonds. Chem. Rev. 1988, 88, 1327–1362. [Google Scholar] [CrossRef]
- Appel, R.; Knoll, F.; Ruppert, I. Phospha-alkenes and Phospha-alkynes, Genesis and Properties of the (p–p) π-Multiple Bond. Angew. Chem. Int. Ed. Engl. 1981, 20, 731–744. [Google Scholar] [CrossRef]
- Cordaro, J.G.; Stein, D.; Rüegger, H.; Grützmacher, H. Making the True “CP” Ligand. Angew. Chem. Int. Ed. 2006, 45, 6159–6162. [Google Scholar] [CrossRef] [PubMed]
- Mansell, S.M.; Green, M.; Kilby, R.J.; Murry, M.; Russell, C.A. Facile preparation of trimethylsilylphosphaalkyne and its conversion to polyphospholide anions. C. R. Chim. 2010, 13, 1073–1081. [Google Scholar] [CrossRef]
- Mansell, S.M.; Green, M.; Russell, C.A. Coordination chemistry of trimethylsilylphosphaalkyne: A phosphaalkyne bearing a reactive substituent. Dalton Trans. 2012, 41, 14360–14368. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Schulten, C.; Stasch, A. The first complexes and cyclodimerisations of methylphosphaalkyne (P ≡CMe). Dalton Trans. 2006, 31, 3733–3735. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Alidori, S.; Puschmann, F.F.; Santiso-Quinones, G.; Benkö, Z.; Li, Z.; Becker, G.; Grützmacher, H.-F.; Grützmacher, H. Sodium Phosphaethynolate as a Building Block for Heterocycles. Angew. Chem. Int. Ed. 2014, 53, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Jupp, A.R.; Goichoechea, J.M. The 2-Phosphaethynolate Anion: A Convenient Synthesis and [2+2] Cycloaddition Chemistry. Angew. Chem. Int. Ed. 2013, 52, 10064–10067. [Google Scholar] [CrossRef] [PubMed]
- Mo, O.; Yanez, M.; Guillemin, J.-C.; Riague, E.H.; Gal, J.-F.; Maria, P.-C.; Poliart, C.D. The Gas-Phase Acidity of HCP, CH3CP, HCAs, and CH3CAs: An Unexpected Enhanced Acidity of the Methyl Group. Chem. Eur. J. 2002, 8, 4919–4924. [Google Scholar] [CrossRef]
- Markovski, L.N.; Romanenko, V.D. Phosphaalkynes and phosphaalkenes. Tetrahedron 1989, 45, 6019–6090. [Google Scholar] [CrossRef]
- Le Floch, P. Phosphaalkene, phospholyl and phosphinine ligands: New tools in coordination chemistry and catalysis. Coord. Chem. Rev. 2006, 250, 627–681. [Google Scholar] [CrossRef]
- De Vaumes, R.; Marinetti, A.; Mathey, F. Catalytic hydrogenation of the phosphorus–carbon double bond in phosphaalkene complexes. J. Organomet. Chem. 1991, 413, 411–417. [Google Scholar] [CrossRef]
- Van der Knaap, T.A.; Bickelhaupt, F.; Krasykamp, J.G.; van Koten, G.; Bernards, J.P.C.; Edzes, H.T.; Veeman, W.S.; de Boer, E.; Baerends, E.J. The η1- and η2-coordination in a (phosphaalkene)platinum(0) complex. Organometallics 1984, 3, 1804–1811. [Google Scholar] [CrossRef]
- Kraajkamp, J.G.; van Koten, G.; van der Knaap, T.A.; Bickelhaupt, F.; Stam, C.H. Influence of steric factors on the coordination mode (η1 or η2) of phosphaalkenes to zerovalent Pt(0)L2 centers. X-ray structure of bis(triphenylphosphine)[(2,6-dimethylphenyl)-9-fluorenylidenephosphine]platinum(0)-toluene. Organometallics 1986, 5, 2014–2020. [Google Scholar] [CrossRef]
- Appel, R.; Casser, C.; Knoch, F. Über niederkoordinierte phosphorverbindungen: XXXX. 2,4,6-tri-t-Butylphenylmethylenphosphan,ein vielseitiger ligand in übergangsmetall-komplexen. J. Organomet. Chem. 1985, 293, 213–217. [Google Scholar]
- Weber, L. Recent developments in the chemistry of metallophosphaalkenes. Coord. Chem. Rev. 2005, 249, 741–763. [Google Scholar] [CrossRef]
- Weber, L. Metallophosphaalkenes—from Exotics to Versatile Building Blocks in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1996, 35, 271–288. [Google Scholar] [CrossRef]
- Weber, L.; Reizig, K.; Boese, R.; Polk, M. Z-[(η5-C5H5)(CO)2Fe-P=C(OSiMe3)(tBu)], a Phosphaalkenyl-Complex with FeP Single Bond. Angew. Chem. Int. Ed. Engl. 1985, 24, 604–605. [Google Scholar] [CrossRef]
- Saunders, A.J.; Crossley, I.R.; Roe, S.M. Aroylphosphanes: Base-Free Synthesis and Their Coordination Chemistry with Platinum-Group Metals. Eur. J. Inorg. Chem. 2016, 25, 4076–4082. [Google Scholar] [CrossRef]
- Saundersa, A.J.; Crossley, I.R. Synthesis of 3-stannyl and 3-silyl propargyl phosphanes and the formation of a phosphinoallene. Dalton Trans. 2016, 45, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Trathen, N.; Leech, M.C.; Crossley, I.R.; Greenacre, V.K.; Roe, S.M. Synthesis and electronic structure of the first cyaphide-alkynyl complexes. Dalton Trans. 2014, 43, 9004–9007. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.J.; Crossley, I.R.; Coles, M.P.; Roe, S.M. Facile self-assembly of the first diphosphametacyclophane. Chem. Commun. 2012, 48, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, V.K.; Trathen, N.; Crossley, I.R. Ruthenaphosphaalkenyls: Synthesis, Structures, and Their Conversion to η2-Phosphaalkene Complexes. Organometallics 2015, 34, 2533–2542. [Google Scholar] [CrossRef]
- Trathen, N.; Greenacre, V.K.; Crossley, I.R.; Roe, S.M. Ambiphilic Reactivity of a Ruthenaphosphaalkenyl: Synthesis of P-Pyrazolylphosphaalkene Complexes of Ruthenium(0). Organometallics 2013, 32, 2501–2504. [Google Scholar] [CrossRef]
- Bedford, R.B.; Hill, A.F.; Jones, C. Phosphaalkyne Hydrometalation: Synthesis of [RuCl(P=CHtBu)(CO)(PPh3)2]. Angew. Chem. Int. Ed. Engl. 1996, 35, 547–549. [Google Scholar] [CrossRef]
- Bedford, R.B.; Hill, A.F.; Jones, C.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. Phosphaalkyne Hydrometalation: Synthesis and Reactivity of the Complexes [Ru(PCHCMe3)Cl(CA)(PPh3)2] (A = O, S). Organometallics 1998, 17, 4744–4753. [Google Scholar] [CrossRef]
- Brym, M.; Jones, C. Synthesis, characterisation and reactivity of the first diphosphaalkyne. Dalton Trans. 2003, 3665–3667. [Google Scholar] [CrossRef]
- Benuvenutti, M.H.A.; Cenac, N.; Nixon, J.F. Hydrozirconation of an η2-ligated phosphaalkyne. A new synthetic route to η2-ligated phosphaalkenes. Chem. Commun. 1997, 1327–1328. [Google Scholar] [CrossRef]
- Bedford, R.B.; Hibbs, D.E.; Hill, A.F.; Hursthouse, M.B.; Abdul Malik, K.M.; Jones, C. Complete metal-mediated reduction of the triple bond of a phosphaalkyne: X-ray structure of [Ru(PHFCH2But)Cl(CO)(CNC6H3Me2-2,6)(PPh3)2]BF4·CH2Cl2. Chem. Commun. 1996, 1895–1896. [Google Scholar] [CrossRef]
- Bedford, R.B.; Hill, A.F.; Jones, C.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. Novel syntheses of heterodinuclear phosphaalkenyl complexes: X-ray structure of [Ru{P(AuPPh3)=CHBut}Cl2(CO)(PPh3)2]. Chem. Commun. 1997, 179–180. [Google Scholar] [CrossRef]
- Hill, A.F.; Jones, C.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. A metallacyclic λ5-phosphaalkenyl complex of ruthenium(II): X-ray structure of [Ru{κ2-P(=O)CButC(=O)}(CNBut)2(PPh3)2]. Chem. Commun. 1998, 367–368. [Google Scholar] [CrossRef]
- Bedford, R.B.; Hill, A.F.; Jones, C.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. Co-ordinative activation of phosphaalkynes: Methyl neopentylidene phosphorane complexes of ruthenium(II); crystal structure of [Ru(MeP=CHBut)Cl(I)(CO)(PPh3)2]. J. Chem. Soc. Dalton Trans. 1997, 139–140. [Google Scholar] [CrossRef]
- Hill, A.F.; Jones, C.; White, A.J.P.; Williams, D.J.; Wilton-Ely, J.D.E.T. Mercuriophosphaalkene-P complexes: Crystal structure of [Ru{P(=CHBut)HgC5H4Fe(η-C5H5)}Cl2(CO)(PPh3)2]. J. Chem. Soc. Dalton Trans. 1998, 1419–1420. [Google Scholar] [CrossRef]
- Greenacre, V.K.; Crossley, I.R. Hydrochlorination of Ruthenaphosphaalkenyls: Unexpectedly Facile Access to Alkylchlorohydrophosphane Complexes. Organometallics 2016. Submitted. [Google Scholar]
- Maciel, G.E.; McIver, J.W., Jr.; Ostlund, N.S.; Pople, J.A. Approximate self-consistent molecular orbital theory of nuclear spin coupling. I. Directly bonded carbon–hydrogen coupling constants. J. Am. Chem. Soc. 1970, 92, 1–11. [Google Scholar] [CrossRef]
- Bohanna, C.; Esteruelas, M.A.; Lahoz, F.J.; Onate, E.; Oro, L.A.; Sola, E. Synthesis of Butadiene-Osmium(0) and -Ruthenium(0) Complexes by Reductive Carbon–Carbon Coupling of Two Alkenyl Fragments. Organometallics 1995, 14, 4825–4831. [Google Scholar] [CrossRef]
- Bolton, P.D.; Grellier, M.; Vautravers, N.; Vendier, L.; Sabo-Etienne, S. Access to Ruthenium(0) Carbonyl Complexes via Dehydrogenation of a Tricyclopentylphosphine Ligand and Decarbonylation of Alcohols. Organometallics 2008, 27, 5088–5093. [Google Scholar] [CrossRef]
- Hill, A.F.; Owen, G.R.; White, A.J.P.; Williams, D.J. The Sting of the Scorpion: A Metallaboratrane. Angew. Chem. Int. Ed. 1999, 38, 2759–2761. [Google Scholar] [CrossRef]
- Christian, D.F.; Roper, W.R. Isocyanide complexes of zerovalent ruthenium. Proton addition to a transition-metal base offering alternative base sites. J. Chem. Soc. D Chem. Commun. 1971, 1271–1272. [Google Scholar] [CrossRef]
- Herberhold, M.; Hill, A.F. The coordination chemistry of iminooxosulphuranes VI. Factors affecting coordination geometry in complexes of tosyliminooxosulphurane. J. Organomet. Chem. 1990, 395, 195–206. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. II 1987, 12, S1–S19. [Google Scholar] [CrossRef]
- Rakita, P.E.; Worsham, L.S. 13C NMR studies of organosilanes: IV. Vinyl- and allyl-silanes. J. Organomet. Chem. 1977, 139, 135–142. [Google Scholar] [CrossRef]
- Rakita, P.E.; Srebro, J.P.; Worsham, L.S. 13C NMR studies of organosilanes: I. Substituent-chemical-shift parameters for phenylsilanes. J. Organomet. Chem. 1976, 104, 27–37. [Google Scholar] [CrossRef]
- Boniface, S.M.; Clark, G.R.; Collins, T.J.; Roper, W.R. Preparation of octahedral hydrido-aquo-ruthenium(II) complexes, and structural characterisation of hydridoaquodicarbonylbis(triphenylphosphine)-ruthenium(II) tetrafluoroborate. J. Organomet. Chem. 1981, 206, 109–117. [Google Scholar] [CrossRef]
- Averre, C.E.; Coles, M.P.; Crossley, I.R.; Day, I.J. The open-chain triphosphanes RMe2SiCH2P(PR′2)2 (R = Me, Ph; R′ = SiMe3, Cy, Ph). Dalton Trans. 2012, 41, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K. Puschmann, H. OLEX2: A complete structure solution, refinement and analysis. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Compound | δP1 | δC2 | δH2 | |||
|---|---|---|---|---|---|---|
| R | P=C | PPh3 | P=C | P=CH (1JCH/Hz)3 | ||
| 24 | 2a | Me | 58.7 | 46.6, 42.0 | 45.1 | 1.59 (137) |
| (pz′ = pz) | 2b | Ph | 57.0 | 47.0, 41.7 | 42.6 | 1.72 (135) |
| 3 | 3a | Me4 | 32.9 | 46.6, 39.2 | 44.9 | 1.62 (123) |
| (pz′ = pzMe2) | 3b | Ph4 | 32.3 | 47.0, 38.9 | 41.8 | 1.77 (128) |
| 3c | C6H4Me-p5 | 32.6 | 46.7, 39.1 | 41.8 | 1.75 (136) | |
| 3d | C6H4CF3-p5 | 32.1 | 46.6, 38.6 | 39.8 | 1.66 (135) | |
| 45 | 4a | Me | 76.6 | 47.7, 41.5 | 47.1 | 1.78 (136) |
| (pz′ = pzCF3) | 4b | Ph | 74.9 | 48.0, 41.3 | 46.7 | 1.91 (136) |
| 4c | C6H4Me-p | 75.0 | 47.9, 41.3 | 45.4 | 1.90 (134) | |
| 4d | C6H4CF3-p | 73.8 | 47.8, 40.9 | 43.8 | 1.82 (134) | |
| 55 | 5a | Me | 64.6 | 46.9, 38.4 | 45.2 | 1.76 (129) |
| (pz′ = pzMe,CF3) | 5b | Ph | 62.7 | 47.2, 38.3 | 41.8 | 1.97 (131) |
| 5c | C6H4Me-p | 61.6 | 47.2, 38.4 | 42.1 | 1.97 (135) | |
| 5d | C6H4CF3-p | 62.0 | 47.1, 37.8 | 40.7 | 1.85 (133) | |
| 65 | 6a | Me | 64.4 | 47.4, 41.8 | 47.5 | 1.74 (137) |
| (pz′ = pzPh) | 6d | C6H4CF3-p | 60.5 | 47.7, 41.3 | 43.7 | 1.78 (136) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greenacre, V.K.; Crossley, I.R. η1:η2-P-Pyrazolylphosphaalkene Complexes of Ruthenium(0). Inorganics 2016, 4, 30. https://doi.org/10.3390/inorganics4040030
Greenacre VK, Crossley IR. η1:η2-P-Pyrazolylphosphaalkene Complexes of Ruthenium(0). Inorganics. 2016; 4(4):30. https://doi.org/10.3390/inorganics4040030
Chicago/Turabian StyleGreenacre, Victoria K., and Ian R. Crossley. 2016. "η1:η2-P-Pyrazolylphosphaalkene Complexes of Ruthenium(0)" Inorganics 4, no. 4: 30. https://doi.org/10.3390/inorganics4040030